Uninterrupted Development of Two Aphid Species Belonging to Cinara Genus during Winter Diapause
Abstract
1. Introduction
2. Materials and Methods
2.1. Aphids and Egg Material
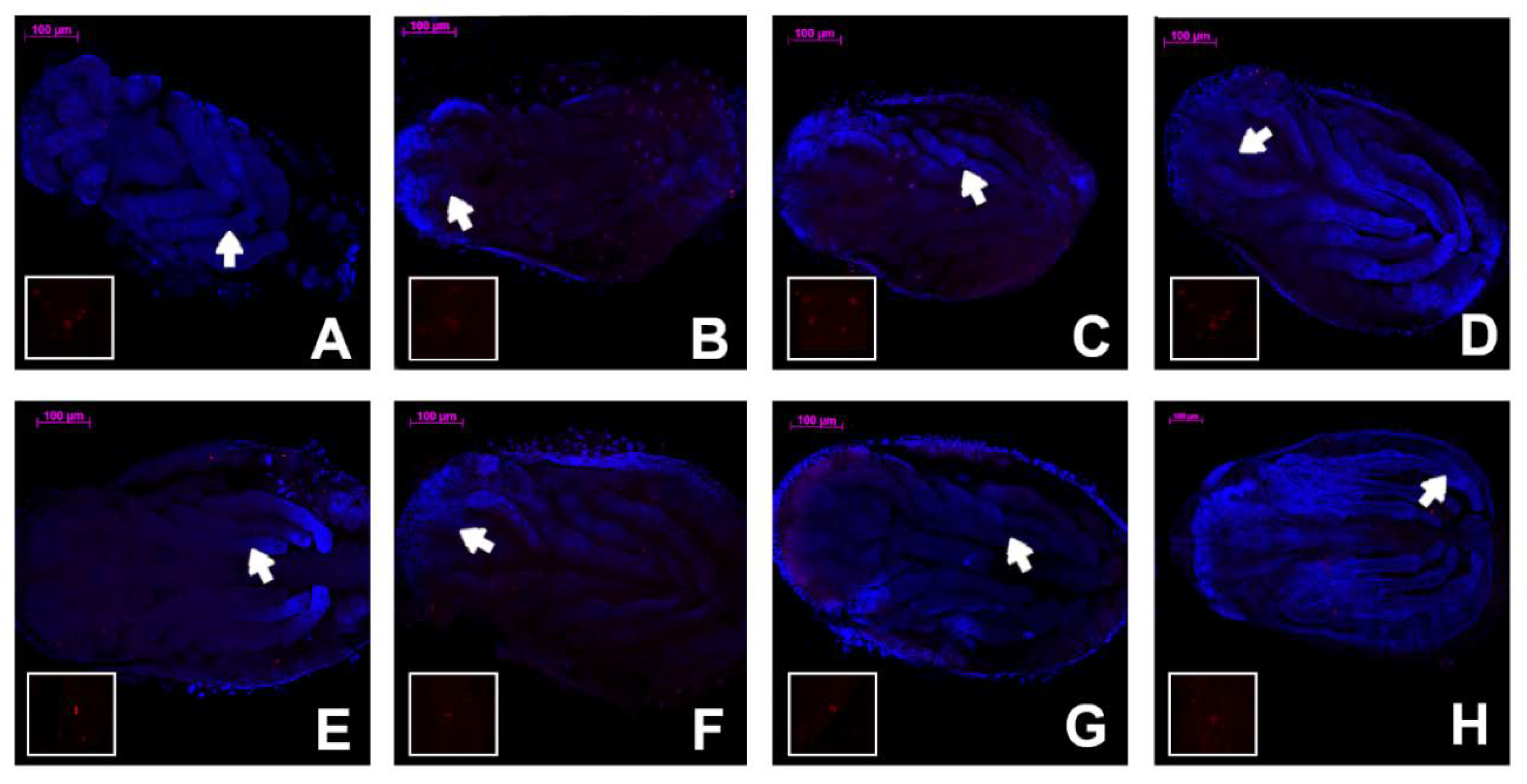
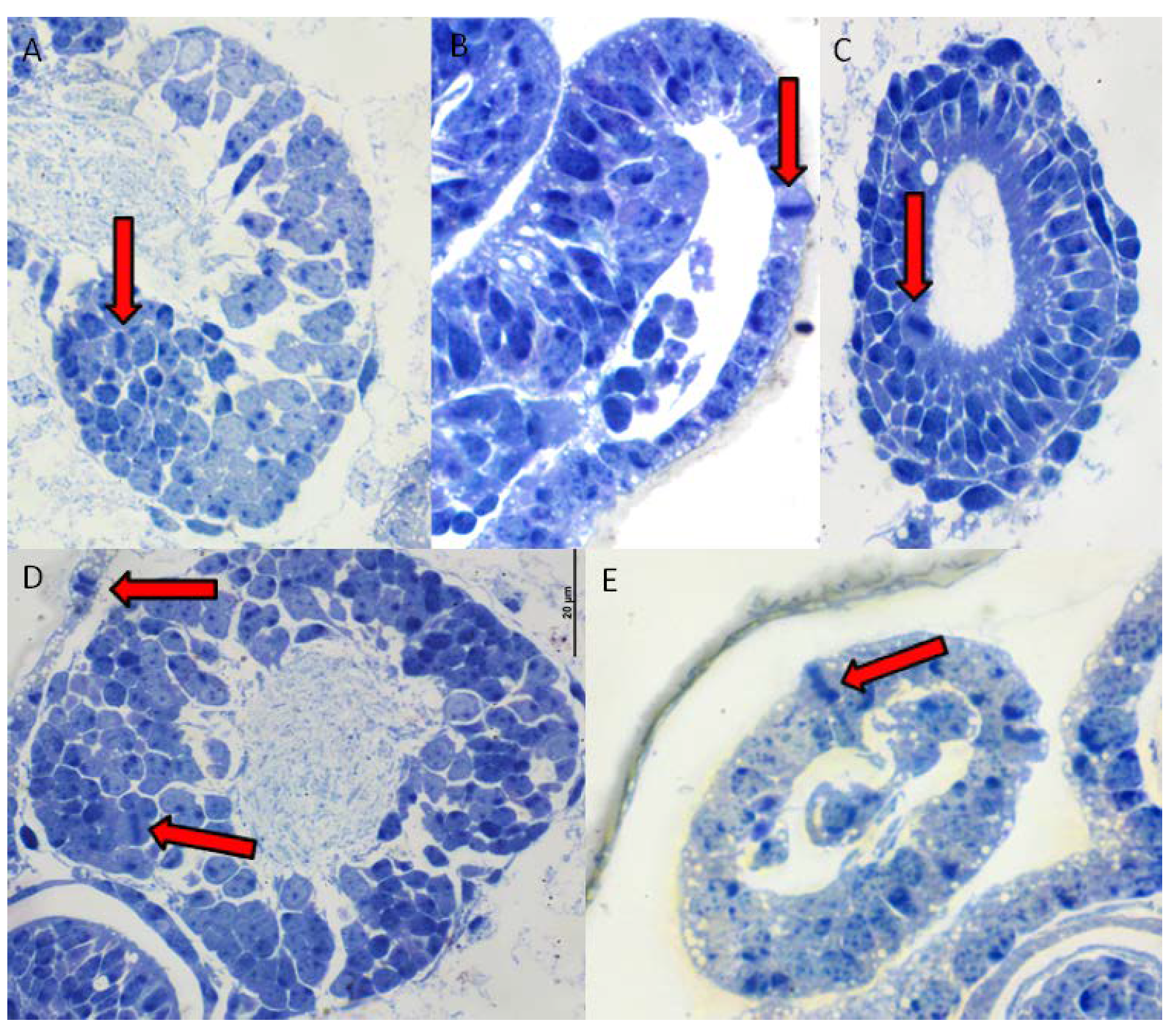
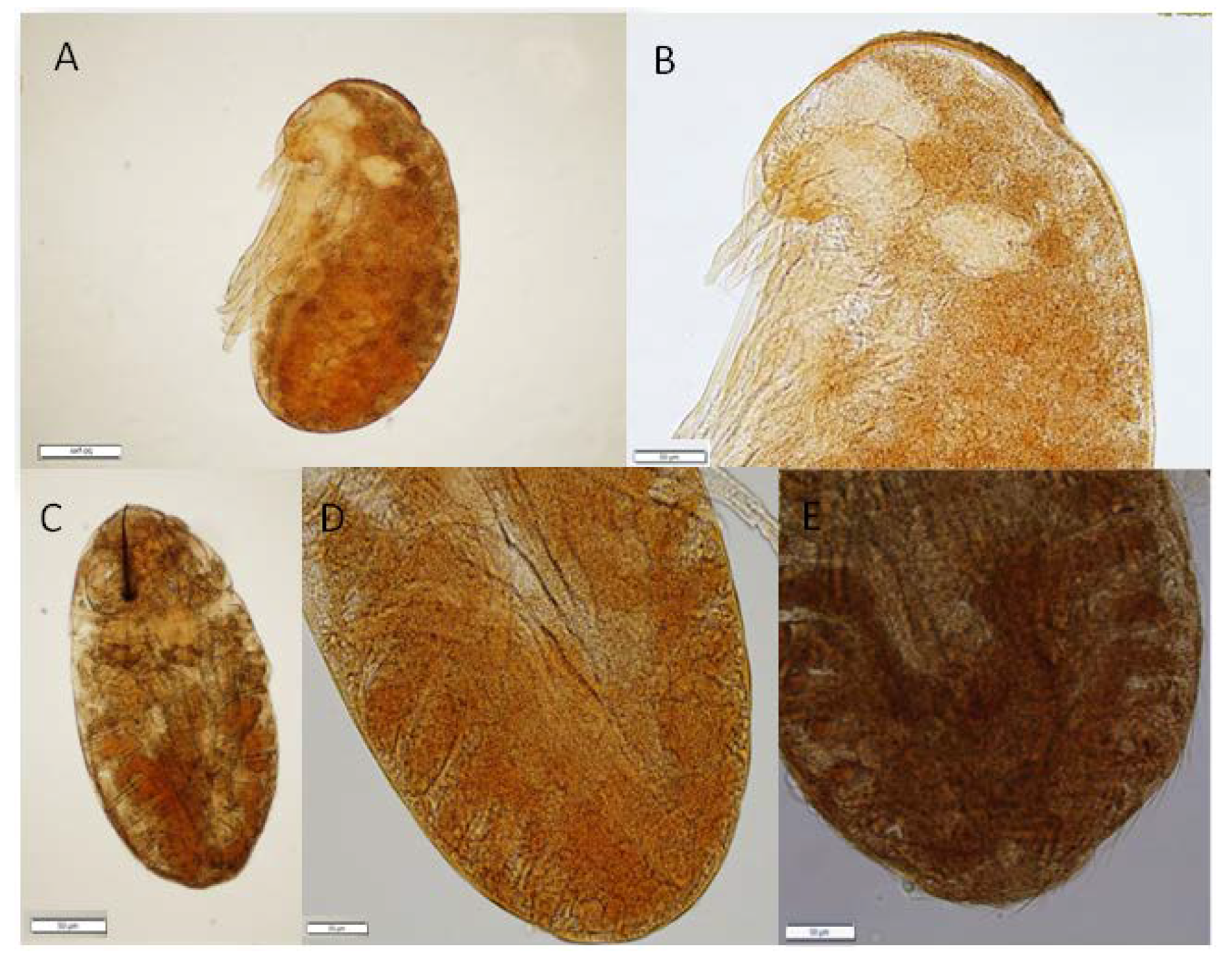
2.2. Fixing the Embryos, Dissection, and Staining
