New Tools for Conservation Biological Control: Testing Ant-Attracting Artificial Nectaries to Employ Ants as Plant Defenders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
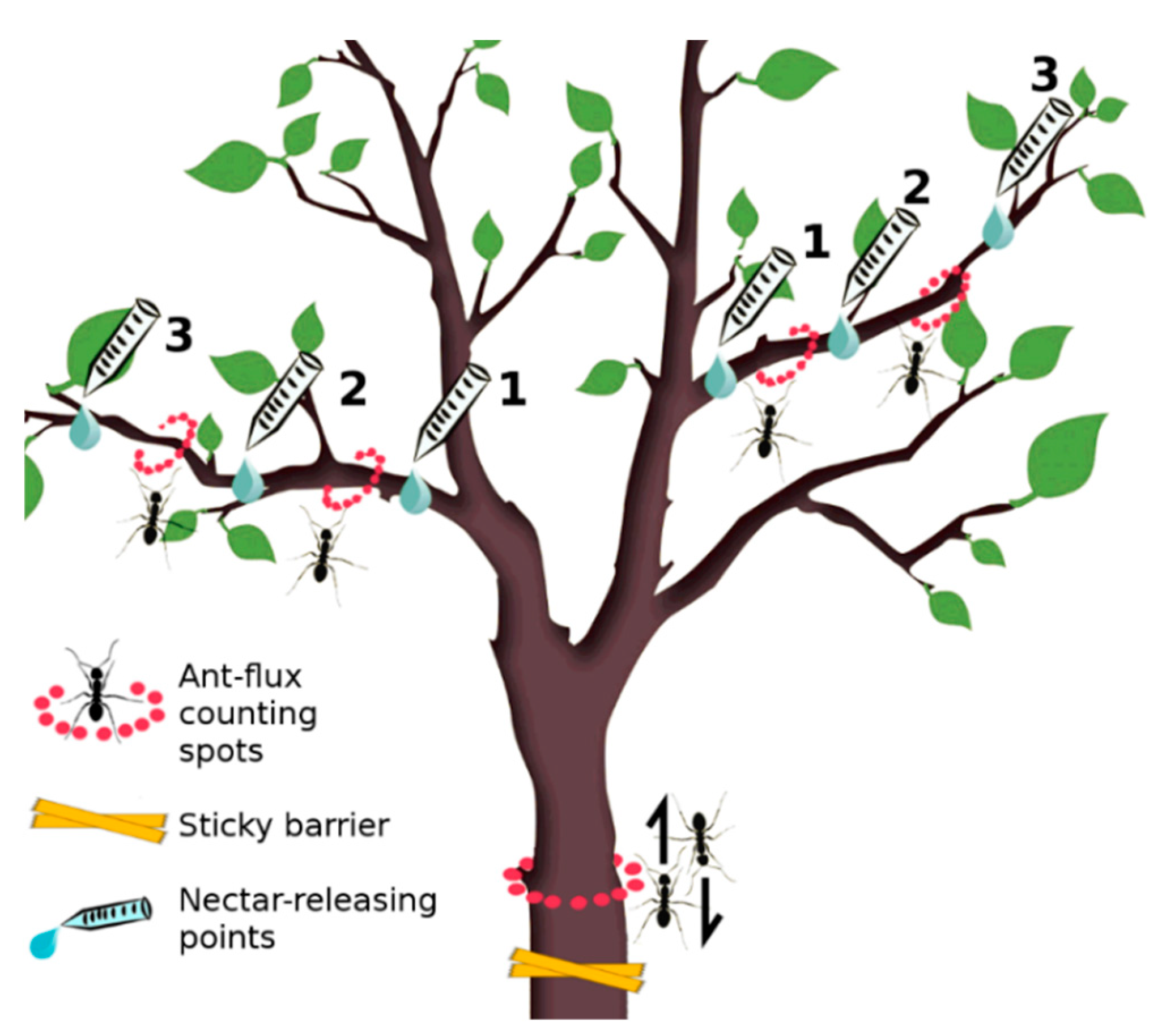
2.2. Artificial Nectaries (ANs)
2.3. Treatments
- Treatment 1 (ANs+/Ants+): ANs were installed and ants were free to climb the tree
- Treatment 2 (ANs−/Ants+): no ANs were installed and ants were free to climb the tree (trees with no manipulations)
- Treatment 3 (ANs+/Ants−): ANs were installed and ants were not free to climb the tree since sticky barriers were installed
- Treatment 4: (ANs−/Ants−): no ANs were installed and ants were not free to climb the tree since sticky barriers were installed
2.4. Data Collection
2.4.1. Ant Activity
2.4.2. Arthropod Abundance
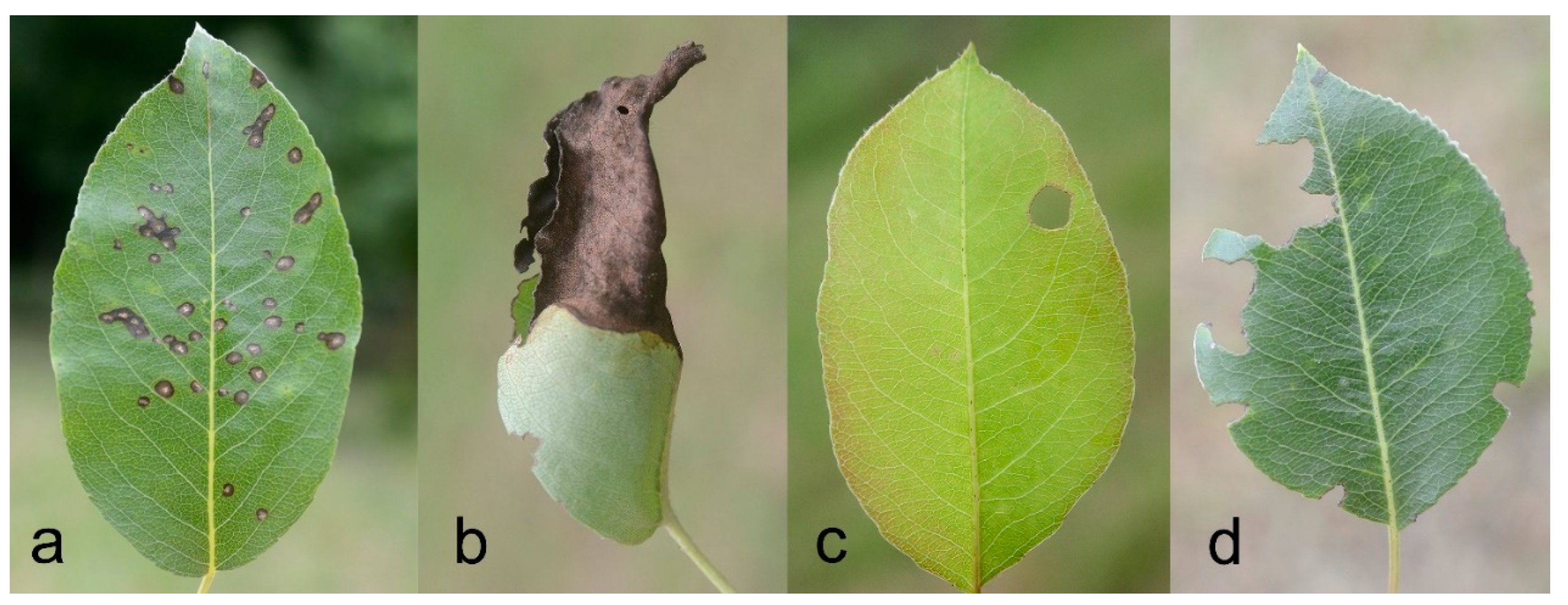
2.4.3. Leaf Damages
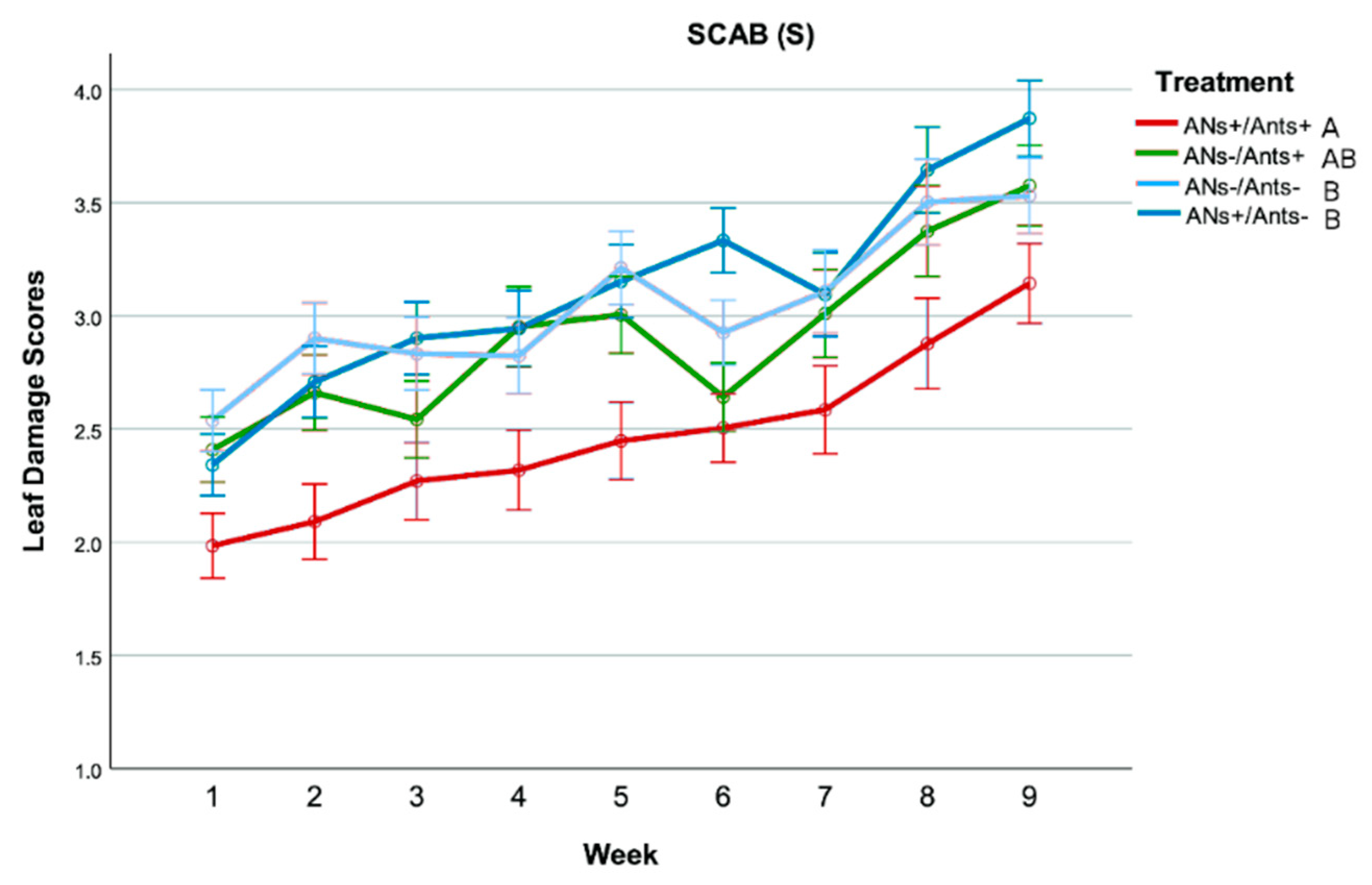
- Scab (S): presence of distinct black spots on the leaf surface, attributed to the fungus Venturia pyrina Aderh. (1896). Scores: 1 = absent; 2 = low (less than half leaf surface interested); 3 = medium (half of the leaf surface interested); 4 = high (more than half leaf surface interested)
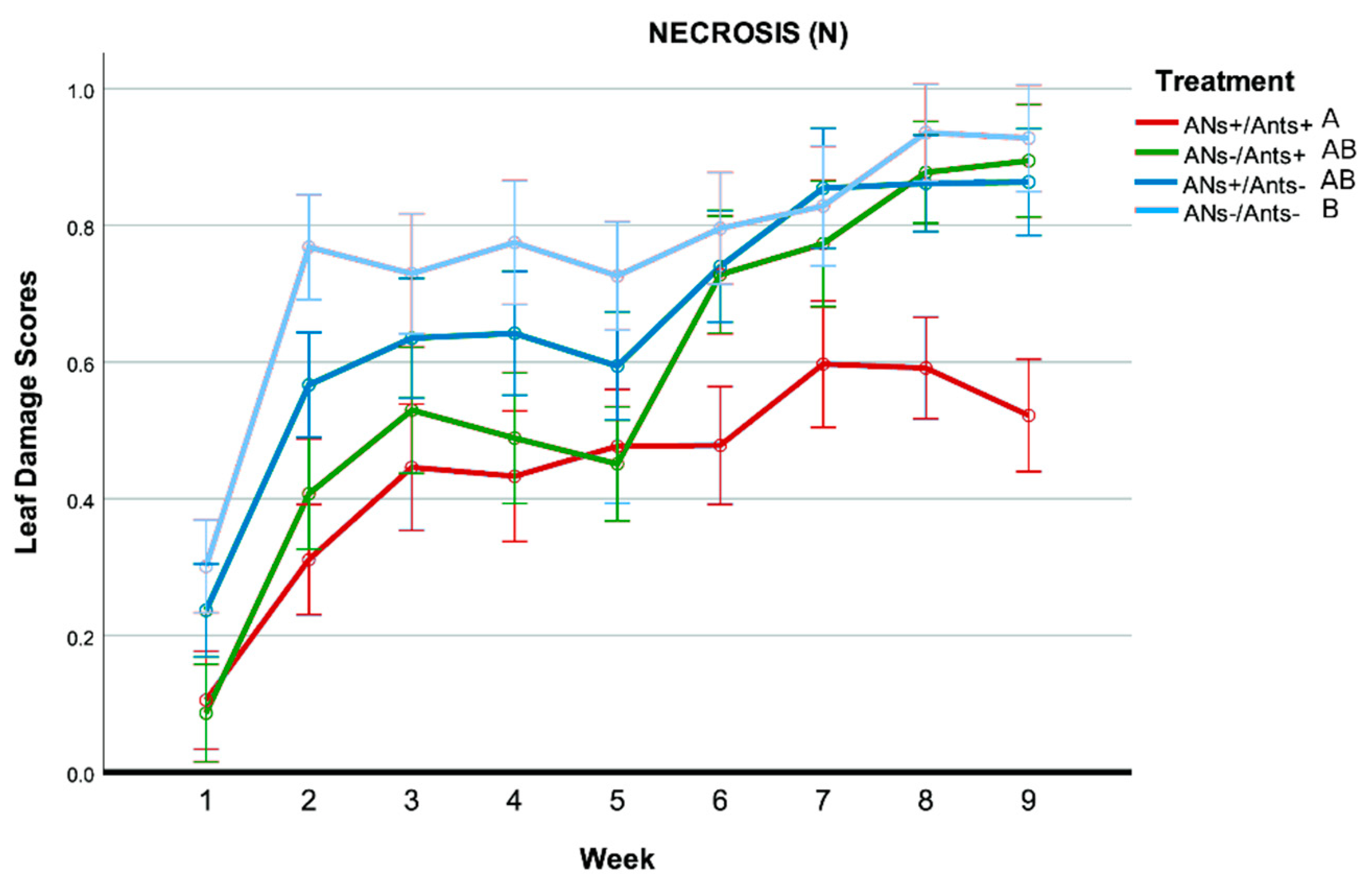
- Necrosis (N): presence of extended necrotic areas on the leaf (surfaces larger than spots and with different shapes). Scores: 0 = absent; 1 = present
- Holes (H): presence of holes due to missing parts of tissue far from the edges. Scores: 0 = absent; 1 = present
- Damaged Edge (DE): presence of altered leaf profile due to missing parts of tissue at the edges; Scores: 1 = absent; 2 = low (less than half leaf edge interested); 3 = medium (half of the leaf edge interested); 4 = high (more than half leaf edge interested)
