Ovitraps Provide a Reliable Estimate of Wolbachia Frequency during wMelBr Strain Deployment in a Geographically Isolated Aedes aegypti Population
Abstract
1. Introduction
2. Materials and Methods
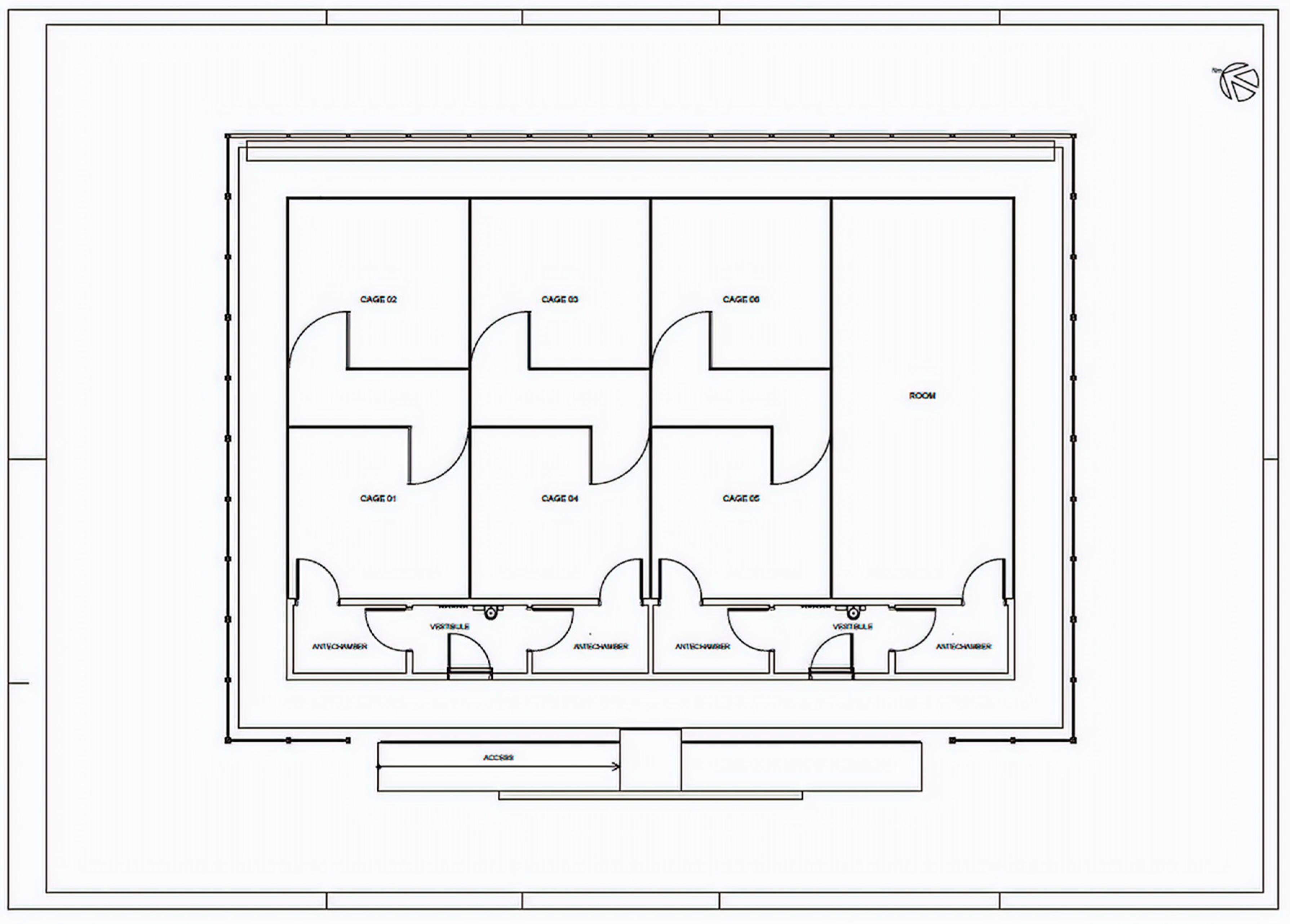
2.1. Semi-Field System (SFS)
2.2. Mosquito Rearing
2.3. Release Treatments in SFS
2.4. Estimating Wolbachia Frequency in SFS
2.5. Estimating Wolbachia Frequency in the Field
2.6. Wolbachia Detection from Semi-Field and Field Experiments
2.7. Statistical Analysis
3. Results
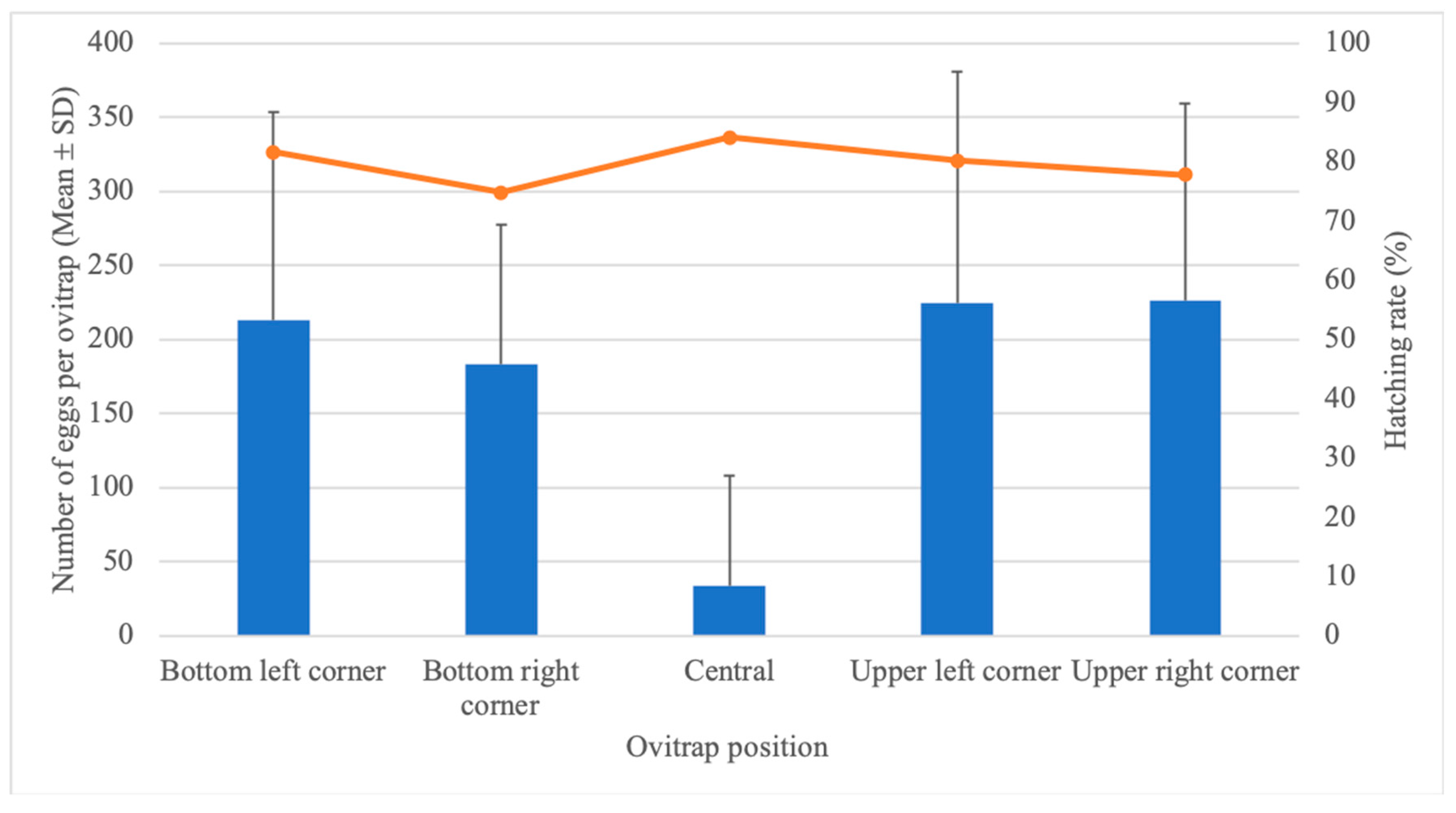
3.1. Effect of Ovitrap Positioning on Egg-Laying Behavior and Hatching Rate
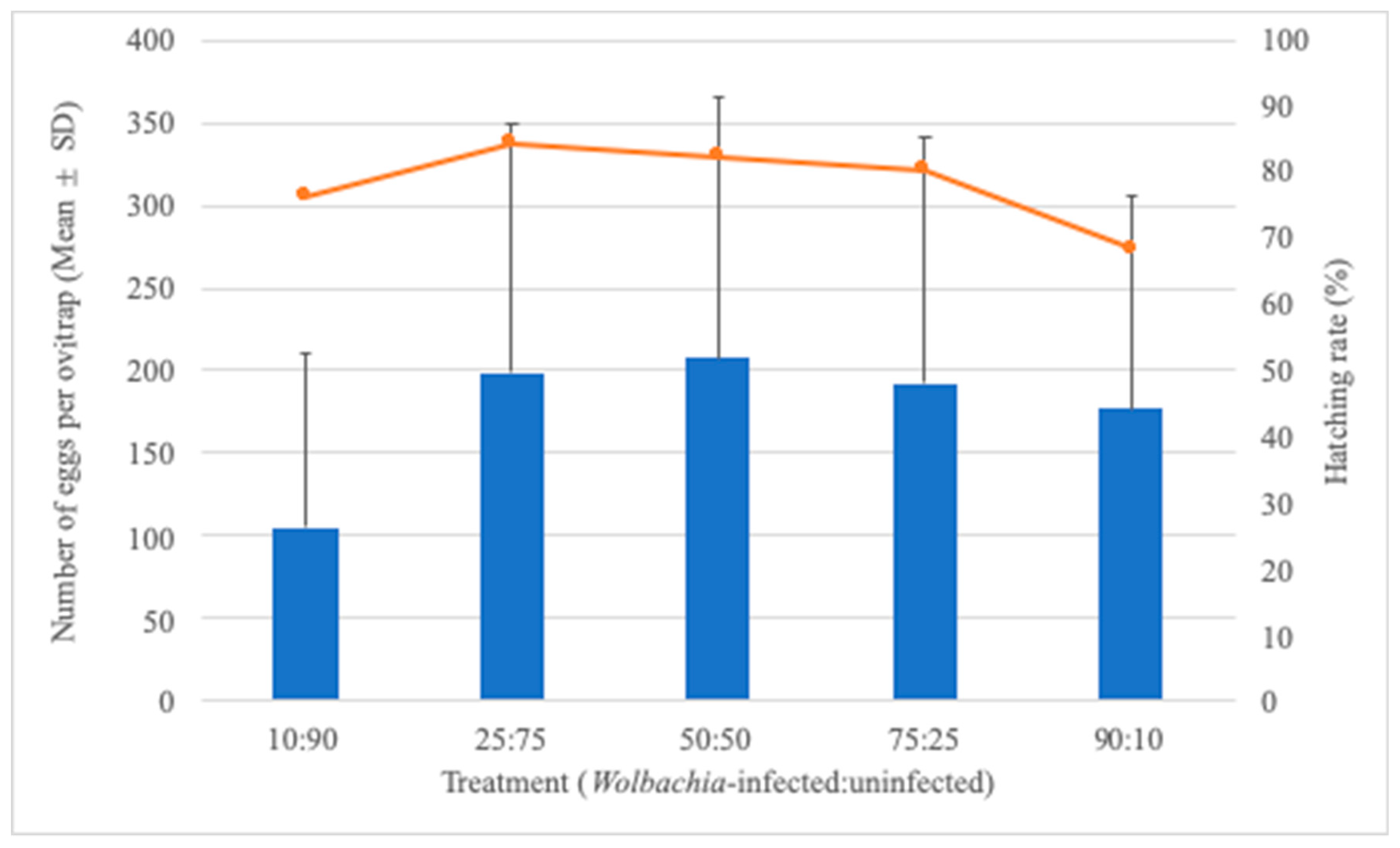
3.2. Effect of Treatment on Egg-Laying Behavior and Hatching Rate
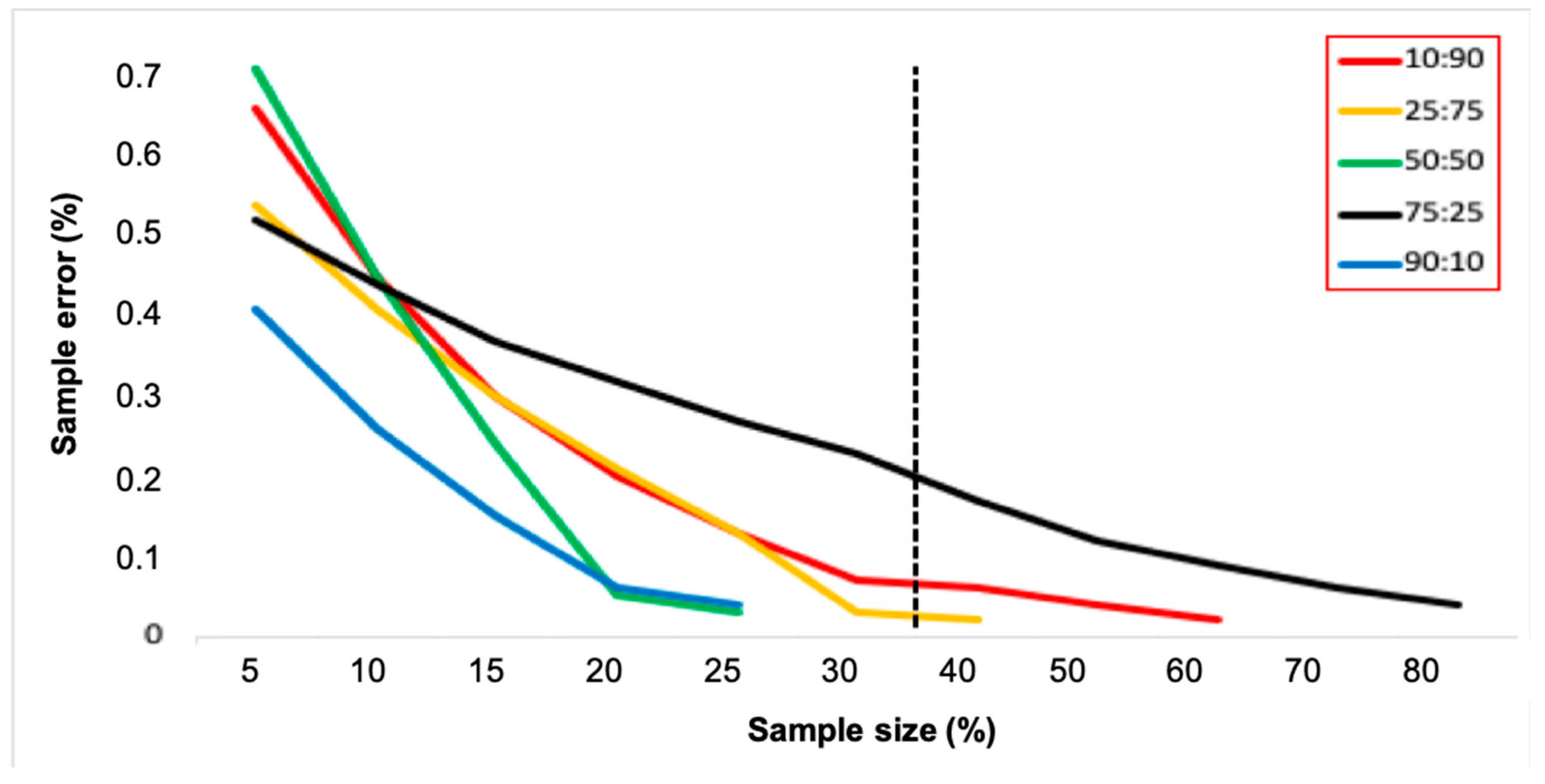
3.3. Sample Size Estimates and Wolbachia Frequency
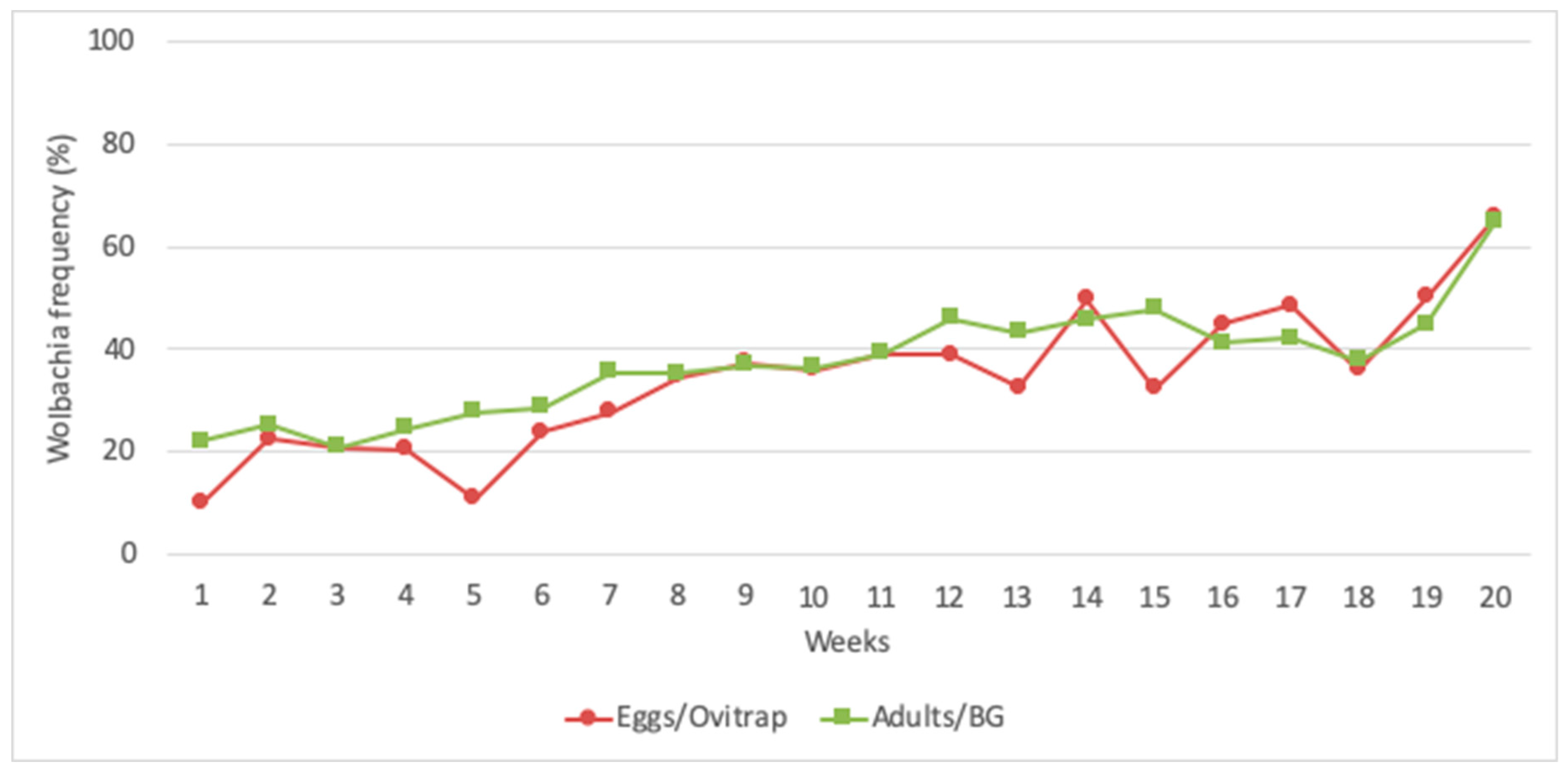
3.4. Wolbachia Frequency in the Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scott, T.W.; Amerasinghe, P.H.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D.; Kittayapong, P.; Edman, J.D. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J. Med. Entomol. 2000, 37, 89–101. [Google Scholar] [CrossRef]
- Braks, M.A.H.; Honório, N.A.; Lourenço-De-Oliveira, R.; Juliano, S.A.; Lounibos, L.P. Convergent Habitat Segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J. Med. Entomol. 2003, 40, 785–794. [Google Scholar] [CrossRef]
