How Does the Male Penisfilum Enter the Female Copulatory Pore in Hangingflies?
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Insect Rearing
2.3. Mating Behavior Observation
2.4. Light and Scanning Electron Microscopy
3. Results
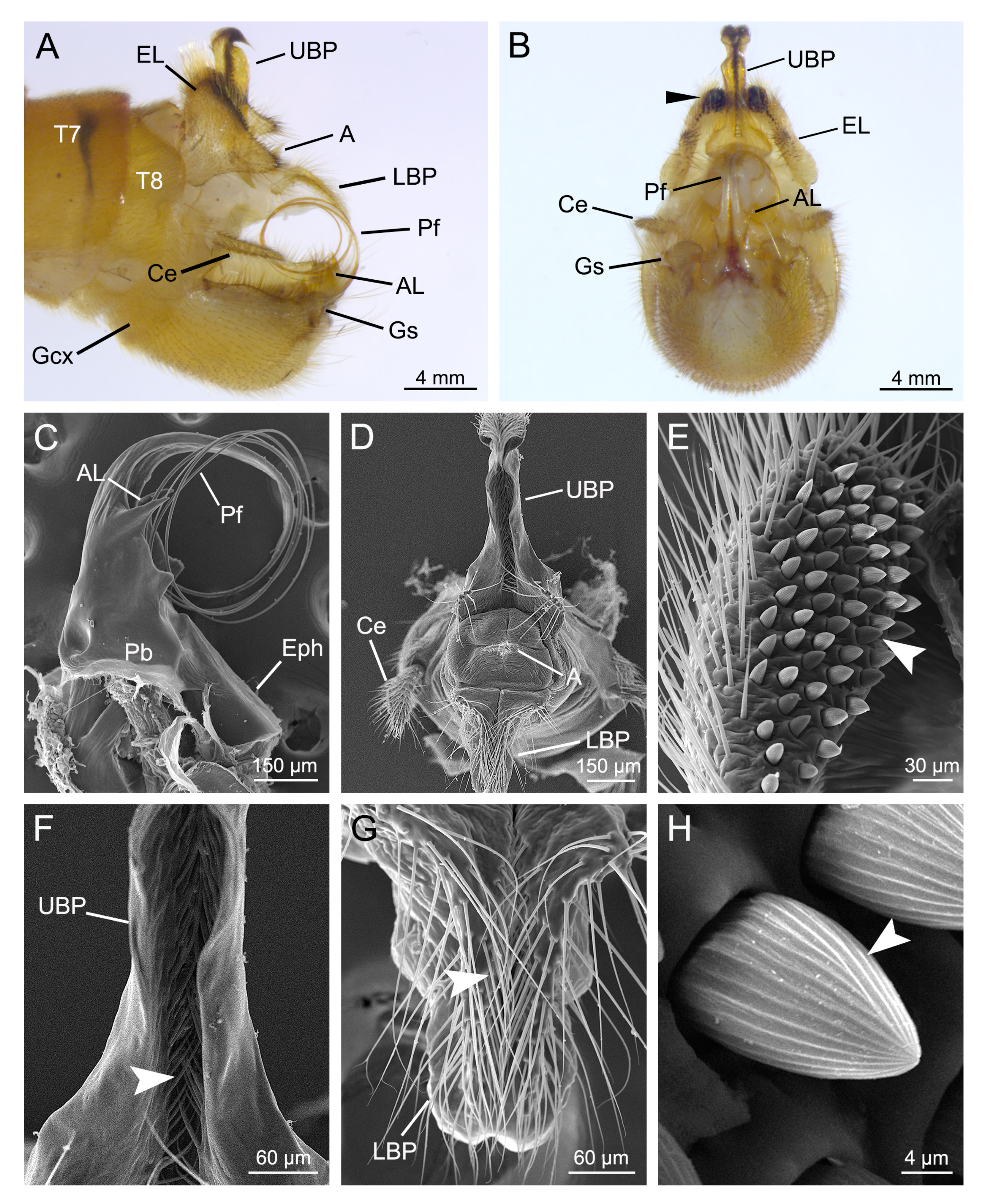
3.1. Genital Structures of the Male
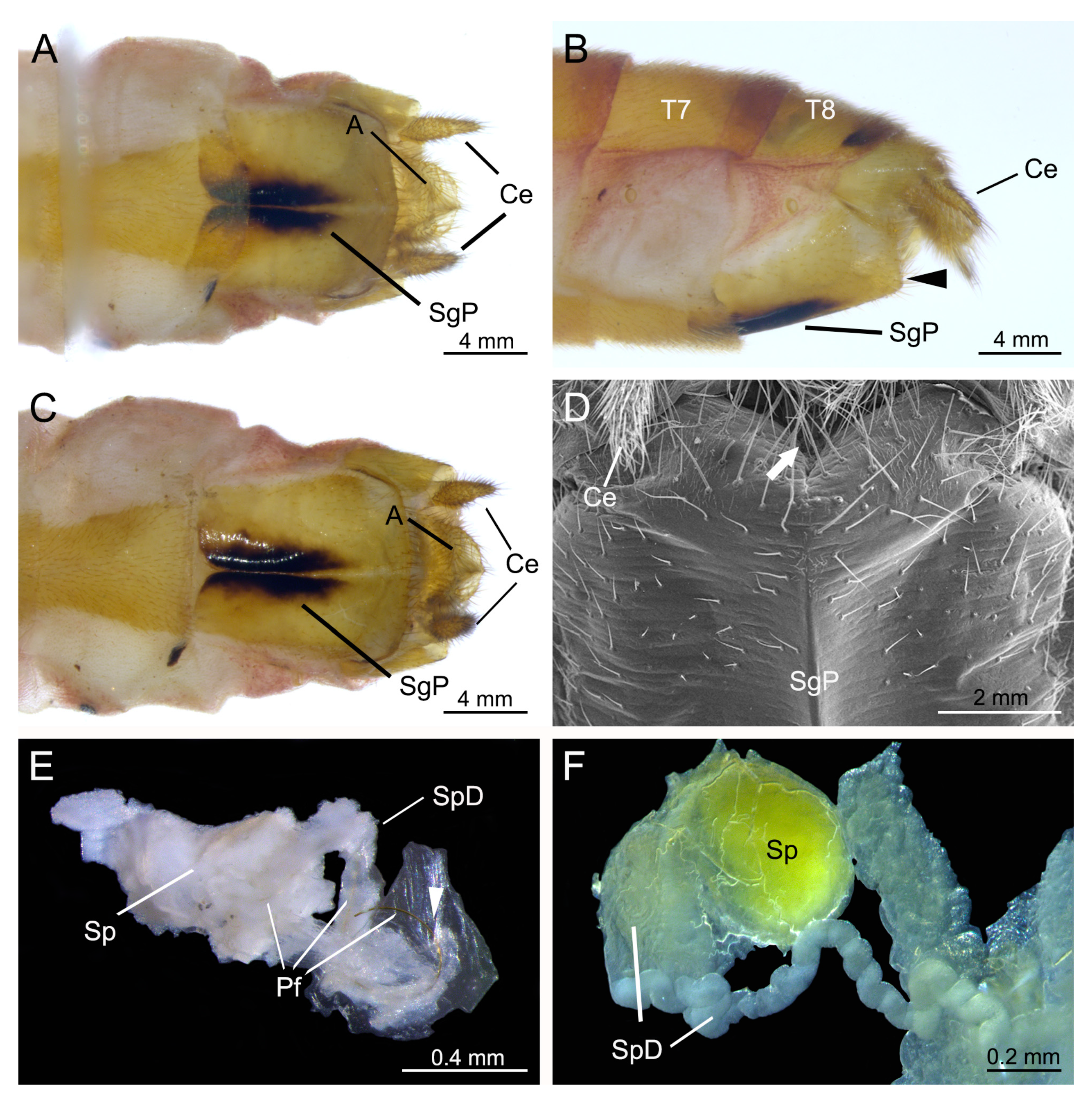
3.2. Genital Structures of the Female
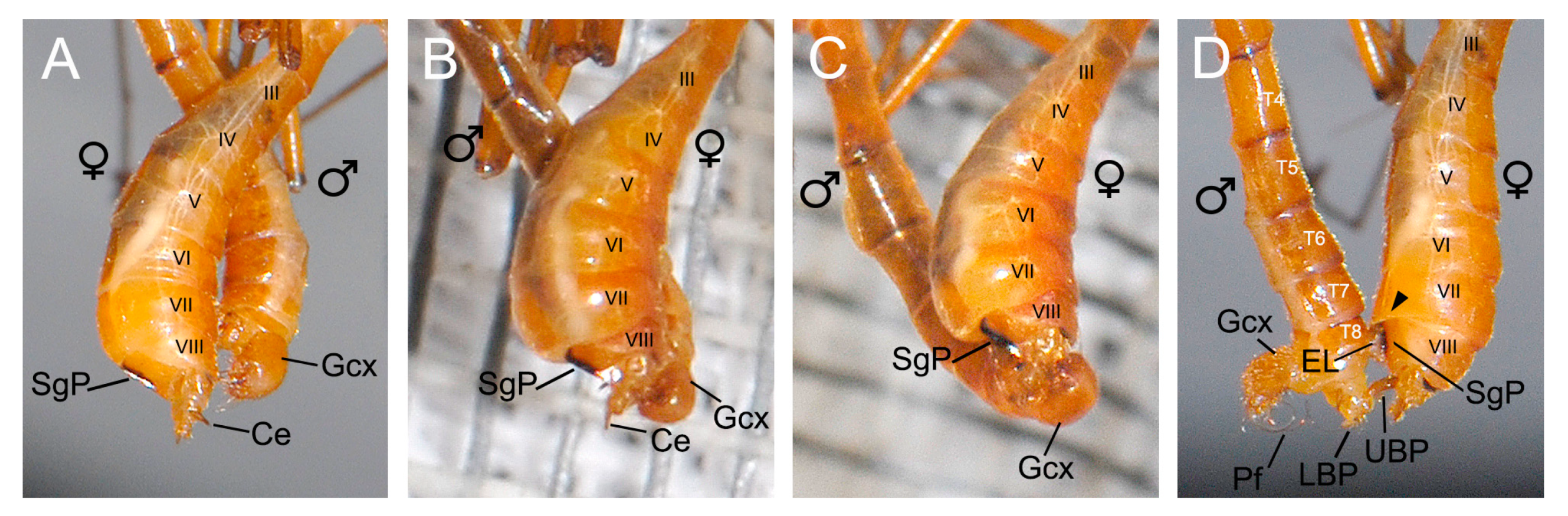
3.3. Mating Behavior
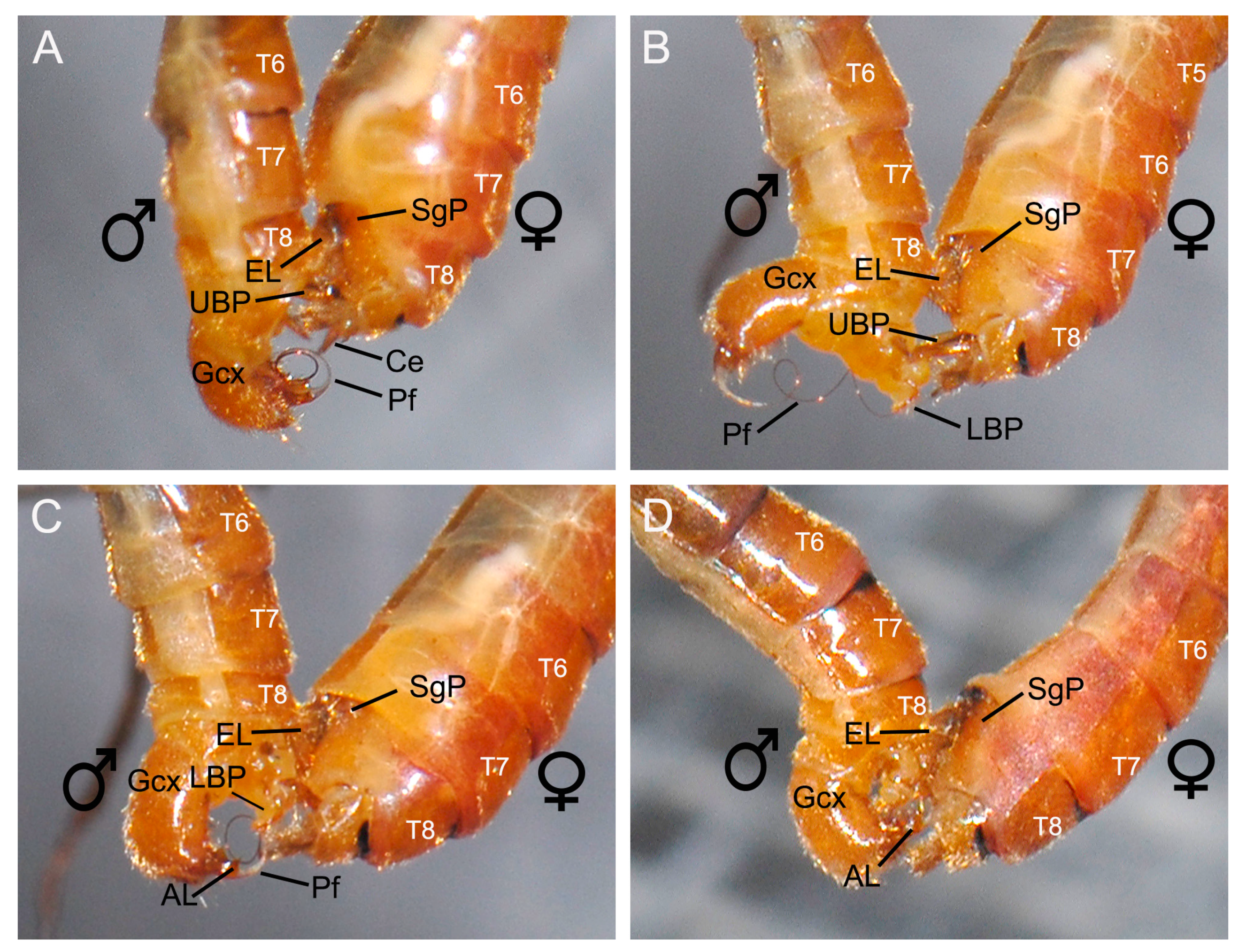
3.4. Entering of the Male Penisfilum
3.4.1. Clamping of the Male Epandrial Lobes
3.4.2. Assistance of the Male Proctiger
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matthews, R.W.; Matthews, J.R. Insect Behavior, 2nd ed.; Springer Science & Business Media: London, UK, 2010; pp. 341–387. [Google Scholar]
- Firman, R.C.; Gasparini, C.; Manier, M.K.; Pizzari, T. Postmating female control: 20 years of cryptic female choice. Trends Ecol. Evol. 2017, 32, 368–382. [Google Scholar] [CrossRef] [PubMed]
- Shuker, D.M.; Simmons, L.W. The Evolution of Insect Mating Systems; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Arnqvist, G. The evolution of animal genitalia: Distinguishing between hypotheses by single species studies. Biol. J. Linn. Soc. 1997, 60, 365–379. [Google Scholar] [CrossRef]
- Arnqvist, G. Comparative evidence for the evolution of genitalia by sexual selection. Nature 1998, 393, 784. [Google Scholar] [CrossRef]