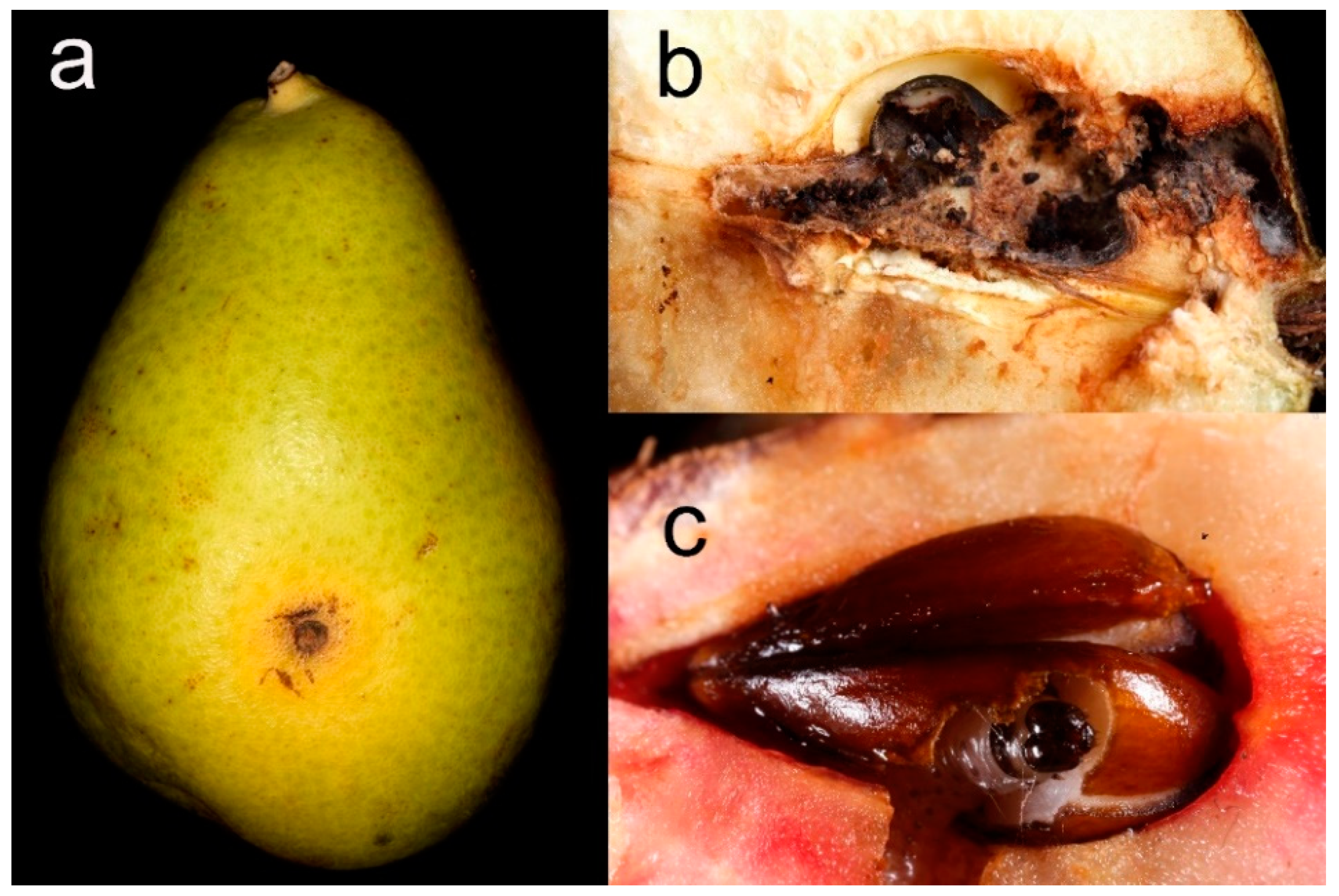
2.4.4. Fruit Damages
- Damage caused by sucking or chewing phytophagous insects (e.g., see [108] for H. halys)
- Holes produced by codling moth Cydia pomonella (Linnaeus, 1758) caterpillars (Lepidoptera, Tortricidae)
- Damage caused by fungi (e.g., V. pyrina)
- Other damage
2.5. Statistical Analyses
3. Results
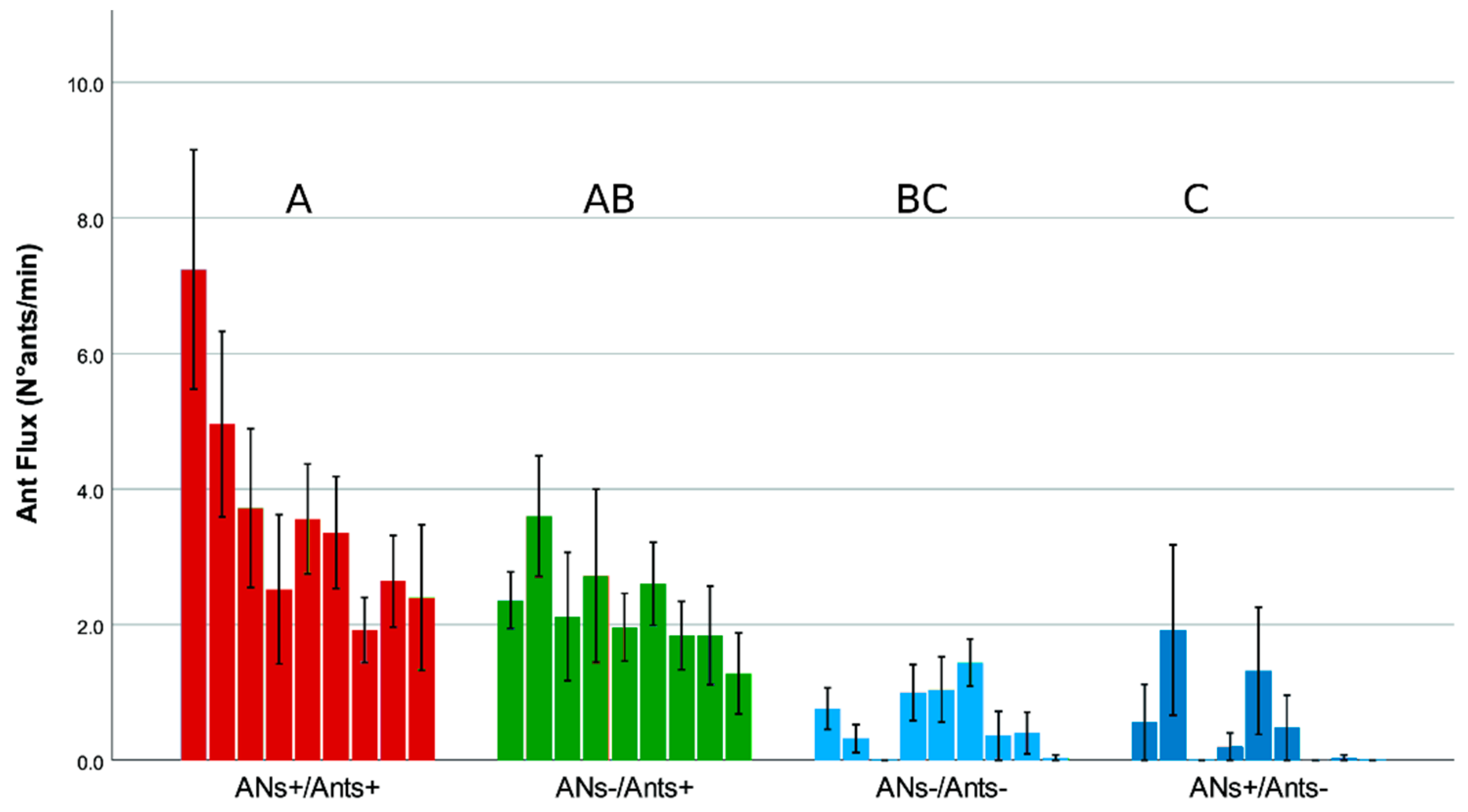
3.1. Ant Activity
3.2. Arthropods Abundance
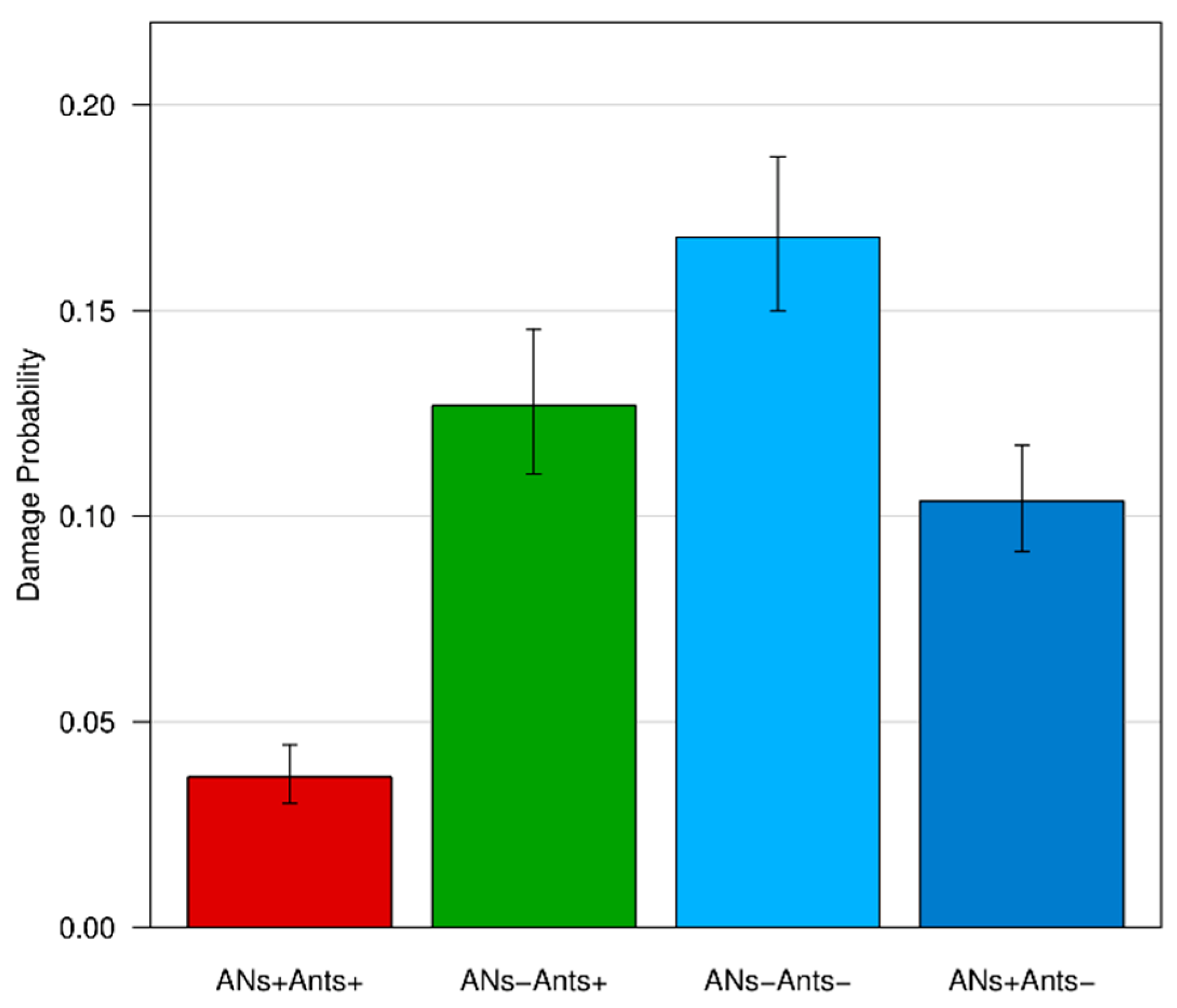
3.3. Leaf Damage
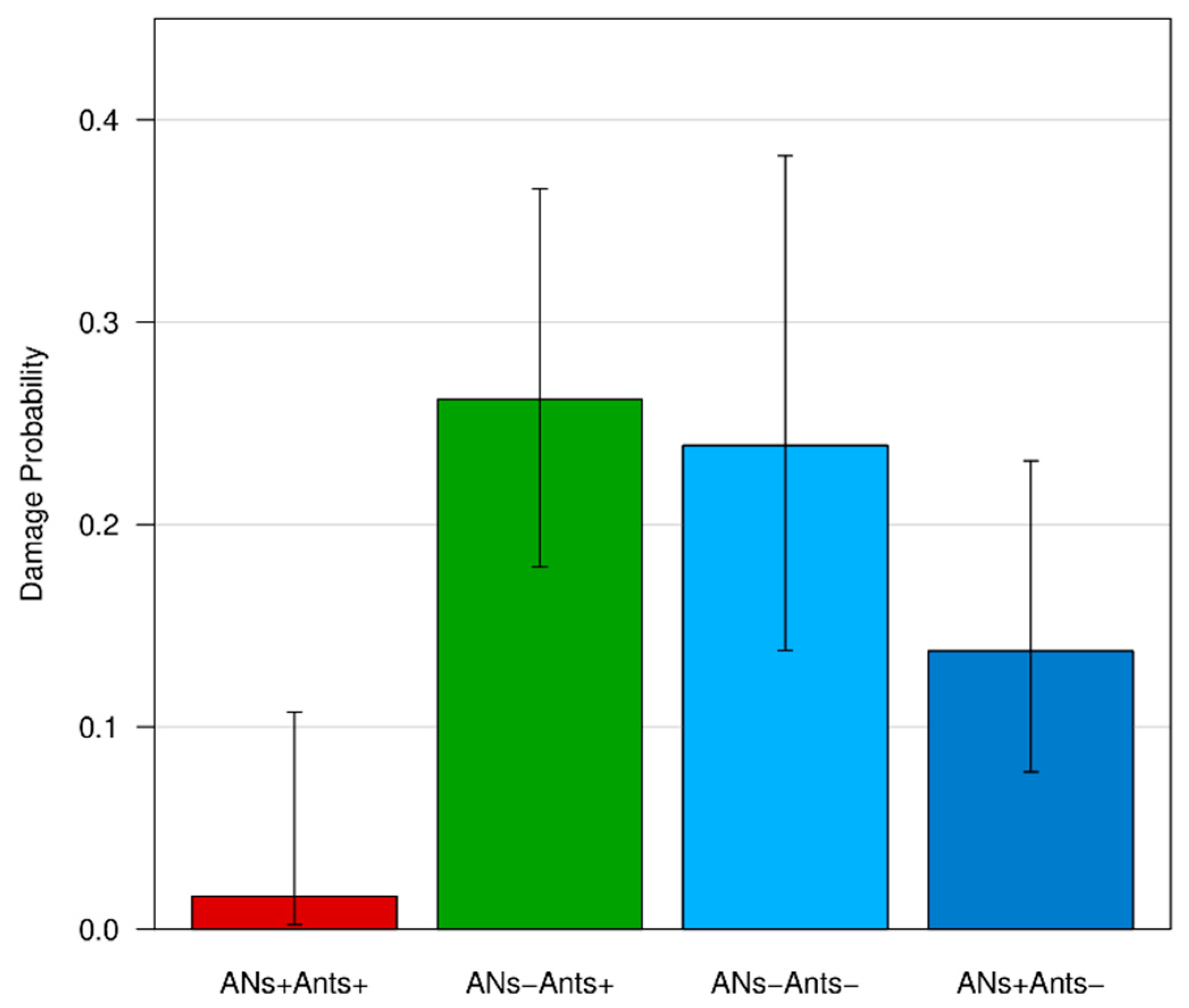
3.4. Fruit Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Superorganism: Beauty, Elegance, and Strangeness of Insect Societies; W.W. Norton & Company: New York, NY, USA, 2008. [Google Scholar]
- Rosumek, F.B.; Silveira, F.A.O.; de, S. Neves, F.; de U. Barbosa, N.P.; Diniz, L.; Oki, Y.; Pezzini, F.; Fernandes, G.W.; Cornelissen, T. Ants on plants: A meta-analysis of the role of ants as plant biotic defenses. Oecologia 2009, 160, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, M.P.; Ree, R.H.; Moreau, C.S. Ant-plant interactions evolved through increasing interdependence. Proc. Natl. Acad. Sci. USA 2018, 115, 12253–12258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico-Gray, V.; Oliveira, P. The Ecology and Evolution of Ant-Plant Interactions; The University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Lach, L.; Lori, L.; Parr, L.; Catherine, L.; Abbot, K. Ant