Thioester-Containing Proteins in the Drosophila melanogaster Immune Response against the Pathogen Photorhabdus
Abstract
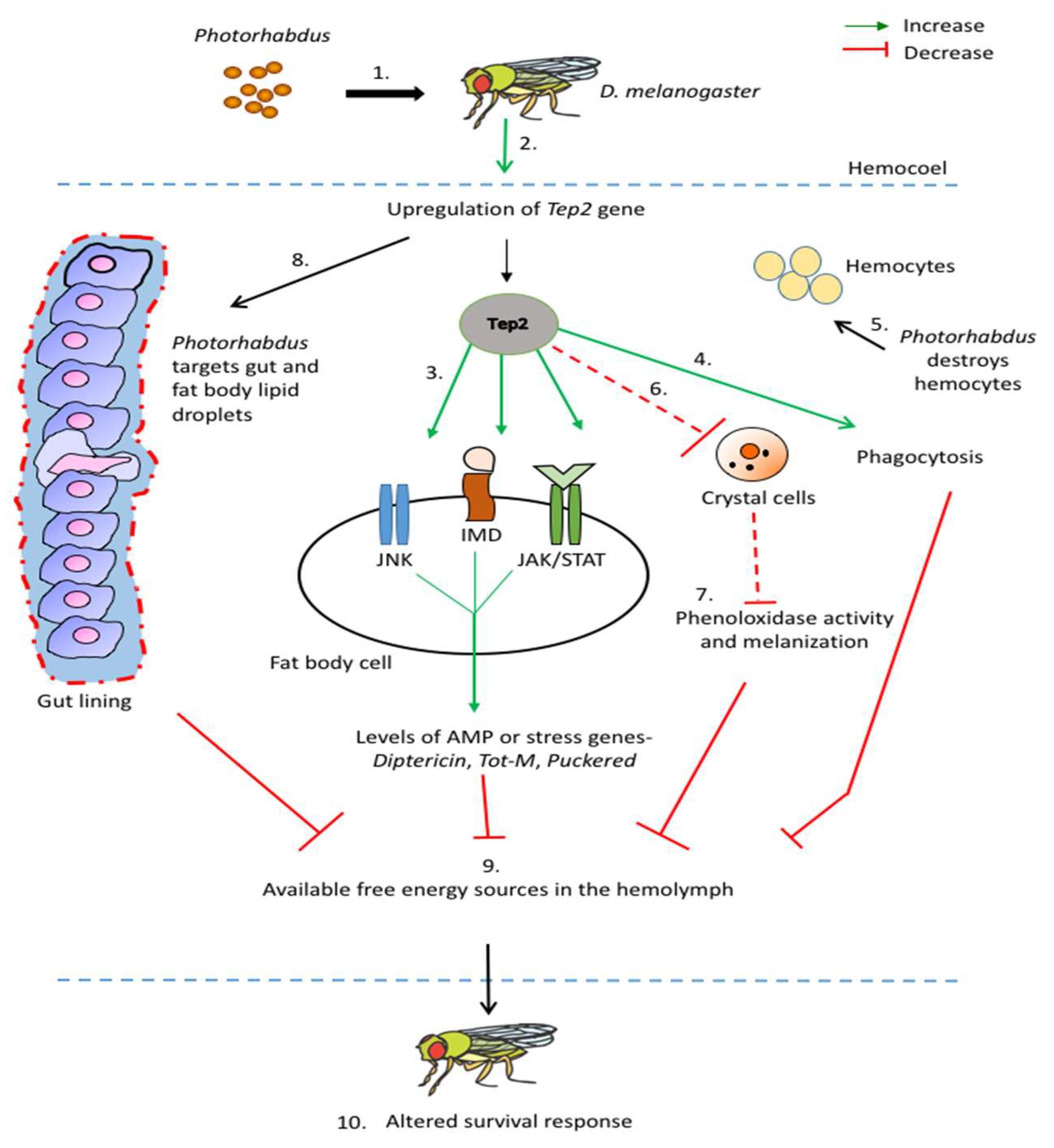
1. Introduction
2. Thioester-Containing Proteins in the Drosophila Humoral Response against Photorhabdus
3. Thioester-Containing Proteins in the Drosophila Cellular Response against Photorhabdus
4. Thioester-Containing Proteins and Drosophila Metabolism during Photorhabdus Infection
5. Thioester-Containing Protein Gene Expression in Drosophila and Stress and Inflammation Response to Photorhabdus
6. Role of Thioester-Containing Proteins in the Drosophila Antinematode Response
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sackton, T.B. Comparative genomics and transcriptomics of host-pathogen interactions in insects: Evolutionary insights and future directions. Curr. Opin. Insect Sci. 2019, 31, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster—From microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Gold, K.S.; Brückner, K. Macrophages and cellular immunity in Drosophila melanogaster. Semin. Immunol. 2015, 27, 357–368. [Google Scholar] [CrossRef]
- Parsons, B.; Foley, E. Cellular immune defenses of Drosophila melanogaster. Dev. Comp. Immunol. 2016, 58, 95–101. [Google Scholar] [CrossRef]
- Keebaugh, E.S.; Schlenke, T.A. Insights from natural host-parasite interactions: The Drosophila model. Dev. Comp. Immunol. 2014, 42, 111–123. [Google Scholar] [CrossRef]
- Lindsay, S.A.; Wasserman, S.A. Conventional and non-conventional Drosophila Toll signaling. Dev. Comp. Immunol. 2014, 42, 16–24. [Google Scholar] [CrossRef]
- Zhai, Z.; Huang, X.; Yin, Y. Beyond immunity: The Imd pathway as a coordinator of host defense, organismal physiology and behavior. Dev. Comp. Immunol. 2018, 83, 51–59. [Google Scholar] [CrossRef]
- Myllymäki, H.; Rämet, M. JAK/STAT pathway in Drosophila immunity. Scand. J. Immunol. 2014, 79, 377–385. [Google Scholar] [CrossRef]
- Delaney, J.R.; Stöven, S.; Uvell, H.; Anderson, K.V.; Engström, Y.; Mlodzik, M. Cooperative control of Drosophila immune responses by the JNK and NF-kappaB signaling pathways. EMBO J. 2006, 25, 3068–3077. [Google Scholar] [CrossRef]
- Brivio, M.F.; Mastore, M. Nematobacterial Complexes and Insect Hosts: Different Weapons for the Same War. Insects 2018, 9, 117. [Google Scholar] [CrossRef]
- Cooper, D.; Eleftherianos, I. Parasitic Nematode Immunomodulatory Strategies: Recent Advances and Perspectives. Pathogens 2016, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.M.; Bode, H.B. Chemical language and warfare of bacterial natural products in bacteria-nematode-insect interactions. Nat. Prod. Rep. 2018, 35, 309–335. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Reynolds, S.E.; Eleftherianos, I. Insect immune responses to nematode parasites. Trends Parasitol. 2011, 27, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; ffrench-Constant, R.H.; Clarke, D.J.; Dowling, A.J.; Reynolds, S.E. Dissecting the immune response to the entomopathogen Photorhabdus. Trends Microbiol. 2010, 18, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, F.; Li, Q.; Zhang, J.; Li, Y.; Tang, T.; Hu, Q.; Yu, X.Q. Pattern recognition receptors in Drosophila immune responses. Dev. Comp. Immunol. 2020, 102, 103468. [Google Scholar] [CrossRef]
