Two Distinct Genotypes of Spissistilus festinus (Say, 1830) (Hemiptera, Membracidae) in the United States Revealed by Phylogenetic and Morphological Analyses
Abstract
1. Introduction
2. Materials and Methods
2.1. Spissistilus festinus Specimen Collection
2.2. Morphological Characteristics of Spissistilus festinus
2.3. DNA Extraction, PCR Amplification, and Sequencing of Spissistilus festinus DNA Fragments
2.4. Sequence Analyses and Phylogenetic Analyses
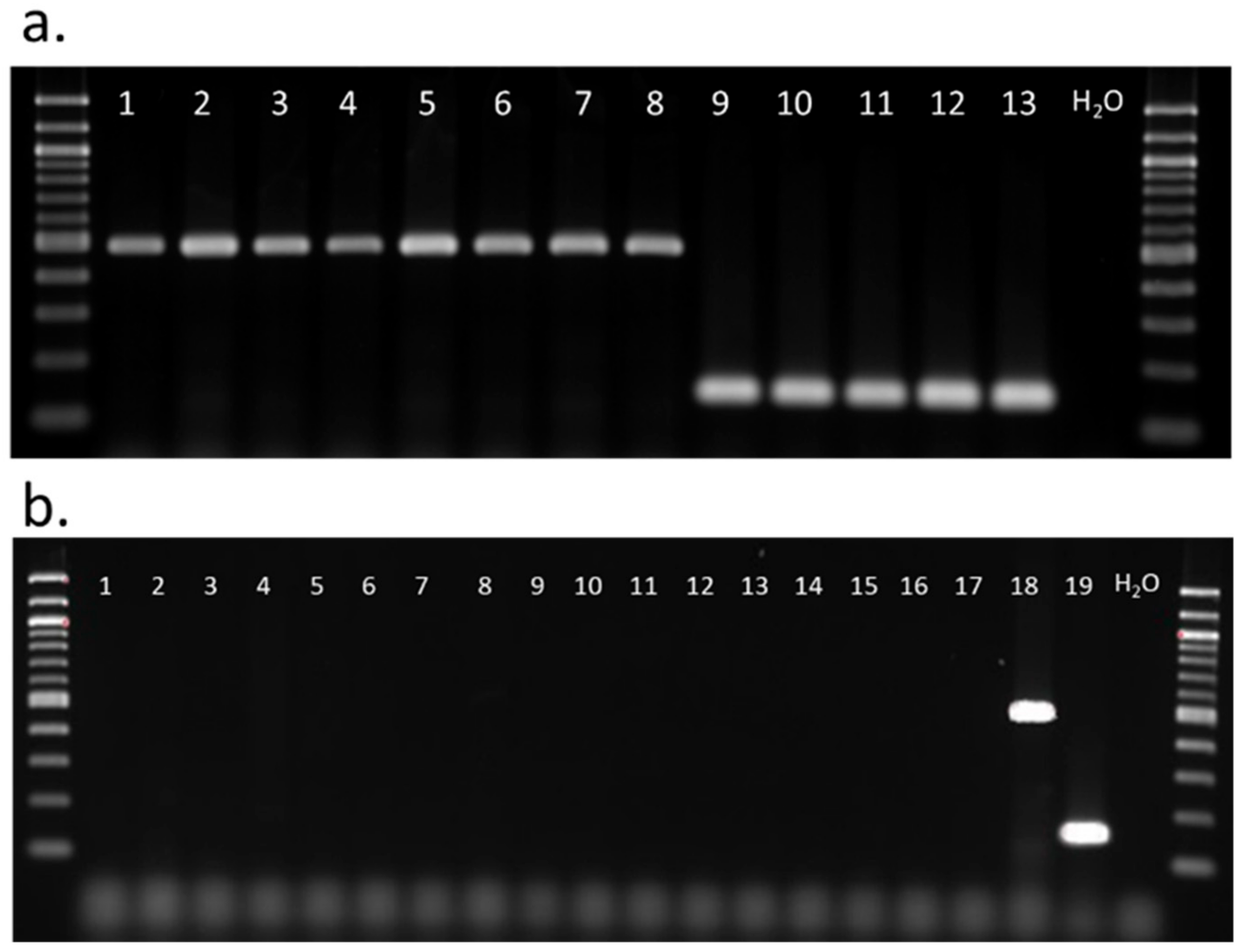
2.5. Diagnostic PCR for Distinction of Spissistilus festinus Genotype
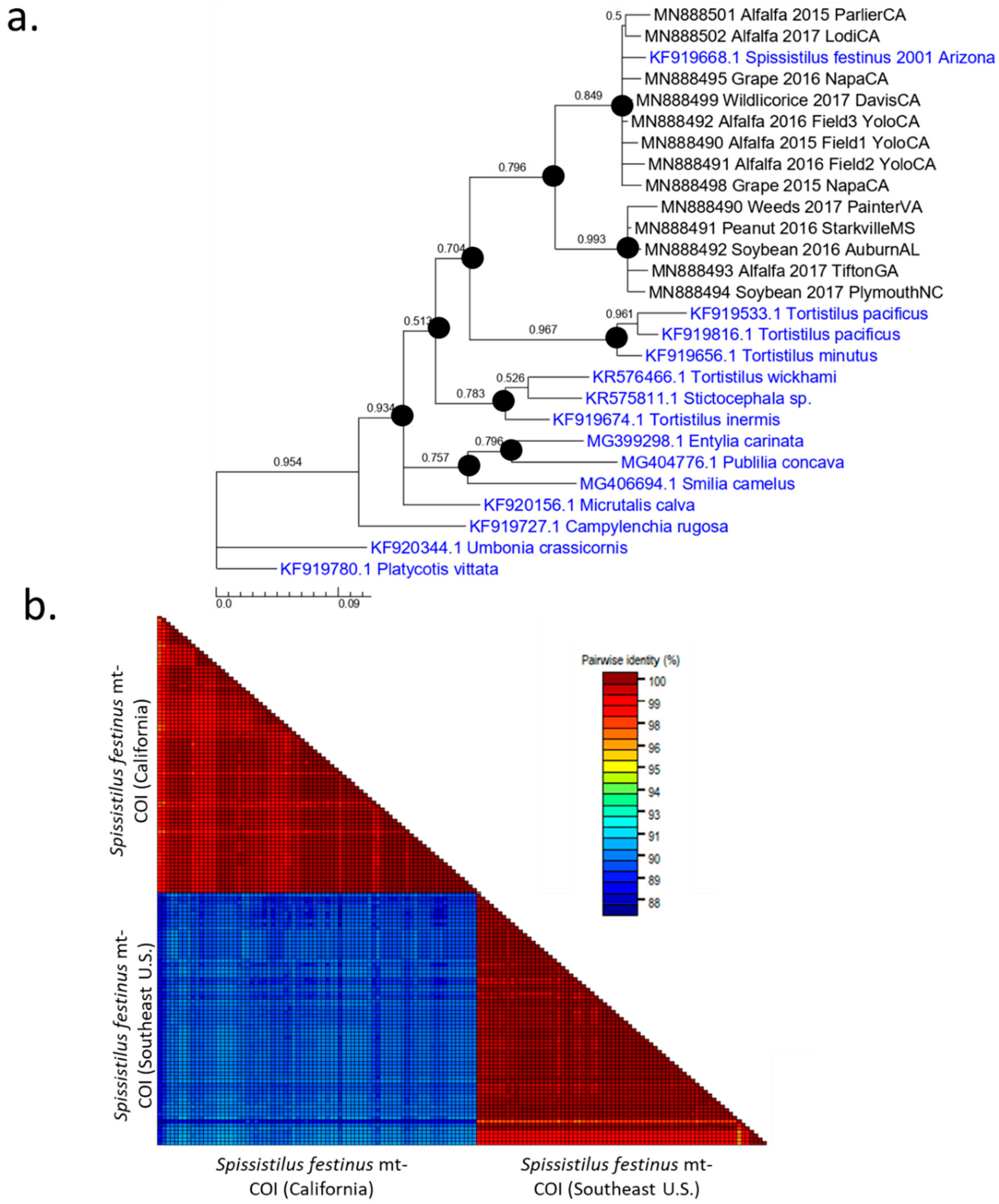
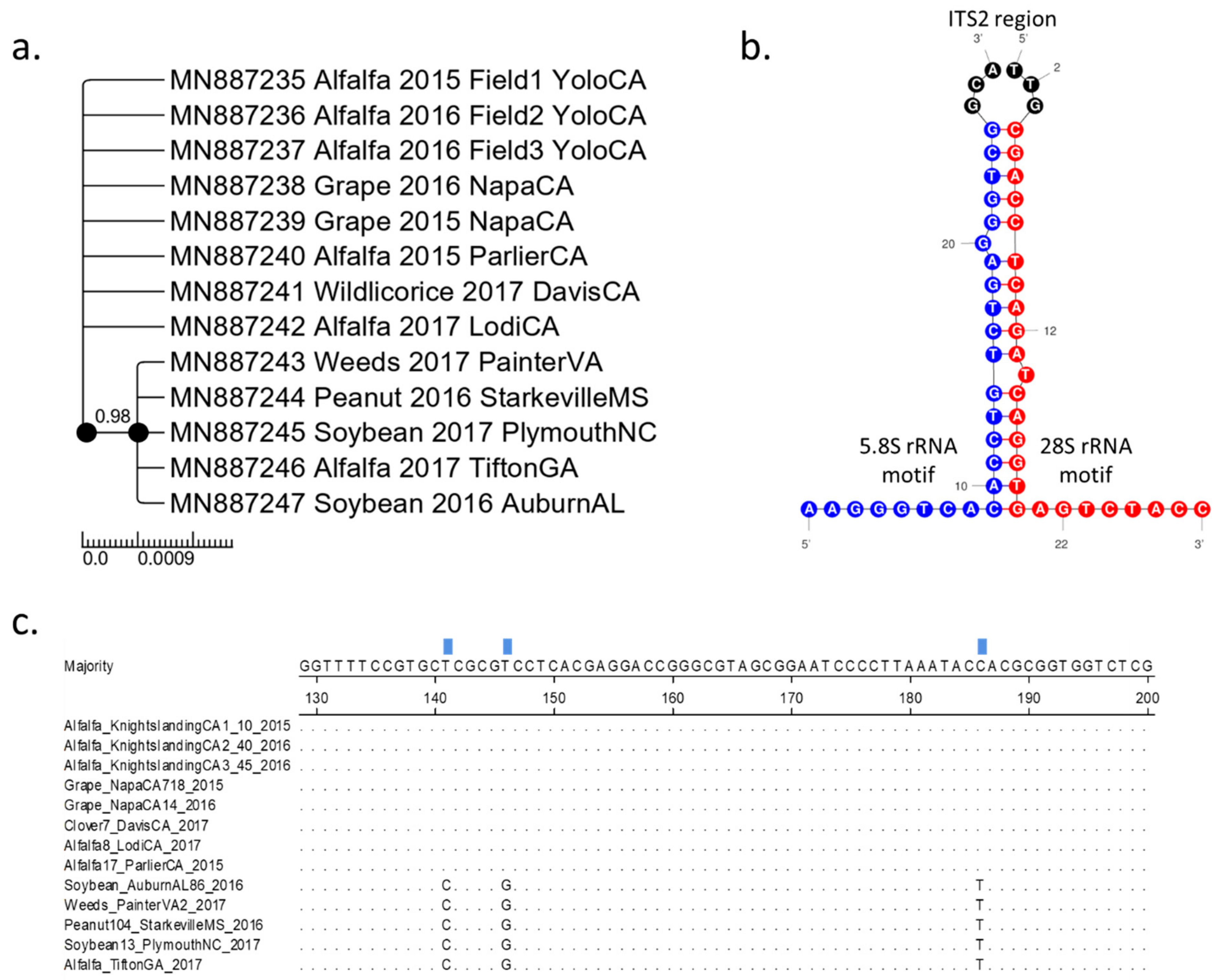
3. Results
3.1. Two Distinct Spissistilus festinus Genotypes Based on Geography
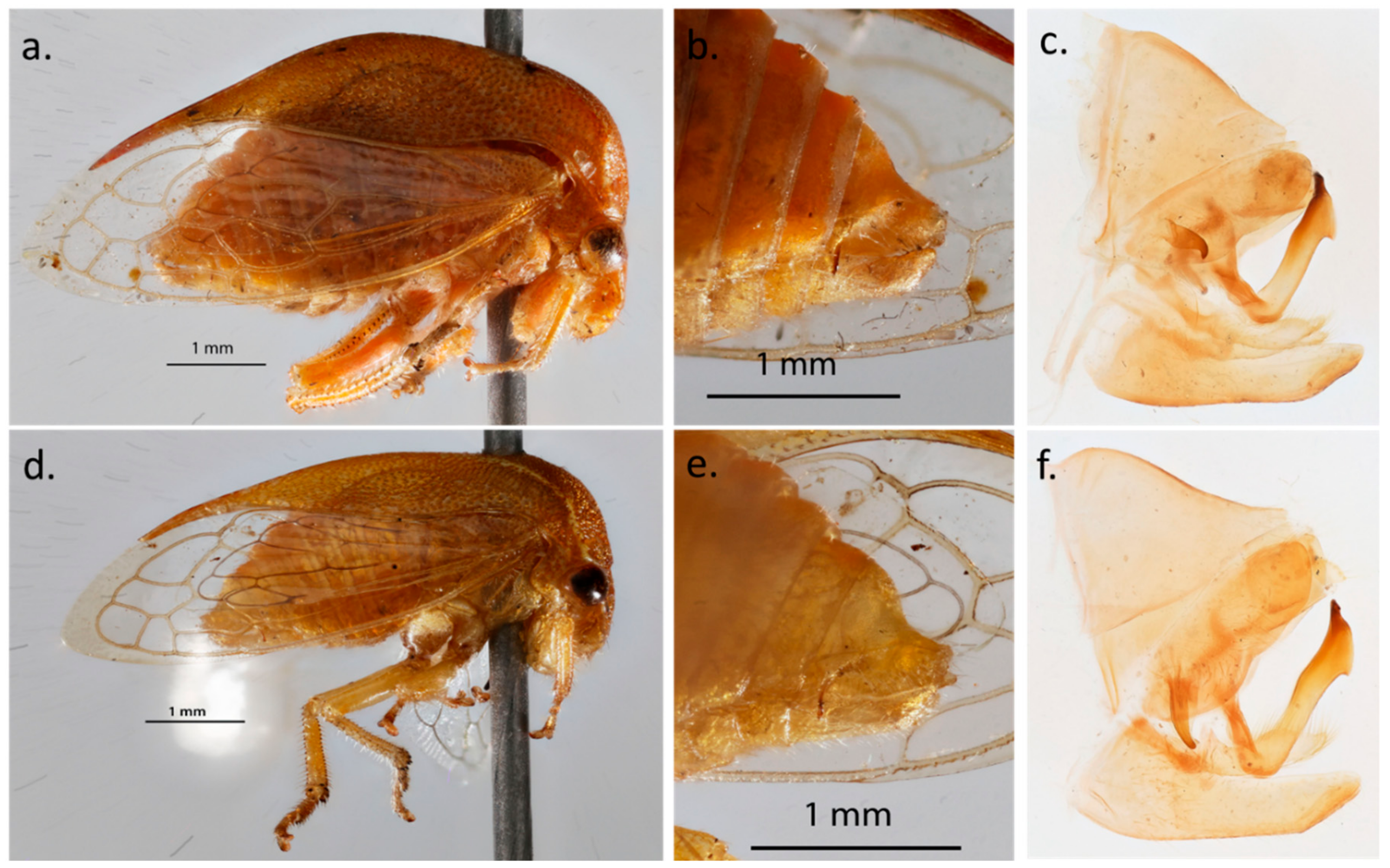
3.2. Phenotypic Characterization of the Two Spissistilus festinus Genotypes
3.3. Diagnostic Polymerase Chain Reaction for Spissistilus festinus Genotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beyer, B.A.; Srinivasan, R.; Roberts, P.M.; Abney, M.R. Biology and Management of the Threecornered Alfalfa Hopper (Hemiptera: Membracidae) in Alfalfa, Soybean, and Peanut. J. Integr. Pest Manag. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Bahder, B.W.; Zalom, F.G.; Jayanth, M.; Sudarshana, M.R. Phylogeny of Geminivirus Coat Protein Sequences and Digital PCR Aid in Identifying Spissistilus festinus as a Vector of Grapevine red blotch-associated virus. Phytopathology 2016, 106, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Cieniewicz, E.J.; Pethybridge, S.J.; Loeb, G.; Perry, K.; Fuchs, M. Insights into the Ecology of Grapevine red blotch virus in a Diseased Vineyard. Phytopathology 2018, 108, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Cieniewicz, E.; Flasco, M.; Brunelli, M.; Onwumelu, A.; Wise, A.; Fuchs, M.F. Differential Spread of Grapevine Red Blotch Virus in California and New York Vineyards. Phytobiomes J. 2019, 3, 203–211. [Google Scholar] [CrossRef]
- Preto, C.R.; Sudarshana, M.R.; Bollinger, M.L.; Zalom, F.G. Vitis vinifera (Vitales: Vitaceae) as a Reproductive Host of Spissistilus festinus (Hemiptera: Membracidae). J. Insect Sci. 2018, 18. [Google Scholar] [CrossRef]
- Kjer, K.; Borowiec, M.L.; Frandsen, P.B.; Ware, J.; Wiegmann, B.M. Advances using molecular data in insect systematics. Curr. Opin. Insect Sci. 2016, 18, 40–47. [Google Scholar] [CrossRef]
- DeSalle, R.; Goldstein, P. Review and Interpretation of Trends in DNA Barcoding. Front. Ecol. Evol. 2019, 7, 302. [Google Scholar] [CrossRef]
- Wang, Y.; Dietrich, C.H.; Zhang, Y. Phylogeny and historical biogeography of leafhopper subfamily Evacanthinae (Hemiptera: Cicadellidae) based on morphological and molecular data. Sci. Rep. 2017, 7, 45387. [Google Scholar] [CrossRef]
- Park, D.-S.; Foottit, R.; Maw, E.; Hebert, P.D.N. Barcoding Bugs: DNA-Based Identification of the True Bugs (Insecta: Hemiptera: Heteroptera). PLoS ONE 2011, 6, e18749. [Google Scholar] [CrossRef]
- Caterino, M.S.; Cho, S.; Sperling, F.A.H. The Current State of Insect Molecular Systematics: A Thriving Tower of Babel. Annu. Rev. Entomol. 2000, 45, 1–54. [Google Scholar] [CrossRef]