- Cayetano, L.; Maklakov, A.A.; Brooks, R.C.; Bonduriansky, R. Evolution of male and female genitalia following release from sexual selection. Evolution 2011, 65, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.A.; Sinclair, B.J.; Schmitt, M. The evolution of asymmetric genitalia in spiders and insects. Biol. Rev. 2007, 82, 647–698. [Google Scholar] [CrossRef]
- Byers, G.W.; Thornhill, R. Biology of the Mecoptera. Annu. Rev. Entomol. 1983, 28, 203–228. [Google Scholar] [CrossRef]
- Cooper, K.W. Sexual biology, chromosomes, development, life histories and parasites of Boreus, especially of B. notoperates. A southern California Boreus, II. (Mecoptera: Boreidae). Psyche 1974, 81, 84–120. [Google Scholar] [CrossRef]
- Tong, X.; Hua, B.Z. Nuptial feeding and genital coupling of Neopanorpa scorpionflies (Insecta: Mecoptera: Panorpidae) with notal organs of various lengths. Contrib. Zool. 2019, 88, 498–512. [Google Scholar] [CrossRef]
- Tong, X.; Zhong, W.; Hua, B.Z. Copulatory mechanism and functional morphology of genitalia and anal horn of the scorpionfly Cerapanorpa dubia (Mecoptera: Panorpidae). J. Morphol. 2018, 279, 1532–1539. [Google Scholar] [CrossRef]
- Zhong, W.; Hua, B.Z. Mating behaviour and copulatory mechanism in the scorpionfly Neopanorpa longiprocessa (Mecoptera: Panorpidae). PLoS ONE 2013, 8, e74781. [Google Scholar] [CrossRef]
- Zhong, W.; Ding, G.; Hua, B.Z. The role of male’s anal horns in copulation of a scorpionfly. J. Zool. 2015, 295, 170–177. [Google Scholar] [CrossRef]
- Zhong, W.; Qi, Z.Y.; Hua, B.Z. Atypical mating in a scorpionfly without a notal organ. Contrib. Zool. 2015, 84, 305–315. [Google Scholar] [CrossRef]