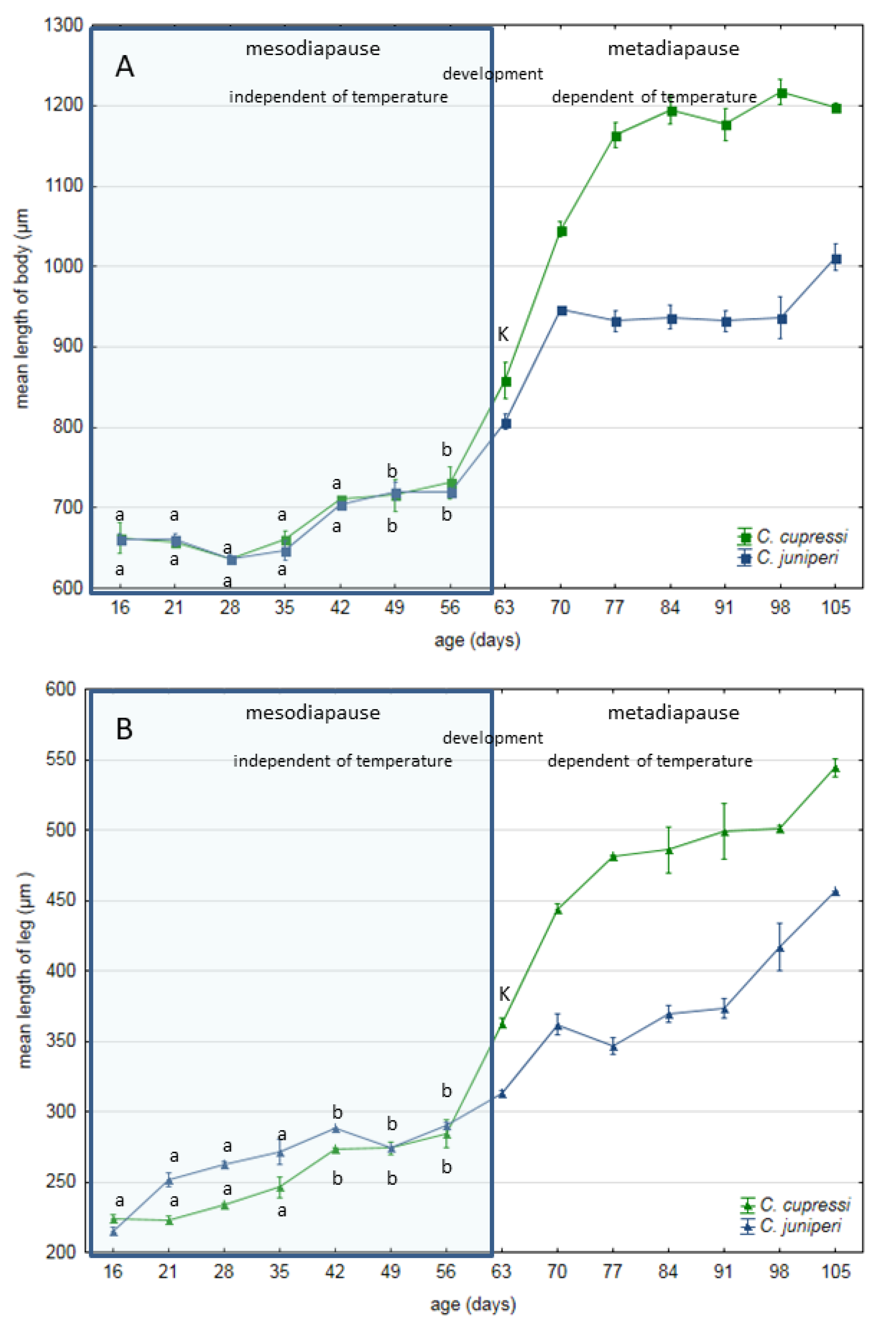
2.3. Embryo Size Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hullé, M.; d’Acier, A.C.; Bankhead-Dromet, S.; Harrington, R.A. Aphids in the face of global changes. Compt. Rend. Biol. 2010, 333, 497–503. Available online: https://www.eje.cz/pdfs/eje/2002/02/04 (accessed on 27 February 2012).