Ecology; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Solida, L.; Grasso, D.A.; Testi, A.; Fanelli, G.; Scalisi, M.; Bartolino, V.; Mori, A.; Fanfani, A. Differences in the nesting sites microhabitat characteristics of two syntopic species of Messor harvester ants in a phytosociological homogeneous grassland area. Ethol. Ecol. Evol. 2011, 23, 229–239. [Google Scholar] [CrossRef]
- Way, M.J. Mutualism between ants and honeydew producing Homoptera. Annu. Rev. Entomol. 1963, 8, 307–344. [Google Scholar] [CrossRef]
- Buckley, R. Interactions involving plants, Homoptera, and ants. Annu. Rev. Ecol. Evol. Syst. 1987, 18, 111–135. [Google Scholar] [CrossRef]
- Flatt, T.; Weisser, W.W. The effects of mutualistic ants on aphid life history traits. Ecology 2000, 81, 3522–3529. [Google Scholar] [CrossRef]
- Delabie, J.H. Trophobiosis between Formicidae and Hemiptera (Sternorrhyncha and Auchenorrhyncha): An overview. Neotrop. Entomol. 2001, 30, 501–516. [Google Scholar] [CrossRef] [Green Version]
- Styrsky, J.D.; Eubanks, M.D. Ecological consequences of interactions between ants and honeydew-producing insects. Proc. Biol. Sci. 2007, 274, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Beattie, A.J. The Evolutionary Ecology of Ant–Plant Mutualisms; Cambridge University Press: Cambridge, UK, 1985. [Google Scholar]
- Davidson, D.W.; McKey, D. The Evolutionary Ecology of Symbiotic Ant-Plant Relationships. J. Hymenopt. Res. 1993, 2, 13–83. [Google Scholar]
- De la Fuente, M.A.S.; Marquis, R.J. The role of ant-tended extrafloral nectaries in the protection and benefit of a Neotropical rainforest tree. Oecologia 1999, 118, 192–202. [Google Scholar] [CrossRef]