- Frohlich, D.R.; Torres-Jerez, I.; Bedford, I.D.; Markham, P.G.; Brown, J.K. A phylogeographical analysis of the Bemisia tabaci species complex based on mitochondrial DNA markers. Mol. Ecol. 1999, 8, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; De Barro, A.P. Refined Global Analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) Mitochondrial Cytochrome Oxidase 1 to Identify Species Level Genetic Boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- Perring, T.M.; Stansly, P.A.; Liu, T.X.; Smith, H.A.; Andreason, S.A. Sustainable Management of Arthropod Pests of Tomato. Whiteflies: Biology, Ecology, and Management; Academic Press: Cambridge, MA, USA, 2018; pp. 73–110. [Google Scholar]
- Bedford, I.D.; Briddon, R.W.; Brown, J.K.; Rosell, R.C.; Markham, P.G. Geminivirus transmission and biological characterisation of Bemisia tabaci (Gennadius) biotypes from different geographic regions. Ann. Appl. Biol. 1994, 125, 311–325. [Google Scholar] [CrossRef]
- Chowda-Reddy, R.; Kirankumar, M.; Seal, S.E.; Muniyappa, V.; Valand, G.B.; Govindappa, M.; Colvin, J. Bemisia tabaci Phylogenetic Groups in India and the Relative Transmission Efficacy of Tomato leaf curl Bangalore virus by an Indigenous and an Exotic Population. J. Integr. Agric. 2012, 11, 235–248. [Google Scholar] [CrossRef]
- Shi, X.; Tang, X.; Zhang, X.; Zhang, D.; Li, F.; Yan, F.; Zhang, Y.; Zhou, X.; Liu, Y. Transmission Efficiency, Preference and Behavior of Bemisia tabaci MEAM1 and MED under the Influence of Tomato Chlorosis Virus. Front. Plant Sci. 2017, 8, 2271. [Google Scholar] [CrossRef]
- Costa, H.S.; Brown, J.K.; Sivasupramaniam, S.; Bird, J. Regional distribution, insecticide resistance, and reciprocal crosses between the A and B biotypes of Bemisia tabaci. Insect Sci. Applic 2019, 14, 255–266. [Google Scholar] [CrossRef]