- Maciel-De-Freitas, R.; Marques, W.A.; Peres, R.C.; Cunha, S.P.; Lourenço De Oliveira, R. Variation in Aedes aegypti (Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Mem. Inst. Oswaldo Cruz 2007, 102, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-de-Brito, A.; Ribeiro, I.P.; Miranda, R.M.; Fernandes, R.S.; Campos, S.S.; Silva, K.A.B.; Castro, M.G.; Bonaldo, M.C.; Brasil, P.; Lourenço-de-Oliveira, R. First detection of natural infection of Aedes aegypti with Zika virus in Brazil and throughout South America. Mem. Inst. Oswaldo Cruz 2016, 111, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Quiroga, D.; Medina-Sánchez, A.; Sánchez-González, J.M.; Eckert, K.A.; Villalobos-Sánchez, E.; Navarro-Zúñiga, A.R.; Sánchez-Tejeda, G.; Correa-Morales, F.; González-Acosta, C.; Arias, C.F.; et al. Zika Virus in Salivary Glands of Five Different Species of Wild-Caught Mosquitoes from Mexico. Sci. Rep. 2018, 8, 809. [Google Scholar] [CrossRef]
- Tun-Lin, W.; Lenhart, A.; Nam, V.S.; Rebollar-Téllez, E.; Morrison, A.C.; Barbazan, P.; Cote, M.; Midega, J.; Sanchez, F.; Manrique-Saide, P.; et al. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: A multi-country non-inferiority cluster randomized trial. Trop. Med. Int. Health 2009, 14, 1143–1153. [Google Scholar] [CrossRef]
- Maciel-de-Freitas, R.; Avendanho, F.C.; Santos, R.; Sylvestre, G.; Araújo, S.C.; Lima, J.B.P.; Martins, A.J.; Coelho, G.E.; Valle, D. Undesirable Consequences of Insecticide Resistance following Aedes aegypti Control Activities due to a Dengue Outbreak. PLoS ONE 2014, 9, e92424. [Google Scholar] [CrossRef]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.-P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, e0005625. [Google Scholar] [CrossRef]