- Heil, M.; McKey, D. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 425–453. [Google Scholar] [CrossRef] [Green Version]
- Offenberg, J.; Damgaard, C. Ants suppressing plant pathogens: A review. Oikos 2019, 128, 1–13. [Google Scholar] [CrossRef]
- Becerra, J.X.I.; Venable, D.L. Extrafloral nectaries: A defense against ant-Homoptera mutualism? Oikos 1989, 55, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Engel, V.; Fischer, M.K.; Wäckers, F.L.; Völkl, W. Interactions between extrafloral nectaries, aphids and ants: Are there competition effects between plant and homopteran sugar sources? Oecologia 2001, 129, 577–584. [Google Scholar] [CrossRef]
- Nagy, C.; Cross, J.V.; Markó, V. Can artificial nectaries outcompete aphids in ant-aphid mutualism? Applying artificial sugar sources for ants to support better biological control of rosy apple aphid, Dysaphis plantaginea Passerini in apple orchards. Crop Prot. 2015, 77, 127–138. [Google Scholar] [CrossRef]
- Beltrà, A.; Navarro-Campos, C.; Calabuig, A.; Estopà, L.; Wäckers, F.L.; Pekas, A.; Soto, A. Association between ants (Hymenoptera: Formicidae) and the vine mealybug (Hemiptera: Pseudococcidae) in table-grape vineyards in Eastern Spain. Pest Manag. Sci. 2017, 73, 2473–2480. [Google Scholar] [CrossRef]
- Weber, M.G.; Keeler, K.H. The phylogenetic distribution of extrafloral nectaries in plants. Ann. Bot. 2013, 111, 1251–1261. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.G.; Porturas, L.D.; Keeler, K.H. World List of Plants with Extrafloral Nectaries. 2015. Available online: www.extrafloralnectaries.org (accessed on 2 November 2019).