- Ryalls, J.M.W.; Harrington, R. Climate and atmospheric change impacts on aphids as vectors of plant diseases. In Global Climate Change and Terrestrial Invertebrates; Johnson, S.N., Jones, T.H., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 148–175. [Google Scholar]
- Trębicki, P.; Dáder, D.; Vassiliadis, S.; Fereres, A. Insect-plant-pathogen interactions as shaped by future climate: Effects on biology, distribution and implications for agriculture. Insect Sci. 2017, 24, 975–989. [Google Scholar] [CrossRef]
- Trębicki, P.; Vandegeer, R.; Bosque-Pérez, N.; Powell, K.; Dáder, B.; Freeman, A.; Yen, A.; Fitzgerald, G.; Luck, J. Virus infection mediates the effects of elevated CO2 on plants and vectors. Sci. Rep. 2016, 6, 22785. [Google Scholar] [CrossRef]
- Harrington, R.; Clark, S.J.; Welham, S.J.; Verrier, P.J.; Denholm, C.H.; Hulle, M. Environmental change and the phenology of European aphids. Glob. Chang. Biol. 2007, 13, 1550–1564. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Good, J.E. Herbivory in a global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 88, 1–16. [Google Scholar] [CrossRef]
- Bell, J.R.; Taylor, M.S.; Shortall, C.R.; Welham, S.J.; Harrington, R. The trait ecology and host plants of aphids and their distribution and abundance over the United Kingdom. Glob. Ecol. Biogeogr. 2012, 21, 405–415. [Google Scholar] [CrossRef]
- Bell, J.R.; Alderson, L.; Izera, D.; Kruger, T.; Parker, S.; Pickup, J.; Shortall, C.R.; Taylor, M.S.; Verrier, P.; Harrington, R. Long-term phenological trends, species accumulation rates, aphid traits and climate: Five decades of change in migrating aphids. J. Anim. Ecol. 2015, 84, 21–34. [Google Scholar] [CrossRef]
- Diniz, D.F.A.; de Albuquerque, C.M.R.; Oliva, L.O.; de Melo-Santos, M.A.V.; Ayres, C.F.J. Diapause and quiescence: Dormancy mechanisms that contribute to the geographical expansion of mosquitoes and their evolutionary success. Parasites Vectors 2017, 10, 310. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; University Press: New York, NY, USA; Oxford, UK, 1986. [Google Scholar]
- Hodek, I. Controversial aspects of diapause development. Eur. J. Entomol. 2002, 99, 163–173. [Google Scholar] [CrossRef]
- Koštál, V.; Šimůnková, P.; Kobelková, A.; Shimada, K. Cell cycle arrest as a hallmark of insect diapause: Changes in gene transcription during diapause induction in the drosophilid fly, Chymomyza costata. Insect Biochem. Molec. Biol. 2009, 39, 875–883. [Google Scholar] [CrossRef]
- Beck, S.D. Photoperiodic induction of diapause in an insect. Biol. Bull. 1962, 122, 1–12. [Google Scholar] [CrossRef]
- Andrewartha, H.G. Diapause in relation to the ecology of insects. Biol. Rev. 1952, 27, 50–107. [Google Scholar] [CrossRef]
- Denlinger, D.L. Regulation of diapauses. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Koštál, V. Eco-physiological Phases of Insect Diapause. J. Insect Physiol. 2006, 52, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bergland, A.O.; Behrman, E.L.; Gregory, B.D.; Petrov, D.A.; Schmidt, P.S. Global transcriptional profiling of diapause and climatic adaptation in Drosophila melanogaster. Mol. Biol. Evol. 2016, 33, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Tougeron, K. Diapause research in insects: Historical review and recent work perspectives. Ent. Exp. App. 2019, 167, 27–36. [Google Scholar] [CrossRef]
- Danks, H.V. The elements of seasonal adaptations in insects. Can. Entomol. 2007, 139, 1–44. [Google Scholar] [CrossRef]
- Williams, I.S.; Dixon, F.G. Life cycles and polymorphism. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CAB International: Wallingford, UK, 2007; pp. 69–81. [Google Scholar]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef]
- Kawada, K. Polymorphism and morph determination. In Aphids: Their Biology, Natural Enemies and Control; Minks, K., Harrewijin, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 2A, pp. 255–268. [Google Scholar]