- Luo, C.; Jones, C.M.; Devine, G.; Zhang, F.; Denholm, I.; Gorman, K. Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot. 2010, 29, 429–434. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol. 2005, 58, 216–225. [Google Scholar] [CrossRef]
- Kopp, D.D.; Yonke, T.R. The Treehoppers of Missouri: Part 1. Subfamilies Centrotinae, Hoplophorioninae, and Membracinae (Homoptera: Membracidae). J. Kansas Entomol. Soc. 1973, 46, 42–64. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Park, D.-S.; Suh, S.-J.; Oh, H.-W.; Hebert, P.D. Recovery of the mitochondrial COI barcode region in diverse Hexapoda through tRNA-based primers. BMC Genom. 2010, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Ji, Y.J.; Zhang, D.X.; He, L.J. Evolutionary conservation and versatility of a new set of primers for amplifying the ribosomal internal transcribed spacer regions in insects and other invertebrates. Mol. Ecol. Notes 2003, 3, 581–585. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef]
- Ankenbrand, M.J.; Keller, A.; Wolf, M.; Schultz, J.; Forster, F. ITS2 Database V: Twice as Much. Mol. Biol. Evol. 2015, 32, 3030–3032. [Google Scholar] [CrossRef]
- Hwang, U.-W.; Kim, W. General properties and phylogenetic utilities of nuclear ribosomal DNA and mitochondrial DAN commonly used in molecular systematics. Korean J. Parasitol. 1999, 37, 215–228. [Google Scholar] [CrossRef]
- Wosula, E.N.; Chen, W.; Fei, Z.; Legg, J.P. Unravelling the genetic diversity among cassava Bemisia tabaci whiteflies using NextRAD sequencing. Genome Biol. Evol. 2017, 9, 2958–2973. [Google Scholar] [CrossRef]
- Dijkstra, E.; Rubio, J.M.; Post, R.J. Resolving relationships over a wide taxonomic range in Delphacidae (Homoptera) using the COI gene. Syst. Entomol. 2003, 28, 89–100. [Google Scholar] [CrossRef]