- Tong, X.; Jiang, L.; Hua, B.Z. A unique mating pattern of Panorpodes kuandianensis (Mecoptera: Panorpodidae). Contrib. Zool. 2017, 86, 229–237. [Google Scholar] [CrossRef]
- Bornemissza, G.F. Observations on the hunting and mating behaviours of two species of scorpion flies (Bittacidae: Mecoptera). Aust. J. Zool. 1966, 14, 371–382. [Google Scholar] [CrossRef]
- Gao, Q.H.; Hua, B.Z. Co-evolution of the mating position and male genitalia in insects: A case study of a hangingfly. PLoS ONE 2013, 8, e80651. [Google Scholar] [CrossRef][Green Version]
- Mickoleit, G.; Mickoleit, E. Zum Kopulationsverhalten des Mückenhaftes Bittacus italicus (Mecoptera: Bittacidae). Entomol. Gen. 1978, 5, 1–15. [Google Scholar]
- Setty, L.R. The biology of Bittacus stigmaterus Say (Mecoptera, Bittacusidae). Ann. Entomol. Soc. Am. 1931, 24, 467–484. [Google Scholar] [CrossRef]
- Setty, L.R. Biology and morphology of some north American Bittacidae (Order Mecoptera). Am. Midl. Nat. 1940, 23, 257–353. [Google Scholar] [CrossRef]
- Tan, J.L.; Hua, B.Z. Behaviors of the hangingfly Bittacus planus. Chin. Bull. Entomol. 2006, 43, 348–351. [Google Scholar]
- Thornhill, R. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am. Nat. 1983, 122, 765–788. [Google Scholar] [CrossRef]
- Thornhill, R. Alternative female choice tactics in the scorpionfly Hylobittacus apicalis (Mecoptera) and their implications. Am. Zool. 1984, 24, 367–383. [Google Scholar] [CrossRef][Green Version]
- Iwasaki, Y. Hunting and mating behavior in the Japanese hangingfly Bittacus mastrillii (Mecoptera: Bittacidae). Ann. Entomol. Soc. Am. 1996, 89, 869–874. [Google Scholar] [CrossRef]