- Ganesan, S.; Aggarwal, K.; Paquette, N.; Silverman, N. NF-kappaB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr. Top. Microbiol. Immunol. 2011, 349, 25–60. [Google Scholar]
- Imler, J.L.; Bulet, P. Antimicrobial peptides in Drosophila: Structures, activities and gene regulation. Chem. Immunol. Allergy 2005, 86, 1–21. [Google Scholar]
- Shokal, U.; Eleftherianos, I. Thioester-Containing Protein-4 Regulates the Drosophila Immune Signaling and Function against the Pathogen Photorhabdus. J. Innate Immun. 2017, 9, 83–93. [Google Scholar] [CrossRef]
- Shokal, U.; Kopydlowski, H.; Eleftherianos, I. The distinct function of Tep2 and Tep6 in the immune defense of Drosophila melanogaster against the pathogen Photorhabdus. Virulence 2017, 8, 1668–1682. [Google Scholar] [CrossRef]
- Wood, W.; Martin, P. Macrophage Functions in Tissue Patterning and Disease: New Insights from the Fly. Dev. Cell 2017, 40, 221–233. [Google Scholar] [CrossRef]
- Honti, V.; Csordás, G.; Kurucz, É.; Márkus, R.; Andó, I. The cell-mediated immunity of Drosophila melanogaster: Hemocyte lineages, immune compartments, microanatomy and regulation. Dev. Comp. Immunol. 2014, 42, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Shokal, U.; Eleftherianos, I. The Drosophila Thioester-Containing Protein-4 participates in the induction of the cellular immune response to the pathogen Photorhabdus. Dev. Comp. Immunol. 2017, 76, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Tang, H. Regulation and function of the melanization reaction in Drosophila. Fly 2009, 3, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Revenis, C. Role and importance of phenoloxidase in insect hemostasis. J. Innate Immun. 2011, 3, 28–33. [Google Scholar] [CrossRef]
- Ganeshan, K.; Chawla, A. Metabolic regulation of immune responses. Annu. Rev. Immunol. 2014, 32, 609–634. [Google Scholar] [CrossRef]
- Vavricka, C.J.; Han, Q.; Mehere, P.; Ding, H.; Christensen, B.M.; Li, J. Tyrosine metabolic enzymes from insects and mammals: a comparative perspective. Insect Sci. 2014, 21, 13–19. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015, 161, 146–160. [Google Scholar] [CrossRef]
- Zmora, N.; Bashiardes, S.; Levy, M.; Elinav, E. The Role of the Immune System in Metabolic Health and Disease. Cell Metab. 2017, 25, 506–521. [Google Scholar] [CrossRef]
- Dionne, M. Immune-metabolic interaction in Drosophila. Fly 2014, 8, 75–79. [Google Scholar] [CrossRef]
- Lee, K.A.; Lee, W.J. Immune-metabolic interactions during systemic and enteric infection in Drosophila. Curr. Opin. Insect Sci. 2018, 29, 21–26. [Google Scholar] [CrossRef]
- Galenza, A.; Foley, E. Immunometabolism: Insights from the Drosophila model. Dev. Comp. Immunol. 2019, 94, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Shokal, U.; Kopydlowski, H.; Harsh, S.; Eleftherianos, I. Thioester-Containing Proteins 2 and 4 Affect the Metabolic Activity and Inflammation Response in Drosophila. Infect. Immun. 2018, 86, e00810-17. [Google Scholar] [CrossRef] [PubMed]