- Wäckers, F.L.; van Rijn, P.C.J.; Bruin, J. Plant Provided Food and Plant-Carnivore Mutualism; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Grasso, D.A.; Pandolfi, C.; Bazihizina, N.; Nocentini, D.; Nepi, M.; Mancuso, S. Extrafloral-nectar-based partner manipulation in plant–ant relationships. AoB PLANTS 2015, 7, plv002. [Google Scholar] [CrossRef] [Green Version]
- Nepi, M.; Grasso, D.A.; Mancuso, S. Nectar in Plant–Insect Mutualistic Relationships: From Food Reward to Partner Manipulation. Front. Pl. Sci. 2018, 9, 1063. [Google Scholar] [CrossRef]
- Pulice, C.E.; Packer, A.A. Simulated herbivory induces extrafloral nectary production in Prunus avium. Funct. Ecol. 2008, 22, 801–807. [Google Scholar] [CrossRef]
- Inui, Y.; Itioka, T. Species-specific leaf volatile compounds of obligate Macaranga myrmecophytes and host-specific aggressiveness of symbiotic Crematogaster ants. J. Chem. Ecol. 2007, 33, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Mayer, V.; Schaber, D.; Hadacek, F. Volatiles of myrmecophytic Piper plants signal stem issue damage to inhabiting Pheidole ant partners. J. Ecol. 2008, 96, 962–970. [Google Scholar] [CrossRef]
- Kost, C.; Heil, M. The defensive role of volatile emission and extrafloral nectar secretion for lima bean in nature. J. Chem. Ecol. 2008, 34, 2–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vittecoq, M.; Djieto-Lordon, C.; Buatois, B.; Dormont, L.; McKey, D.; Blatrix, R. The evolution of communication in two ant-plant mutualisms. Evol. Biol. 2011, 38, 360–369. [Google Scholar] [CrossRef]
- Schettino, M.; Grasso, D.A.; Weldegergis, B.T.; Castracani, C.; Mori, A.; Dicke, M.; Van Lenteren, J.C.; Van Loon, J.J. Response of a predatory ant to volatiles emitted by aphid-and caterpillar-infested cucumber and potato plants. J. Chem. Ecol. 2017, 43, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Quimby, P.C.; King, L.R.; Grey, W.E. Biological control as a means of enhancing the sustainability of crop/land management systems. Agricult. Ecosys. Environ. 2002, 88, 147–152. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Luna, J.M. Multi-function agricultural biodiversity: Pest management and other benefits. Basic Appl. Ecol. 2003, 4, 107–116. [Google Scholar] [CrossRef]
- Bianchi, F.J.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [Green Version]
- Van Mele, P. A historical review of research on the weaver ant Oecophylla in biological control. Agric. Forest Entomol. 2008, 10, 13–22. [Google Scholar] [CrossRef]
- Peng, R.; Christian, K. Box 7.2 Ants as biological-control agents in the horticultural industry. In Ant Ecology; Lach, L., Lori, L., Parr, L., Catherine, L., Abbot, K., Eds.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Offenberg, J. Ants as tools in sustainable agriculture. J. Appl. Ecol. 2015, 52, 1197–1205. [Google Scholar] [CrossRef]
- Peck, S.L.; Mcquaid, B.; Campbell, C.L. Using ant species (Hymenoptera: Formicidae) as a biological indicator of agroecosystem condition. Environ. Entomol. 1998, 27, 1102–1110. [Google Scholar] [CrossRef] [Green Version]
- De Bruyn, L.A.L. Ants as bioindicators of soil function in rural environments. Agric. Ecosyst. Environ. 1999, 74, 425–441. [Google Scholar] [CrossRef]
- Risch, S.J.; Carroll, C.R. The ecological role of ants in two Mexican agroecosystems. Oecologia 1982, 55, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Way, M.J.; Khoo, K.C. Role of ants in pest management. Annu. Rev. Entomol. 1992, 37, 479–503. [Google Scholar] [CrossRef]
- Benckiser, G. Ants and sustainable agriculture. A review. Agron. Sustain. Dev. 2010, 30, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Choate, B.; Drummond, F. Ants as biological control agents in agricultural cropping systems. Terr. Arthropod Rev. 2011, 4, 157–180. [Google Scholar] [CrossRef]
- Seguni, Z.S.K.; Way, M.J.; Van Mele, P. The effect of ground vegetation management on competition between the ants Oecophylla longinoda and Pheidole megacephala and implications for conservation biological control. Crop Prot. 2011, 30, 713–717. [Google Scholar] [CrossRef]
- Offenberg, J. The use of artificial nests by weaver ants: A preliminary field observation. Asian Myrmecol. 2014, 6, 119–128. [Google Scholar] [CrossRef]
- Philpott, S.M.; Foster, P.F. Nest-site limitation in coffee agroecosystems: Artificial nests maintain diversity of arboreal ants. Ecol. Appl. 2005, 15, 1478–1485. [Google Scholar] [CrossRef]
- Offenberg, J.; Nielsen, J.S.; Damgaard, C. Wood Ant (Formica polyctena) Services and Disservices in a Danish Apple Plantation. Sociobiology 2019, 66, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Jones, I.M.; Koptur, S.; von Wettberg, E.J. The use of extrafloral nectar in pest management: Overcoming context dependence. J. Appl. Ecol. 2017, 54, 489–499. [Google Scholar] [CrossRef]
- James, D.G.; Stevens, M.; O’Malley, K.J.; Faulder, R.J. Ant foraging reduces the abundance of beneficial and incidental arthropods in citrus canopies. Biol. Control 1999, 14, 121–126. [Google Scholar] [CrossRef]
- Daane, K.M.; Sime, K.R.; Fallon, J.; Cooper, M.L. Impacts of Argentine ants on mealybugs and their natural enemies in California’s coastal vineyards. Ecol. Entomol. 2007, 32, 583–596. [Google Scholar] [CrossRef]
- Pekas, A.; Tena, A.; Aguilar, A.; Garcia-Marí, F. Effect of Mediterranean ants (Hymenoptera: Formicidae) on California red scale (Hemiptera: Diaspididae) populations in citrus orchards. Environ. Entomol. 2010, 39, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Pekas, A.; Tena, A.; Aguilar, A.; Garcia-Marí, F. Spatio-temporal patterns and interactions with honeydew producing hemiptera of ants in a Mediterranean citrus orchard. Agric. Forest. Entomol. 2011, 13, 89–97. [Google Scholar] [CrossRef]
- Calabuig, A.; Garcia-Marí, F.; Pekas, A. Ants affect the infestation levels but not the parasitism of honeydew and non-honeydew producing pests in citrus. Bull. Entomol. Res. 2013, 104, 405–417. [Google Scholar] [CrossRef]
- Yoo, H.J.S.; Kizner, M.C.; Holway, D.A. Ecological effects of multi-species, ant hemipteran mutualisms in citrus. Ecol. Entomol. 2013, 38, 505–514. [Google Scholar] [CrossRef]
- Dao, H.T.; Meats, A.; Beattie, G.A.C.; Spooner-Hart, R. Ant-coccid mutualism in citrus canopies and its effect on natural enemies of red scale, Aonidiella aurantii (Maskell) (Hemiptera: Diaspididae). Bull. Entomol. Res. 2014, 104, 137–142. [Google Scholar] [CrossRef]
- Klotz, J.H.; Rust, M.K.; Gonzalez, D.; Greenberg, L.; Costa, H.; Phillips, P.; Gispert, C.; Reierson, D.A.; Kido, K. Directed Sprays and Liquid Baits to Manage Ants in Vineyards and Citrus Groves. J. Agric. Urban Entomol. 2003, 20, 31–40. [Google Scholar]