- Bahder, B.W.; Bartlett, C.R.; Barrantes Barrantes, E.A.; Zumbado Echavarria, M.A.; Humphries, A.R.; Helmick, E.E.; Ascunce, M.S.; Goss, E.M. A new species of Omolicna (Hemiptera: Auchenorrhyncha: Fulgoroidea: Derbidae) from coconut palm in Costa Rica and new country records for Omolicna brunnea and Omolicna triata. Zootaxa 2019, 4577, 501–514. [Google Scholar] [CrossRef]
- Caldwell, J.S. A generic revision of the treehoppers of the tribe Ceresini in America north of Mexico based on a study of the male genitalia. Proc. U. S. Natl. Mus. 1949, 98, 491–521. [Google Scholar] [CrossRef]
- Ebsen, J.R.; Boomsma, J.J.; Nash, D.R. Phylogeography and cryptic speciation in the Myrmica scabrinodis Nylander, 1846 species complex (Hymenoptera: Formicidae), and their conservation implications. Insect Conserv. Divers. 2019, 12, 467–480. [Google Scholar] [CrossRef]
- Iftikhar, R.; Ashfaq, M.; Rasool, A.; Hebert, P.D.N. DNA barcode analysis of thrips (Thysanoptera) diversity in Pakistan reveals cryptic species complexes. PLoS ONE 2016, 11, e0146014. [Google Scholar] [CrossRef]
- Tyagi, K.; Kumar, V.; Singha, D.; Chandra, K.; Laskar, B.A.; Kundu, S.; Chakraborty, R.; Chatterjee, S. DNA Barcoding studies on Thrips in India: Cryptic species and Species complexes. Sci. Rep. 2017, 7, 4898. [Google Scholar] [CrossRef] [PubMed]
- Minard, G.; Tran Van, V.; Tran, F.H.; Melaun, C.; Klimpel, S.; Koch, L.K.; Ly Huynh Kim, K.; Huynh Thi Thuy, T.; Tran Ngoc, H.; Potier, P.; et al. Identification of sympatric cryptic species of Aedes albopictus subgroup in Vietnam: New perspectives in phylosymbiosis of insect vector. Parasites Vectors 