- Garcia, G.D.A.; David, M.R.; Martins, A.D.J.; Maciel-de-Freitas, R.; Linss, J.G.B.; Araújo, S.C.; Lima, J.B.P.; Valle, D. The impact of insecticide applications on the dynamics of resistance: The case of four Aedes aegypti populations from different Brazilian regions. PLoS Negl. Trop. Dis. 2018, 12, e0006227. [Google Scholar] [CrossRef]
- Maciel-de-Freitas, R.; Valle, D. Challenges encountered using standard vector control measures for dengue in Boa Vista, Brazil. Bull. World Health Organ. 2014, 92, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Yakob, L.; Walker, T. Zika virus outbreak in the Americas: The need for novel. Lancet Glob. Health 2016, 4, e148–e149. [Google Scholar] [CrossRef]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Caragata, E.; Dutra, H.; Moreira, L. Inhibition of Zika virus by Wolbachia in Aedes aegypti. Microb. Cell 2016, 3, 293–295. [Google Scholar] [CrossRef]
- Dutra, H.L.C.; Rocha, M.N.; Dias, F.B.S.; Mansur, S.B.; Caragata, E.P.; Moreira, L.A. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe 2016, 19, 771–774. [Google Scholar] [CrossRef]
- Aliota, M.T.; Walker, E.C.; Uribe Yepes, A.; Dario Velez, I.; Christensen, B.M.; Osorio, J.E. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004677. [Google Scholar] [CrossRef]
- Turelli, M.; Barton, N.H. Deploying dengue-suppressing Wolbachia: Robust models predict slow but effective spatial spread in Aedes aegypti. Theor. Popul. Biol. 2017, 115, 45–60. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Ryan, P.A.; Turley, A.P.; Wilson, G.; Retzki, K.; Iturbe-Ormaetxe, I.; Dong, Y.; Kenny, N.; Paton, C.J.; Ritchie, S.A.; et al. Scaled deployment of Wolbachia to protect the community from dengue and other Aedes transmitted arboviruses. Gates Open Res. 2018, 2, 36. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- Nazni, W.A.; Hoffmann, A.A.; Noor Afizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, M.R.G.; Arif, A.K.M.; Thohir, H.; Nur Syamimi, H.S.; et al. Establishment of Wolbachia strain AlbB in Malaysian populations of Aedes aegypti for dengue control. bioRxiv 2019. [Google Scholar] [CrossRef]
- Krockel, U.; Rose, A.; Eiras, A.E.; Geier, M. New tools for surveillance of adult yellow fever mosquitoes: Comparison of trap catches with human landing rates in an urban environment. J. Am. Mosq. Control Assoc. 2006, 22, 229–238. [Google Scholar] [CrossRef]
- Maciel-de-Freitas, R.; Eiras, Á.E.; Lourenço-de-Oliveira, R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz 2006, 101, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Codeço, C.T.; Lima, A.W.S.; Araújo, S.C.; Lima, J.B.P.; Maciel-de-Freitas, R.; Honório, N.A.; Galardo, A.K.R.; Braga, I.A.; Coelho, G.E.; Valle, D. Surveillance of Aedes aegypti: Comparison of House Index with Four Alternative Traps. PLoS Negl. Trop. Dis. 2015, 9, e0003475. [Google Scholar] [CrossRef]
- Garcia, G.D.A.; Sylvestre, G.; Aguiar, R.; da Costa, G.B.; Martins, A.J.; Lima, J.B.P.; Petersen, M.T.; Lourenço-de-Oliveira, R.; Shadbolt, M.F.; Rašić, G.; et al. Matching the genetics of released and local Aedes aegypti populations is critical to assure Wolbachia invasion. PLoS Negl. Trop. Dis. 2019, 13, e0007023. [Google Scholar] [CrossRef]