- Iwasaki, Y. Variation in hunting and mating behavior in two populations of the Japanese hangingfly Bittacus mastrillii (Mecoptera: Bittacidae). Ann. Entomol. Soc. Am. 1998, 91, 235–238. [Google Scholar] [CrossRef]
- Thornhill, R. The comparative predatory and sexual behavior of hanging flies (Mecoptera: Bittacidae). Occas. Pap. Mus. Zool. Univ. Mich. 1977, 677, 1–43. [Google Scholar]
- Thornhill, R. Sexually selected predatory and mating behavior of the hangingfly, Bittacus stigmaterus (Mecoptera: Bittacidae). Ann. Entomol. Soc. Am. 1978, 71, 597–601. [Google Scholar] [CrossRef]
- Thornhill, R. Sexual selection in the black-tipped hangingfly. Sci. Am. 1980, 242, 162–172. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function, 5th ed.; Cambridge University Press: Cambridge, UK, 2013; pp. 269–343. [Google Scholar]
- Eberhard, W.G. The functional morphology of species-specific clasping structures on the front legs of male Archisepsis and Palaeosepsis flies (Diptera, Sepsidae). Zool. J. Linn. Soc. 2001, 133, 335–368. [Google Scholar] [CrossRef]
- Richmond, M.P.; Park, J.; Henry, C.S. The function and evolution of male and female genitalia in Phyllophaga Harris scarab beetles (Coleoptera: Scarabaeidae). J. Evol. Biol. 2016, 29, 2276–2288. [Google Scholar] [CrossRef]
- McPeek, M.A.; Shen, L.; Farid, H. The correlated evolution of three-dimensional reproductive structures between male and female damselflies. Evolution 2009, 63, 73–83. [Google Scholar] [CrossRef]