- Tollerup, K.E.; Rust, M.K.; Dorschner, K.; Phillips, P.; Klotz, J.H. Low-toxicity baits control ants in citrus orchards and grape vineyards. Calif. Agric. 2004, 58, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Tollerup, K.; Rust, M.K.; Klotz, J.H. Formica perpilosa, an Emerging Pest in Vineyards. J. Agric. Urban Entomol. 2007, 24, 147–158. [Google Scholar] [CrossRef]
- Greenberg, L.; Tollerup, K.E.; Rust, M.K. Control of Argentine Ants (Hymenoptera: Formicidae) in Citrus using Methoprene and Imidacloprid Delivered in Liquid Bait Stations. Fla. Entomol. 2013, 96, 1023–1029. [Google Scholar] [CrossRef]
- Daane, K.M.; Sime, K.R.; Hogg, B.N.; Bianchi, M.L.; Cooper, M.L.; Rust, M.K.; Klotz, J.H. Effects of liquid insecticide baits on Argentine ants in California’s coastal vineyards. Crop Prot. 2006, 25, 592–603. [Google Scholar] [CrossRef]
- Nondillo, A.; Andzeiewski, S.; Bello Fialho, F.; Bueno, O.C.; Botton, M. Control of Linepithema micans (Hymenoptera: Formicidae) and Eurhizococcus brasiliensis (Hemiptera: Margarodidae) in vineyards using toxic baits. J. Econ. Entomol. 2016, 109, 1660–1666. [Google Scholar] [CrossRef]
- Westermann, F.L.; Bell, V.A.; Suckling, D.M.; Lester, P.J. Synthetic pheromones as a management technique—Dispensers reduce Linepithema humile activity in a commercial vineyard. Pest. Manag. Sci. 2016, 72, 719–724. [Google Scholar] [CrossRef]
- Cooper, M.L.; Hobbs, M.B.; Boser, C.L.; Varela, L.G. Argentine Ant Management: Using Toxin-Laced Polyacrylamide Crystals to Target Ant Colonies in Vineyards. Catal. Discov. Pract. 2019. [Google Scholar] [CrossRef]
- Chong, C.S.; D’Alberto, C.F.; Thomson, L.J.; Hoffmann, A.A. Influence of native ants on arthropod communities in a vineyard. Agric. Forest Entomol. 2010, 12, 223–232. [Google Scholar] [CrossRef]
- Samways, M.J.; Nel, M.; Prins, A.J. Ants (Hymenoptera: Formicidae) foraging in citrus trees and attending honeydew-producing Homoptera. Phytophylactica 1982, 14, 155–157. [Google Scholar]
- Samways, M.J.; Grout, T.J.; Prins, A.J. Ants as Citrus Pests. In Citrus Pests in the Republic of South Africa; Bedford, E.C.G., Van Den Berg, M.A., Villiers, E.A., Eds.; Institute for Tropical and Subtropical Crops: Nelspruit, South Africa, 1998. [Google Scholar]
- Pavan, M. Attivitá Italiana per la Lotta Biologica Con Formiche del Gruppo Formica rufa Contro Gli Insetti Dannosi Alle Foreste; Ministero dell’Agricoltura e delle Foreste, Collana verde: Rome, Italy, 1959; Volume 4, pp. 1–80. [Google Scholar]
- Adlung, K.G. A critical evaluation of the European research on use of red wood ants (Formica rufa group) for the protection of forests against harmful insects. Z. Angew. Entomol. 1966, 57, 167–189. [Google Scholar] [CrossRef]
- Nielsen, J.S.; Nielsen, M.G.; Offenberg, J. Experiences in Transplanting Wood Ants into Plantations for Integrated Pest Management. Sociobiology 2018, 65, 403–414. [Google Scholar] [CrossRef]
- Castracani, C.; Mori, A. The role of permanent grasslands on ant community structure: Ants (Hymenoptera: Formicidae) as ecological indicators in the agro-ecosystems of the Taro River Regional Park (Italy). Myrmecol. Nachr. 2006, 9, 47–54. [Google Scholar]
- La Pergola, A.; Alicata, A.; Longo, S. Survey of the ants (Hymenoptera Formicidae) in citrus orchards with different types of crop management in Sicily. IOBC/WPRS Bull. 2008, 38, 233–237. [Google Scholar]
- Ottonetti, L.; Tucci, L.; Chelazzi, G.; Santini, G. Stable isotopes analysis to assess the trophic role of ants in a Mediterranean agroecosystem. Agric. Forest Entomol. 2008, 10, 29–36. [Google Scholar] [CrossRef]
- Santini, G.; Ramsay, P.M.; Tucci, L.; Ottonetti, L.; Frizzi, F. Spatial patterns of the ant Crematogaster scutellaris in a model ecosystem. Ecol. Entomol. 2011, 36, 625–634. [Google Scholar] [CrossRef]
- Campolo, O.; Palmeri, V.; Malacrinò, A.; Laudani, F.; Castracani, C.; Mori, A.; Grasso, D.A. Interaction between ants and the Mediterranean fruit fly: New insights for biological control. Biol. Control 2015, 90, 120–127. [Google Scholar] [CrossRef]
- Castracani, C.; Maienza, A.; Grasso, D.A.; Genesio, L.; Malcevschi, A.; Miglietta, F.; Vaccari, F.P.; Mori, A. Biochar–macrofauna interplay: Searching for new bioindicators. Sci. Total Environ. 2015, 536, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Masoni, A.; Frizzi, F.; Brühl, C.; Zocchi, N.; Palchetti, E.; Chelazzi, G.; Santini, G. Management matters: A comparison of ant assemblages in organic and conventional vineyards. Agric. Ecosyst. Environ. 2017, 246, 175–183. [Google Scholar] [CrossRef]
- Castracani, C.; Bulgarini, G.; Giannetti, D.; Spotti, F.A.; Maistrello, L.; Mori, A.; Grasso, D.A. Predatory ability of the ant Crematogaster scutellaris on the brown marmorated stink bug Halyomorpha halys. J. Pest Sci. 2017, 90, 1181–1190. [Google Scholar] [CrossRef]
- Giannetti, D.; Castracani, C.; Spotti, F.A.; Mori, A.; Grasso, D.A. Gall-colonizing ants and their role as plant defenders: From ‘bad job’ to ‘useful service’. Insects 2019, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Baraibar, B.; Carrión, E.; Recasens, J.; Westerman, P.R. Unravelling the process of weed seed predation: Developing options for betterweed control. Biol. Control 2011, 56, 85–90. [Google Scholar] [CrossRef]
- Le Moli, F.; Mori, A.; Parmigiani, S. Agonistic behaviour of Formica rufa L. (Hymenoptera Formicidae). Ital. J. Zool. 1982, 16, 325–331. [Google Scholar]
- Grasso, D.A.; Mori, A.; Le Moli, F. Chemical communication during foraging in the harvesting ant Messor capitatus (Hymenoptera, Formicidae). Insectes Soc. 1998, 45, 85–96. [Google Scholar] [CrossRef]
- Grasso, D.A.; Mori, A.; Le Moli, F. Recruitment and trail communication in two species of Messor ants (Hymenoptera, Formicidae). Ital. J. Zool. 1999, 66, 373–378. [Google Scholar] [CrossRef]
- Mori, A.; Grasso, D.A.; Le Moli, F. Raiding and foraging behavior of the blood-red ant, Formica sanguinea Latr. (Hymenoptera, Formicidae). J. Insect Behav. 2000, 13, 421–430. [Google Scholar] [CrossRef]
- Barbosa, P.A. Conservation Biological Control; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Jonsson, M.; Wratten, S.D.; Landis, D.A.; Gurr, G.M. Recent advances in conservation biological control of arthropods by arthropods. Biol. Control 2008, 45, 172–175. [Google Scholar] [CrossRef]
- Bell, R.L. Pears (Pyrus). Acta Hortic. 1991, 290, 657–700. [Google Scholar] [CrossRef]
- Marino, P.; Schicchi, R.; Barone, E.; Raimondo, F.M.; Domina, G. First results on the phenotypic analysis of wild and cultivated species of Pyrus in Sicily. Fl. Medit. 2013, 23, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Husain, M.; Rathore, J.P.; Sharma, A.; Raja, A.; Qadri, I.; Wani, A.W. Description and management strategies of important pests of pear: A review. J. Entomol. Zool. Stud. 2018, 6, 677–683. [Google Scholar]