- Dutra, H.L.C.; dos Santos, L.M.B.; Caragata, E.P.; Silva, J.B.L.; Villela, D.A.M.; Maciel-de-Freitas, R.; Moreira, L.A. From Lab to Field: The Influence of Urban Landscapes on the Invasive Potential of Wolbachia in Brazilian Aedes aegypti Mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003689. [Google Scholar] [CrossRef]
- De Oliveira, S.; Villela, D.A.M.; Dias, F.B.S.; Moreira, L.A.; Maciel de Freitas, R. How does competition among wild type mosquitoes influence the performance of Aedes aegypti and dissemination of Wolbachia pipientis? PLoS Negl. Trop. Dis. 2017, 11, e0005947. [Google Scholar] [CrossRef]
- Honório, N.A.; Castro, M.G.; de Barros, F.S.M.; Magalhães, M.D.A.F.M.; Sabroza, P.C. The spatial distribution of Aedes aegypti and Aedes albopictus in a transition zone, Rio de Janeiro, Brazil. Cad. Saude Publica 2009, 25, 1203–1214. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Johnson, P.H.; Freeman, A.J.; Odell, R.G.; Graham, N.; DeJong, P.A.; Standfield, G.W.; Sale, R.W.; O’Neill, S.L. A Secure Semi-Field System for the Study of Aedes aegypti. PLoS Negl. Trop. Dis. 2011, 5, e988. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Hue Kien, D.T.; Clapham, H.; Aguas, R.; Trung, V.T.; Bich Chau, T.N.; Popovici, J.; Ryan, P.A.; O’Neill, S.L.; McGraw, E.A.; et al. Modeling the impact on virus transmission of Wolbachia -mediated blocking of dengue virus infection of Aedes aegypti. Sci. Transl. Med. 2015, 7, 279ra37. [Google Scholar] [CrossRef]
- Daniel, W.W. Biostatistics: A Foundation for Analysis in the Health Sciences, 10th ed.; Daniel, W.W., Ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-10582-5. [Google Scholar]