- Arnqvist, G.; Rowe, L. Sexual conflict and arms races between the sexes: A morphological adaptation for control of mating in a female insect. Proc. R. Soc. Lond. B 1995, 261, 123–127. [Google Scholar]
- Arnqvist, G.; Rowe, L. Correlated evolution of male and female morphologies in water striders. Evolution 2002, 56, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.C.; Rowe, L. Sexual conflict and antagonistic coevolution across water strider populations. Evolution 2012, 66, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.; Arnqvist, G. Sexual selection and the evolution of genital shape and complexity in water striders. Evolution 2012, 66, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, J.L.; Hua, B.Z. Review of the Chinese Bittacus (Mecoptera: Bittacidae) with descriptions of three new species. J. Nat. Hist. 2013, 47, 1463–1480. [Google Scholar] [CrossRef]
- Du, W.; Hua, B.Z. Two new species of the genus Terrobittacus Tan & Hua, 2009 (Mecoptera: Bittacidae) from southwestern China with a key to species. Eur. J. Taxon. 2017, 294, 1–13. [Google Scholar]
- Tan, J.L.; Hua, B.Z. Bicaubittacus, a new genus of the Oriental Bittacidae (Mecoptera) with descriptions of two new species. Zootaxa 2009, 2221, 27–40. [Google Scholar] [CrossRef]
- Tan, J.L.; Hua, B.Z. Terrobittacus, a new genus of the Chinese Bittacidae (Mecoptera) with descriptions of two new species. J. Nat. Hist. 2009, 43, 2937–2954. [Google Scholar] [CrossRef]
- Briceno, R.D.; Eberhard, W.G. Experimental modifications imply a stimulatory function for male tsetse fly genitalia, supporting cryptic female choice theory. J. Evol. Biol. 2009, 22, 1516–1525. [Google Scholar] [CrossRef]