- Folkina, M.Y. A case of mass extermination of the San Jose scale (Quadraspidiotus perniciosus) by the ant Crematogaster subdentata. Zool. Zhurnal 1978, 57, 301. [Google Scholar]
- Paulson, G.S.; Akre, D.K. Introducing Ants (Hymenoptera: Formicidae) Into Pear Orchards for the Control of Pear Psylla, Cacopsylla pyricola (Foerster) (Homoptera: Psyllidae). J. Agric. Entomol. 1992, 9, 37–39. [Google Scholar]
- Paulson, G.S.; Akre, D.K. Role of Predaceous Ants in Pear Psylla (Homoptera: Psyllidae) Management: Estimating Colony Size and Foraging Range of Formica neoclara (Hymenoptera: Formicidae) through a Mark-Recapture Technique. J. Econ. Entomol. 1991, 84, 1437–1440. [Google Scholar] [CrossRef]
- Paulson, G.S.; Akre, D.K. Evaluating the Effectiveness of Ants as Biological Control Agents of Pear Psylla (Homoptera: Psyllidae). J. Econ. Entomol. 1992, 85, 70–73. [Google Scholar] [CrossRef]
- Erler, F. Natural enemies of the pear psylla Cacopsylla pyri in treated vs. untreated pear orchards in Antalya, Turkey. Phytoparasitica 2004, 32, 295–304. [Google Scholar] [CrossRef]
- Sanchez, J.; Ortín-Angulo, M.C. Abundance and population dynamics of Cacopsylla pyri (Hemiptera: Psyllidae) and its potential natural enemies in pear orchards in southern Spain. Crop Prot. 2007, 32, 24–29. [Google Scholar] [CrossRef]
- Novak, H. The influence of ant attendance on larval parasitism in hawthorn psyllids (Homoptera: Psyllidae). Oecologia 1994, 99, 72–78. [Google Scholar] [CrossRef]
- Offenberg, J. Balancing between mutualism and exploitation: The symbiotic interaction between Lasius ants and aphids. Behav. Ecol. Sociobiol. 2001, 49, 304–310. [Google Scholar] [CrossRef]
- Carabalí-Banguero, D.J.; Wyckhuys, K.A.G.; Montoya-Lerma, J.; Kondo, T.; Lundgren, J.G. Do additional sugar sources affect the degree of attendance of Dysmicoccus brevipes by the fire ant Solenopsis geminata? Entomol. Exp. Appl. 2013, 148, 65–73. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Alberola, J.S.; Garcia-Marí, F.; Pekas, A. Attract and distract: Manipulation of a food-mediated protective mutualism enhances natural pest control. Agric. Ecosyst. Environ. 2017, 246, 168–174. [Google Scholar] [CrossRef]
- Pacelhe, F.T.; Costa, F.V.; Neves, F.S.; Bronstein, J.; Mello, M.A. Nectar quality affects ant aggressiveness and biotic defense provided to plants. Biotropica 2019, 51, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Piñol, J.; Espadaler, X.; Cañellas, N.; Martínez-Vilalta, J.; Barrientos, J.A.; Sol, D. Ant versus bird exclusion effects on the arthropod assemblage of an organic citrus grove. Ecol. Entomol. 2010, 35, 367–376. [Google Scholar] [CrossRef]
- Piñol, J.; Espadaler, X.; Cañellas, N. Eight years of ant-exclusion from citrus canopies: Effects on the arthropod assemblage and on fruit yield. Agric. Forest Entomol. 2012, 14, 49–57. [Google Scholar] [CrossRef]
- Radchenko, A.G.; Elmes, G.W. Myrmica ants of the Old World. Fauna Mundi 2010, 3, 89–93. [Google Scholar]
- Seifert, B. The Ants of Central and North Europe; Lutra Verlags- und Vertriebsgesellschaft: Tauer, Germany, 2018. [Google Scholar]
- Chinery, M. Guide to the Insects of Britain and Western Europe; Harper-Collins: London, UK, 1986. [Google Scholar]
- Leraut, P. Le Guide entomologique; Delachaux et Niestlé: Paris, France, 2003. [Google Scholar]
- Araneae—Spiders of Europe. Available online: https://www.araneae.nmbe.ch (accessed on 31 January 2019).
- Bariselli, M.; Bugiani, R.; Maistrello, L. Distribution and damage caused by Halyomorpha halys in Italy. EPPO Bull. 2016, 46, 332–334. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; Sage Publications Ltd.: London, UK, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 31 January 2019).
- Arnan, X.; Andersen, A.N.; Gibb, H.; Parr, C.L.; Sanders, N.J.; Dunn, R.R.; Angulo, E.; Baccaro, F.B.; Bishop, T.R.; Boulay, R.; et al. Dominance–diversity relationships in ant communities differ with invasion. Glob. Chang. Biol. 2018, 24, 4614–4625. [Google Scholar] [CrossRef] [PubMed]
- Baroni Urbani, C. Catalogo delle specie di Formicidae d’Italia. Mem. Soc. Entom. Ital. 1971, 50, 5–287. [Google Scholar]
- Lachaud, J.P. Foraging activity and diet in some neotropical ponerine ants. I. Ectatomma ruidum Roger (Hymenoptera, Formicidae). Folia Entomol. Mex. 1990, 78, 241–256. [Google Scholar]
- Cornelius, M.L.; Grace, J.K. Influence of Brood on the Nutritional Preferences of the Tropical Ant Species, Pheidole megacephala (F.) and Ochetellus glaber (Mayr). J. Entomol. Sci. 1997, 32, 421–429. [Google Scholar] [CrossRef]
- Piñol, J.; Ribes, E.; Ribes, J.; Espadaler, X. Longterm changes and ant-exclusion effects on the true bugs (Hemiptera: Heteroptera) of an organic citrus grove. Agric. Ecosyst. Environ. 2012, 158, 127–131. [Google Scholar] [CrossRef]
- Del-Claro, K.; Oliveira, P.S. Ant-Homoptera interaction: Do alternative sugar sources distract tending ants? Oikos 1993, 2, 202–206. [Google Scholar] [CrossRef] [Green Version]
- López, R.; Potter, D.A. Ant Predation on Eggs and Larvae of the Black Cutworm (Lepidoptera: Noctuidae) and Japanese Beetle (Coleoptera: Scarabaeidae) in Turfgrass. Environ. Entomol. 2000, 29, 116–125. [Google Scholar] [CrossRef]
- Vandermeer, J.; Perfecto, I.; Nuñez, G.I.; Phillpott, S.; Ballinas, A.G. Ants (Azteca sp.) as potential biological control agents in shade coffee production in Chiapas, Mexico. Agrofor. Syst. 2002, 56, 271–276. [Google Scholar] [CrossRef]
- Nay, J.E.; Perring, T.M. Impact of Ant Predation and Heat on Carob Moth (Lepidoptera: Pyralidae) Mortality in California Date Gardens. J. Econ. Entomol. 2005, 98, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Eskafi, F.M.; Kolbe, M.M. Predation on larval and pupal Ceratitis capitata (Diptera: Tephritidae) by the ant Solenopsis geminata (Hymenoptera: Formicidae) and other predators in Guatemala. Environ. Entom. 1990, 19, 148–153. [Google Scholar] [CrossRef]
- Van Mele, P.; Vayssières, J.F.; Van Tellingen, E.; Vrolijks, J. Effects of an African weaver ant, Oecophylla longinoda, in controlling mango fruit flies (Diptera: Tephritidae) in Benin. J. Econ. Entomol. 2007, 100, 695–701. [Google Scholar] [CrossRef]
- El Keroumi, A.; Naamani, K.; Dahbi, A.; Luque, I.; Carvajal, A.; Cerda, X.; Boulay, R. Effect of ant predation and abiotic factors on the mortality of medfly larvae, Ceratitis capitata, in the Argan forest of Western Morocco. Biocontrol Sci. Technol. 2010, 20, 751–762. [Google Scholar] [CrossRef]
- Van Mele, P.; Vayssieres, J.F.; Adandonon, A.; Sinzogan, A. Ant cues affect the oviposition behaviour of fruit flies (Diptera: Tephritidae) in Africa. Physiol. Entomol. 2009, 34, 256–261. [Google Scholar] [CrossRef]