- Ritchie, S.A.; Long, S.; Smith, G.; Pyke, A.; Knox, T.B. Entomological Investigations in a Focus of Dengue Transmission in Cairns, Queensland, Australia, by Using the Sticky Ovitraps. J. Med. Entomol. 2004, 41, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D.; Ritchie, S.A. Efficacy of sticky and standard ovitraps for Aedes aegypti in Trinidad, West Indies. J. Vector Ecol. 2010, 35, 395–400. [Google Scholar] [CrossRef]
- Degener, C.M.; Eiras, Á.E.; Ázara, T.M.F.; Roque, R.A.; Rösner, S.; Codeço, C.T.; Nobre, A.A.; Rocha, E.S.O.; Kroon, E.G.; Ohly, J.J.; et al. Evaluation of the Effectiveness of Mass Trapping with BG-Sentinel Traps for Dengue Vector Control: A Cluster Randomized Controlled Trial in Manaus, Brazil. J. Med. Entomol. 2014, 51, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Iturbe-Ormaetxe, I.; Callahan, A.G.; Phillips, B.L.; Billington, K.; Axford, J.K.; Montgomery, B.; Turley, A.P.; O’Neill, S.L. Stability of the wMel Wolbachia Infection following Invasion into Aedes aegypti Populations. PLoS Negl. Trop. Dis. 2014, 8, e3115. [Google Scholar] [CrossRef] [PubMed]
- Colton, Y.M.; Chadee, D.D.; Severson, D.W. Natural skip oviposition of the mosquito Aedes aegypti indicated by codominant genetic markers. Med. Vet. Entomol. 2003, 17, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.C.; Edman, J.D. Indirect Evidence against Delayed “Skip-Oviposition” Behavior by Aedes aegypti (Diptera: Culicidae) in Thailand. J. Med. Entomol. 2001, 38, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Roque, R.A.; Eiras, Á.E. Calibration and evaluation of field cage for oviposition study with Aedes (Stegomyia) aegypti female (L.) (Diptera: Culicidae). Neotrop. Entomol. 2008, 37, 478–485. [Google Scholar] [CrossRef][Green Version]