2017, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Kanturski, M.; Lee, Y.; Choi, J.; Lee, S. DNA barcoding and a precise morphological comparison revealed a cryptic species in the Nippolachnus piri complex (Hemiptera: Aphididae: Lachninae). Sci. Rep. 2018, 8, 8998. [Google Scholar] [CrossRef] [PubMed]
- Preto, C.R.; Bahder, B.W.; Bick, E.N.; Sudarshana, M.R.; Zalom, F.G. Seasonal Dynamics of Spissistilus festinus (Hemiptera: Membracidae) in a Californian Vineyard. J. Econ. Entomol. 2019, 112, 1138–1144. [Google Scholar] [CrossRef]
| Location | Latitude | Longitude | Host | Date Collected | Primers for mt-COI PCR and Sequencing | NCBI GenBank Accession Numbers | |
|---|---|---|---|---|---|---|---|
| mt-COI Sequences | ITS2 Sequences | ||||||
| Davis, CA | 38.5381 | −121.8813 | Clover and wild licorice | October 2017 | HCO1298/LCO1490 a | MN888499 | MN887241 |
| Knights Landing, CA | 38.7914 | −121.7338 | Alfalfa | October 2015 | HCO1298/LCO1490 | MN888490 | MN887235 |
| Knights Landing, CA | 38.7894 | −121.7259 | Alfalfa | October 2016 | HCO1298/LCO1490 | MN888491 | MN887236 |
| Knights Landing, CA | 38.7660 | −121.7911 | Alfalfa | October 2016 | HCO1298/LCO1490 | MN888492 | MN887237 |
| Lodi, CA | 38.1152 | −121.4343 | Alfalfa | October 2017 | HCO1298/LCO1490 | MN888502 | MN887242 |
| Rutherford, CA | 38.4572 | −122.4103 | Grape | July 2015 | HCO1298/LCO1490 | MN888498 | MN887239 |
| Rutherford, CA | 38.4572 | −122.4103 | Grape | July 2016 | HCO1298/LCO1490 | MN888495 | MN887238 |
| Parlier, CA | 36.5956 | −119.5117 | Alfalfa | November 2015 | HCO1298/LCO1490 | MN888501 | MN887240 |
| Auburn, AL | 32.5927 | −85.4858 | Soybean | October 2016 | SETCAHfor/LepR1 b | MN888492 | MN887247 |