- Durak, R. The overwintering strategy of the anholocyclic aphid Cinara tujafilina. Physiol. Entomol. 2014, 39, 313–321. [Google Scholar] [CrossRef]
- Strathdee, A.T.; Howling, G.G.; Bale, J.S. Cold hardiness of overwintering aphid eggs. J. Insect Physiol. 1995, 41, 653–657. [Google Scholar] [CrossRef]
- Behrendt, K. Uber die Eidepause von Aphis fabae Scop. (Homoptera, Aphididae). Zool. Jahrb. Physiol. 1963, 70, 309–398. [Google Scholar]
- Vilcinskas, A. Biology and Ecology of Aphids; CRC Press Taylor and Francis Group: Abingdon, UK, 2016. [Google Scholar]
- Blackman, R. Reproduction, Cytogenetics and Development. In Aphids: Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 2A, pp. 163–195. [Google Scholar]
- Visscher, S.N. The embryonic diapause of Aulocara elliotti (Orthoptera, Acrididiae). Cell Tissue Res. 1980, 174, 433–452. [Google Scholar] [CrossRef]
- Bohmel, W.; Jancke, O. Beitrag zur Embryonalentwicklung der Wintereier von Aphiden. Z. Angew. Entomol. 1942, 29, 636–658. [Google Scholar] [CrossRef]
- Lushai, D.; Hardie, J.; Harrington, R. Diapause termination and egg hatch in the bird cherry aphis, Rhopalosiphum padi. Ent. Exp. App. 1996, 81, 113–115. [Google Scholar] [CrossRef]
- Komazaki, S. Difference of egg diapause in two host races of the spirea aphid, Aphis spiraecola. Ent. Exp. App. 1998, 89, 201–205. [Google Scholar] [CrossRef]
- Shingleton, A.; Sisk, G.C.; Stern, D. Diapause in the pea aphid (Acyrthosiphon pisum) is a slowing but not a cessation of development. BMC Dev. Biol. 2003, 8, 3–7. [Google Scholar] [CrossRef]
- Durak, R.; Borowiak-Sobkowiak, B.; Socha, M. Bionomy and ecology of Cinara cupressi (Buckton, 1881) (Hemiptera, Aphidoidea). Pol. J. Ent. 2007, 76, 107–113. [Google Scholar]
- Durak, R.; Durak, T. Redescription of males of the aphid species Cinara (Cupressobium) tujafilina and Cinara (Cupressobium) cupressi (Hemiptera, Lachninae). Zootaxa 2015, 4032, 209–214. [Google Scholar] [CrossRef]
- Durak, R.; Węgrzyn, E.; Leniowski, K. Do all aphids benefit from climate warming?—An effect of temperature increase on a native species of temperate climatic zone Cinara juniperi. Ethol. Ecol. Evol. 2016, 28, 188–201. [Google Scholar] [CrossRef]
- Miura, T.; Braendle, C.; Shingleton, A.; Sisk, G.; Kambhampati, S.; Stern, D.L. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Exp. Zool. 2003, 295B, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and Chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Mian, R.; Mittapalli, O.; Michel, A.P. Characterization of a chitin synthase encoding gene and effect of diflubenzuron in soybean aphid, Aphis glycines. Int. J. Biol. Sci. 2012, 8, 1323–1334. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, H.W.; Huang, H.J.; Xue, J.; Wu, W.J.; Bao, Y.Y.; Xu, H.J.; Zhu, Z.R.; Cheng, J.A.; Zhang, C.X. Chitin synthase 1 gene and its two alternative splicing variants from two sap-sucking insects, Nilaparvata lugens and Laodelphax striatellus (Hemiptera: Delphacidae). Insect Biochem. Mol. Biol. 2012, 42, 637–646. [Google Scholar] [CrossRef]
- Arakane, Y.; Specht, C.A.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2008, 38, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Gagou, M.E.; Kapsetaki, M.; Turberg, A.; Kafetzopoulos, D. Stage-specific expression of the chitin synthase DmeChSA and DmeChSB genes during the onset of Drosophila metamorphosis. Insect Biochem. Mol. Biol. 2002, 32, 141–146. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Zhu, K.Y. Characterization of a chitin synthase cDNA and its increased mRNA level associated with decreased chitin synthesis in Anopheles quadrimaculatus exposed to diflubenzuron. Insect Biochem. Mol. Biol. 2006, 36, 712–725. [Google Scholar] [CrossRef]
- Saulich, A.K. The effects of photoperiod and density on the development of noctuids Cirphus unipuncta Haw. and Laphygma exigua Hb. (Lepidoptera, Noctuidae). Ent. Rev. 1975, 34, 52–59. [Google Scholar]