- De Abreu, F.V.S.; Morais, M.M.; Ribeiro, S.P.; Eiras, Á.E. Influence of breeding site availability on the oviposition behaviour of Aedes aegypti. Mem. Inst. Oswaldo Cruz 2015, 110, 669–676. [Google Scholar] [CrossRef]
- Farnesi, L.C.; Belinato, T.A.; Gesto, J.S.M.; Martins, A.J.; Bruno, R.V.; Moreira, L.A. Embryonic development and egg viability of wMel-infected Aedes aegypti. Parasites Vectors 2019, 12, 211. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Townsend, M.; Paton, C.J.; Callahan, A.G.; Hoffmann, A.A. Application of wMelPop Wolbachia Strain to Crash Local Populations of Aedes aegypti. PLoS Negl. Trop. Dis. 2015, 9, e0003930. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, H.L.; Nguyen, T.Y.; Vu, S.N.; Tran, N.D.; Le, T.N.; Vien, Q.M.; Bui, T.C.; Le, H.T.; Kutcher, S.; et al. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasites Vectors 2015, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.N.; dos Santos, L.M.B.; Chouin-Carneiro, T.; Pavan, M.G.; Garcia, G.A.; David, M.R.; Beier, J.C.; Dowell, F.E.; Maciel-de-Freitas, R.; Sikulu-Lord, M.T. Rapid, noninvasive detection of Zika virus in Aedes aegypti mosquitoes by near-infrared spectroscopy. Sci. Adv. 2018, 4, eaat0496. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.D.S.; Cassimiro, A.P.A.; De Oliveira, C.D.; Rodrigues, N.B.; Moreira, L.A. Wolbachia detection in insects through LAMP: Loop mediated isothermal amplification. Parasites Vectors 2014, 7, 228. [Google Scholar] [CrossRef] [PubMed]
| Estimate | Std Error | Z Value | p | |
|---|---|---|---|---|