| Painter, VA | 37.5859 | −75.7821 | Weeds | July 2017 | SETCAHfor/LepR1 | MN888490 | MN887243 |
| Plymouth, NC | 35.6172 | −76.7568 | Soybean | August 2017 | SETCAHfor/LepR1 | MN888494 | MN887245 |
| Starkville, MS | 33.4815 | −88.7841 | Peanut | October 2016 | SETCAHfor/LepR1 | MN888491 | MN887244 |
| Tifton, GA | 31.4887 | −83.5413 | Alfalfa | August 2017 | SETCAHfor/LepR1 | MN888493 | MN887246 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieniewicz, E.; Poplaski, V.; Brunelli, M.; Dombroskie, J.; Fuchs, M. Two Distinct Genotypes of Spissistilus festinus (Say, 1830) (Hemiptera, Membracidae) in the United States Revealed by Phylogenetic and Morphological Analyses. Insects 2020, 11, 80. https://doi.org/10.3390/insects11020080
Cieniewicz E, Poplaski V, Brunelli M, Dombroskie J, Fuchs M. Two Distinct Genotypes of Spissistilus festinus (Say, 1830) (Hemiptera, Membracidae) in the United States Revealed by Phylogenetic and Morphological Analyses. Insects. 2020; 11(2):80. https://doi.org/10.3390/insects11020080
Chicago/Turabian StyleCieniewicz, Elizabeth, Victoria Poplaski, Melina Brunelli, Jason Dombroskie, and Marc Fuchs. 2020. "Two Distinct Genotypes of Spissistilus festinus (Say, 1830) (Hemiptera, Membracidae) in the United States Revealed by Phylogenetic and Morphological Analyses" Insects 11, no. 2: 80. https://doi.org/10.3390/insects11020080
APA StyleCieniewicz, E., Poplaski, V., Brunelli, M., Dombroskie, J., & Fuchs, M. (2020). Two Distinct Genotypes of Spissistilus festinus (Say, 1830) (Hemiptera, Membracidae) in the United States Revealed by Phylogenetic and Morphological Analyses. Insects, 11(2), 80. https://doi.org/10.3390/insects11020080