- Urbaneja, A.; Marí, F.G.; Tortosa, D.; Navarro, C.; Vanaclocha, P.; Bargues, L.; Castañera, P. Influence of ground predators on the survival of the Mediterranean fruit fly pupae, Ceratitis capitata, in Spanish citrus orchards. BioControl 2006, 51, 611–626. [Google Scholar] [CrossRef]
- Unruh, T.R.; Lacey, L.A. Control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae), with Steinernema carpocapsae: Effects of supplemental wetting and pupation site on infection rate. Biol. Control 2001, 20, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Mathews, C.R.; Bottrell, D.G.; Brown, M.W. Habitat manipulation of the apple orchard floor to increase ground-dwelling predators and predation of Cydia pomonella (L.) (Lepidoptera: Tortricidae). Biol. Control 2004, 30, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Lacey, L.A.; Unruh, T.R. Biological control of codling moth (Cydia pomonella, Lepidoptera: Tortricidae) and its role in integrated pest management, with emphasis on entomopathogens. Vedalia 2005, 12, 33–60. [Google Scholar] [CrossRef] [Green Version]
- Reyes, M.; Franck, P.; Charmillot, P.J.; Ioriatti, C.; Olivares, J.; Pasqualini, E.; Sauphanor, B. Diversity of insecticide resistance mechanisms and spectrum in European populations of the codling moth, Cydia pomonella. Pest Manag. Sci. 2007, 63, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Franck, P.; Olivares, J.; Margaritopoulos, J.; Knight, A.; Sauphanor, B. Worldwide variability of insecticide resistance mechanisms in the codling moth, Cydia pomonella L. (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2009, 99, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickett, C.H.; Clark, W.D. The function of extrafloral nectaries in Opuntia acanthocarpa (Cactaceae). Am. J. Bot. 1979, 66, 618–625. [Google Scholar] [CrossRef]
- Passos, F.; Leal, L.C. Protein matters: Ants remove herbivores more frequently from extrafloral nectary-bearing plants when habitats are protein poor. Biol. J. Linnean Soc. 2019, blz033. [Google Scholar] [CrossRef]

| Taxon | Subfamily, Tribe | Trees (n = 10) | Records (n = 450) | Counted Individuals (n = 450) |
|---|---|---|---|---|
| Camponotus piceus (Leach, 1825) | Formicinae, Camponotini | 90% | 11.3% | 79 |
| Camponotus vagus (Scopoli, 1763) | Formicinae, Camponotini | 30% | 0.9% | 4 |
| Dolichoderus quadripunctatus (Linnaeus, 1771) | Dolichoderinae, Dolichoderini | 10% | 6.4% | 113 |
| Formica cunicularia Latreille, 1798 | Formicinae, Formicini | 100% | 12.9% | 105 |
| Lasius niger (Linnaeus, 1758) | Formicinae, Lasiini | 40% | 16.4% | 335 |
| Lasius paralienus Seifert, 1992 | Formicinae, Lasiini | 60% | 22.4% | 553 |
| Myrmica sabuleti Meinert, 1861 | Myrmicinae, Myrmicini | 50% | 2.0% | 21 |
| Plagiolepis pygmaea (Latreille, 1798) | Formicinae, Plagiolepidini | 70% | 9.1% | 368 |
| Tapinoma subboreale Seifert, 2012 | Dolichoderinae, Tapinomini | 10% | 0.2% | 1 |
| Temnothorax italicus (Consani, 1952) | Myrmicinae, Crematogastrini | 10% | 0.2% | 1 |
| Class | Order | Family/Species | Total | H(3) | p | Treatment | Mean | SE |
|---|---|---|---|---|---|---|---|---|
| Arachnida | Araneae | 29 | 6.268 | 0.099 | ANs+/Ants+ | 0.04 | 0.02 | |
| ANs−/Ants+ | 0.03 | 0.02 | ||||||
| ANs+/Ants− | 0.14 | 0.05 | ||||||
| ANs−/Ants− | 0.10 | 0.03 | ||||||
| Insecta | Coleoptera | 33 | 1.759 | 0.624 | ANs+/Ants+ | 0.07 | 0.03 | |
| ANs−/Ants+ | 0.09 | 0.07 | ||||||
| ANs+/Ants− | 0.13 | 0.05 | ||||||
| ANs−/Ants− | 0.08 | 0.03 | ||||||
| Dermaptera | 1 | - | - | - | - | - | ||
| Diptera | 35 | 0.727 | 0.867 | ANs+/Ants+ | 0.07 | 0.03 | ||
| ANs−/Ants+ | 0.13 | 0.05 | ||||||
| ANs+/Ants− | 0.09 | 0.03 | ||||||
| ANs−/Ants− | 0.10 | 0.04 | ||||||
| Hemiptera | M. pruinosa | 65 | 3.402 | 0.334 | ANs+/Ants+ | 0.03 | 0.02 | |
| ANs−/Ants+ | 0.19 | 0.08 | ||||||
| ANs+/Ants− | 0.17 | 0.06 | ||||||
| ANs−/Ants− | 0.33 | 0.22 | ||||||
| S. pyri | 192 | 9.094 | 0.028 | ANs+/Ants+ | 0.00 | 0.00 | ||
| ANs−/Ants+ | 0.40 | 0.20 | ||||||
| ANs+/Ants− | 1.73 | 1.19 | ||||||
| ANs−/Ants− | 0.00 | 0.00 | ||||||
| others | 32 | 2.348 | 0.503 | ANs+/Ants+ | 0.08 | 0.03 | ||
| ANs−/Ants+ | 0.08 | 0.03 | ||||||
| ANs+/Ants− | 0.03 | 0.01 | ||||||
| ANs−/Ants− | 0.08 | 0.03 | ||||||
| Hymenoptera | Vespula sp. | 37 | 37.749 | <0.001 | (A)ANs+/Ants+ | 0.14 | 0.04 | |
| (B)ANs−/Ants+ | 0.00 | 0.00 | ||||||
| (A)ANs+/Ants− | 0.27 | 0.06 | ||||||
| (B)ANs−/Ants− | 0.00 | 0.00 | ||||||
| others | 4 | - | - | - | - | - | ||
| Lepidoptera | H. cunea | 631 | 9.050 | 0.029 | ANs+/Ants+ | 0.00 | 0.00 | |
| ANs−/Ants+ | 0.00 | 0.00 | ||||||
| ANs+/Ants− | 0.00 | 0.00 | ||||||
| ANs−/Ants− | 7.01 | 4.92 | ||||||
| others | 4 | - | - | - | - | - | ||
| Neuroptera | Chrysopidae | 7 | - | - | - | - | - | |
| Chrysopidae (eggs) | 40 | 6.642 | 0.503 | ANs+/Ants+ | 0.02 | 0.02 | ||
| ANs−/Ants+ | 0.10 | 0.04 | ||||||
| ANs+/Ants− | 0.11 | 0.04 |
| Variable | B | SE | Odds Ratio | Sig. |
|---|---|---|---|---|
| Constant | −1.04 | 0.25 | −4.18 | <0.001 *** |
| ANs+/Ants+ | −3.06 | 1.04 | −2.95 | 0.003 ** |
| ANs+/Ants− | −0.80 | 0.41 | −1.96 | 0.05 |
| ANs−/Ants− | −0.12 | 0.43 | −0.29 | 0.76 |
| χ2 | 22.18, df = 3, p < 0.001 *** | |||
| Variable | B | SE | Odds Ratio | Sig. |
|---|---|---|---|---|
| Constant | −1.93 | 0.08 | 0.15 | <0.001 *** |
| ANs+/Ants+ | −1.34 | 0.13 | 0.26 | <0.001 *** |
| ANs+/Ants− | −0.23 | 0.11 | 0.80 | 0.03 * |
| ANs−/Ants− | 0.33 | 0.11 | 1.39 | 0.002 ** |
| χ2 | 231.09, df = 3, p < 0.001 *** | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schifani, E.; Castracani, C.; Giannetti, D.; Spotti, F.A.; Reggiani, R.; Leonardi, S.; Mori, A.; Grasso, D.A. New Tools for Conservation Biological Control: Testing Ant-Attracting Artificial Nectaries to Employ Ants as Plant Defenders. Insects 2020, 11, 129. https://doi.org/10.3390/insects11020129
Schifani E, Castracani C, Giannetti D, Spotti FA, Reggiani R, Leonardi S, Mori A, Grasso DA. New Tools for Conservation Biological Control: Testing Ant-Attracting Artificial Nectaries to Employ Ants as Plant Defenders. Insects. 2020; 11(2):129. https://doi.org/10.3390/insects11020129
Chicago/Turabian StyleSchifani, Enrico, Cristina Castracani, Daniele Giannetti, Fiorenza Augusta Spotti, Roberto Reggiani, Stefano Leonardi, Alessandra Mori, and Donato Antonio Grasso. 2020. "New Tools for Conservation Biological Control: Testing Ant-Attracting Artificial Nectaries to Employ Ants as Plant Defenders" Insects 11, no. 2: 129. https://doi.org/10.3390/insects11020129
APA StyleSchifani, E., Castracani, C., Giannetti, D., Spotti, F. A., Reggiani, R., Leonardi, S., Mori, A., & Grasso, D. A. (2020). New Tools for Conservation Biological Control: Testing Ant-Attracting Artificial Nectaries to Employ Ants as Plant Defenders. Insects, 11(2), 129. https://doi.org/10.3390/insects11020129