| Position (Bottom left corner) | 1.438 | 0.048 | 29.894 | <0.0001 |
| Position (Bottom right corner) | 1.216 | 0.049 | 24.767 | <0.0001 |
| Position (Upper left corner) | 1.424 | 0.048 | 29.513 | <0.0001 |
| Position (Upper right corner | 1.451 | 0.047 | 30.391 | <0.0001 |
| Week | −0.167 | 0.006 | −26.527 | <0.0001 |
| Treatment | 0.173 | 0.064 | 2.712 | 0.0067 |
| Ratio | 0.273 | 0.059 | 4.596 | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Jesus, C.P.; Dias, F.B.S.; Villela, D.M.A.; Maciel-de-Freitas, R. Ovitraps Provide a Reliable Estimate of Wolbachia Frequency during wMelBr Strain Deployment in a Geographically Isolated Aedes aegypti Population. Insects 2020, 11, 92. https://doi.org/10.3390/insects11020092
de Jesus CP, Dias FBS, Villela DMA, Maciel-de-Freitas R. Ovitraps Provide a Reliable Estimate of Wolbachia Frequency during wMelBr Strain Deployment in a Geographically Isolated Aedes aegypti Population. Insects. 2020; 11(2):92. https://doi.org/10.3390/insects11020092
Chicago/Turabian Stylede Jesus, Camila P., Fernando B.S. Dias, Daniel M.A. Villela, and Rafael Maciel-de-Freitas. 2020. "Ovitraps Provide a Reliable Estimate of Wolbachia Frequency during wMelBr Strain Deployment in a Geographically Isolated Aedes aegypti Population" Insects 11, no. 2: 92. https://doi.org/10.3390/insects11020092
APA Stylede Jesus, C. P., Dias, F. B. S., Villela, D. M. A., & Maciel-de-Freitas, R. (2020). Ovitraps Provide a Reliable Estimate of Wolbachia Frequency during wMelBr Strain Deployment in a Geographically Isolated Aedes aegypti Population. Insects, 11(2), 92. https://doi.org/10.3390/insects11020092