- Danks, H.V. Insect Dormancy: An Ecological Perspective; Biological Survey of Canada Monograph Series: Ottawa, ON, Canada, 1987. [Google Scholar]
- Kalushkov, P.; Hodkova, M.; Nedved, O.; Hodek, I. Effect of thermoperiod on diapause intensity in Pyrrhocoris apterus (Heteroptera Pyrrhocoridae). J. Insect Physiol. 2001, 47, 55–61. [Google Scholar] [CrossRef]
- Tatar, M.; Kopelman, A.; Epstein, D.; Tu, M.P.; Yin, C.M.; Garofalo, R.S. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001, 292, 107–110. [Google Scholar] [CrossRef]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001, 11, 213–221. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, Y.; Zheng, J.; Liang, L.; Hoffmann, A.A.; Ma, C.S. Response of heat shock protein genes of the oriental fruit moth under diapause and thermal stress reveals multiple patterns dependent on the nature of stress exposure. Cell Stress Chaperones 2016, 21, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, Z.; Rebeca, C.L.; Zhu, X.; Guo, Y.; Lin, Q.; Zhang, F. De novo characterization of the pine aphid Cinara pinitabulaeformis Zhang et Zhang transcriptome and analysis of genes relevant to pesticides. PLoS ONE 2017, 12, e0178496. [Google Scholar] [CrossRef] [PubMed]
- Diaz, F.; Orobio, R.F.; Chavarriaga, P.; Toro-Perea, N. Differential expression patterns among heat-shock protein genes and thermal responses in the whitefly Bemisia tabaci (MEAM 1). J. Thermal. Biol. 2015, 52, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.H.; Hegedus, D.; Soroka, J.; Coutu, C.; Bekkaoui, D.; Dosdall, L. Survival and Hsp70 gene expression in Plutella xylostella and its larval parasitoid Diadegma insulare varied between slowly ramping and abrupt extreme temperature regimes. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Hepat, R.; Kim, Y. RNA interference of a heat shock protein, Hsp70, loses its protection role in indirect chilling injury to the beet armyworm, Spodoptera exigua. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 168, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Li, A.Q.; Popova-Butler, A.; Dean, D.H.; Denlinger, D.L. Proteomics of the flesh fly brain reveals an abundance of upregulated heat shock proteins during pupal diapause. J. Insect Physiol. 2007, 53, 385–391. [Google Scholar] [CrossRef]
- Ragland, G.; Denlinger, D.; Hahn, D. Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc. Natl. Acad. Sci. USA 2010, 107, 14909–14914. [Google Scholar] [CrossRef]
- Colinet, H.; Renault, D.; Charoy-Guevel, B.; Com, E. Metabolic and proteomic profiling of diapause in the aphid parasitoid Praon volucre. PLoS ONE 2012, 7, e32606. [Google Scholar] [CrossRef]
- MacRae, T. Gene expression, metabolic regulation and stress tolerance during diapause. Cell Mol. Life Sci. 2010, 67, 2405–2424. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durak, R.; Dampc, J.; Dampc, J.; Bartoszewski, S.; Michalik, A. Uninterrupted Development of Two Aphid Species Belonging to Cinara Genus during Winter Diapause. Insects 2020, 11, 150. https://doi.org/10.3390/insects11030150
Durak R, Dampc J, Dampc J, Bartoszewski S, Michalik A. Uninterrupted Development of Two Aphid Species Belonging to Cinara Genus during Winter Diapause. Insects. 2020; 11(3):150. https://doi.org/10.3390/insects11030150
Chicago/Turabian StyleDurak, Roma, Jan Dampc, Jagoda Dampc, Sławomir Bartoszewski, and Anna Michalik. 2020. "Uninterrupted Development of Two Aphid Species Belonging to Cinara Genus during Winter Diapause" Insects 11, no. 3: 150. https://doi.org/10.3390/insects11030150
APA StyleDurak, R., Dampc, J., Dampc, J., Bartoszewski, S., & Michalik, A. (2020). Uninterrupted Development of Two Aphid Species Belonging to Cinara Genus during Winter Diapause. Insects, 11(3), 150. https://doi.org/10.3390/insects11030150