- Chaudhury, M.F.; Dhadialla, T.S. Evidence of hormonal control of ovulation in tsetse flies. Nature 1976, 260, 243–244. [Google Scholar] [CrossRef]
- Thornhill, R. Rape in Panorpa scorpionflies and a general rape hypothesis. Anim. Behav. 1980, 28, 52–59. [Google Scholar] [CrossRef]
- Myers, S.S.; Buckley, T.R.; Holwell, G.I. Male genital claspers influence female mate acceptance in the stick insect Clitarchus hookeri. Behav. Ecol. Sociobiol. 2016, 70, 1547–1556. [Google Scholar] [CrossRef]
- Bickel, D. Sex with a twist in the tail. New Sci. 1990, 127, 34–37. [Google Scholar]
- Crampton, G.C. The genitalia of male Diptera and Mecoptera compared with those of related insects, from the standpoint of phylogeny. Trans. Am. Entomol. Soc. 1922, 48, 207–225. [Google Scholar]
- dos Santos, E.A.; Silva-Torres, C.S.A.; Barbosa, P.R.R.; Torres, J.B.; Blassioli-Moraes, M.C. Sexual behavior in ladybird beetles: Sex with lights on and a twist for Tenuisvalvae notata (Coleoptera: Coccinellidae). Behav. Process. 2017, 144, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Giunti, G.; Campolo, O.; Laudani, F.; Palmeri, V. Male courtship behaviour and potential for female mate choice in the black soldier fly Hermetia illucens L. (Diptera: Stratiomyidae). Entomol. Gen. 2018, 38, 29–46. [Google Scholar] [CrossRef]
- Huber, B.A. Mating positions and the evolution of asymmetric insect genitalia. Genetica 2010, 138, 19–25. [Google Scholar] [CrossRef]
- Mair, J.; Blackwell, A. Mating behavior of Culicoides nubeculosus (Diptera: Ceratopogonidae). J. Med. Entomol. 1996, 33, 856–858. [Google Scholar] [CrossRef]