- Bou Aoun, R.; Hetru, C.; Troxler, L.; Doucet, D.; Ferrandon, D.; Matt, N. Analysis of thioester-containing proteins during the innate immune response of Drosophila melanogaster. J. Innate Immun. 2011, 3, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.A.; Overend, G.; Sebastian, S.; Cundall, M.; Cabrero, P.; Dow, J.A.; Terhzaz, S. Immune and stress response ‘cross-talk’ in the Drosophila Malpighian tubule. J. Insect Physiol. 2012, 58, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Asri, R.M.; Salim, E.; Nainu, F.; Hori, A.; Kuraishi, T. Sterile induction of innate immunity in Drosophila melanogaster. Front. Biosci. 2019, 24, 1390–1400. [Google Scholar]
- Dow, J.A.; Romero, M.F. Drosophila provides rapid modeling of renal development, function and disease. Am. J. Physiol. Renal Physiol. 2010, 299, F1237–F1244. [Google Scholar] [CrossRef]
- Nielsen-LeRoux, C.; Gaudriault, S.; Ramarao, N.; Lereclus, D.; Givaudan, A. How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Curr. Opin. Microbiol. 2012, 15, 220–231. [Google Scholar] [CrossRef]
- Ming, M.; Obata, F.; Kuranaga, E.; Miura, M. Persephone/Spätzle pathogen sensors mediate the activation of Toll receptor signaling in response to endogenous danger signals in apoptosis-deficient Drosophila. J. Biol. Chem. 2014, 289, 7558–7568. [Google Scholar] [CrossRef]
- Song, Z.; McCall, K.; Steller, H. DCP-1, a Drosophila cell death protease essential for development. Science 1997, 275, 536–540. [Google Scholar] [CrossRef]
- Arefin, B.; Kucerova, L.; Dobes, P.; Markus, R.; Strnad, H.; Wang, Z.; Hyrsl, P.; Zurovec, M.; Theopold, U. Genome-wide transcriptional analysis of Drosophila larvae infected by entomopathogenic nematodes shows involvement of complement, recognition and extracellular matrix proteins. J. Innate Immun. 2014, 6, 192–204. [Google Scholar] [CrossRef]
- Castillo, J.C.; Creasy, T.; Kumari, P.; Shetty, A.; Shokal, U.; Tallon, L.J.; Eleftherianos, I. Drosophila anti-nematode and antibacterial immune regulators revealed by RNA-seq. BMC Genom. 2015, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Daugherty, S.; Shetty, A.C.; Eleftherianos, I. RNAseq analysis of the Drosophila response to the entomopathogenic nematode Steinernema. G3 (Bethesda) 2017, 7, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Shokal, U.; Eleftherianos, I. Evolution and Function of Thioester-Containing Proteins and the Complement System in the Innate Immune Response. Front. Immunol. 2017, 8, 759. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleftherianos, I.; Sachar, U. Thioester-Containing Proteins in the Drosophila melanogaster Immune Response against the Pathogen Photorhabdus. Insects 2020, 11, 85. https://doi.org/10.3390/insects11020085
Eleftherianos I, Sachar U. Thioester-Containing Proteins in the Drosophila melanogaster Immune Response against the Pathogen Photorhabdus. Insects. 2020; 11(2):85. https://doi.org/10.3390/insects11020085
Chicago/Turabian StyleEleftherianos, Ioannis, and Upasana Sachar. 2020. "Thioester-Containing Proteins in the Drosophila melanogaster Immune Response against the Pathogen Photorhabdus" Insects 11, no. 2: 85. https://doi.org/10.3390/insects11020085
APA StyleEleftherianos, I., & Sachar, U. (2020). Thioester-Containing Proteins in the Drosophila melanogaster Immune Response against the Pathogen Photorhabdus. Insects, 11(2), 85. https://doi.org/10.3390/insects11020085