- Philips, T.K.; Hancock, R.G.; Foster, W.A. Epigamic display and unique mating position in Wyeomyia arthrostigma (Diptera: Culicidae). J. Insect Behav. 1996, 9, 739–753. [Google Scholar] [CrossRef]
- Hardy, G.H. The copulation and the terminal segments of Diptera. Proc. R. Entomol. Soc. Lond. A 1944, 19, 52–65. [Google Scholar] [CrossRef]
- Inatomi, M.; Shin, D.; Lai, Y.T.; Matsuno, K. Proper direction of male genitalia is prerequisite for copulation in Drosophila, implying cooperative evolution between genitalia rotation and mating behavior. Sci. Rep. 2019, 9, 210. [Google Scholar] [CrossRef]
- Lukashevich, E.D. Male terminalia and their rotation in Tanyderidae (Insecta, Diptera, Nematocera) since the Mesozoic. Hist. Biol. 2018, 1–14. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Tong, X.; Hua, B.-Z. How Does the Male Penisfilum Enter the Female Copulatory Pore in Hangingflies? Insects 2020, 11, 123. https://doi.org/10.3390/insects11020123
Wei Z, Tong X, Hua B-Z. How Does the Male Penisfilum Enter the Female Copulatory Pore in Hangingflies? Insects. 2020; 11(2):123. https://doi.org/10.3390/insects11020123
Chicago/Turabian StyleWei, Zheng, Xin Tong, and Bao-Zhen Hua. 2020. "How Does the Male Penisfilum Enter the Female Copulatory Pore in Hangingflies?" Insects 11, no. 2: 123. https://doi.org/10.3390/insects11020123
APA StyleWei, Z., Tong, X., & Hua, B.-Z. (2020). How Does the Male Penisfilum Enter the Female Copulatory Pore in Hangingflies? Insects, 11(2), 123. https://doi.org/10.3390/insects11020123




