Within-Stand Distribution of Tree Mortality Caused by Mountain Pine Beetle, Dendroctonus ponderosae Hopkins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling
2.3. Data Analysis
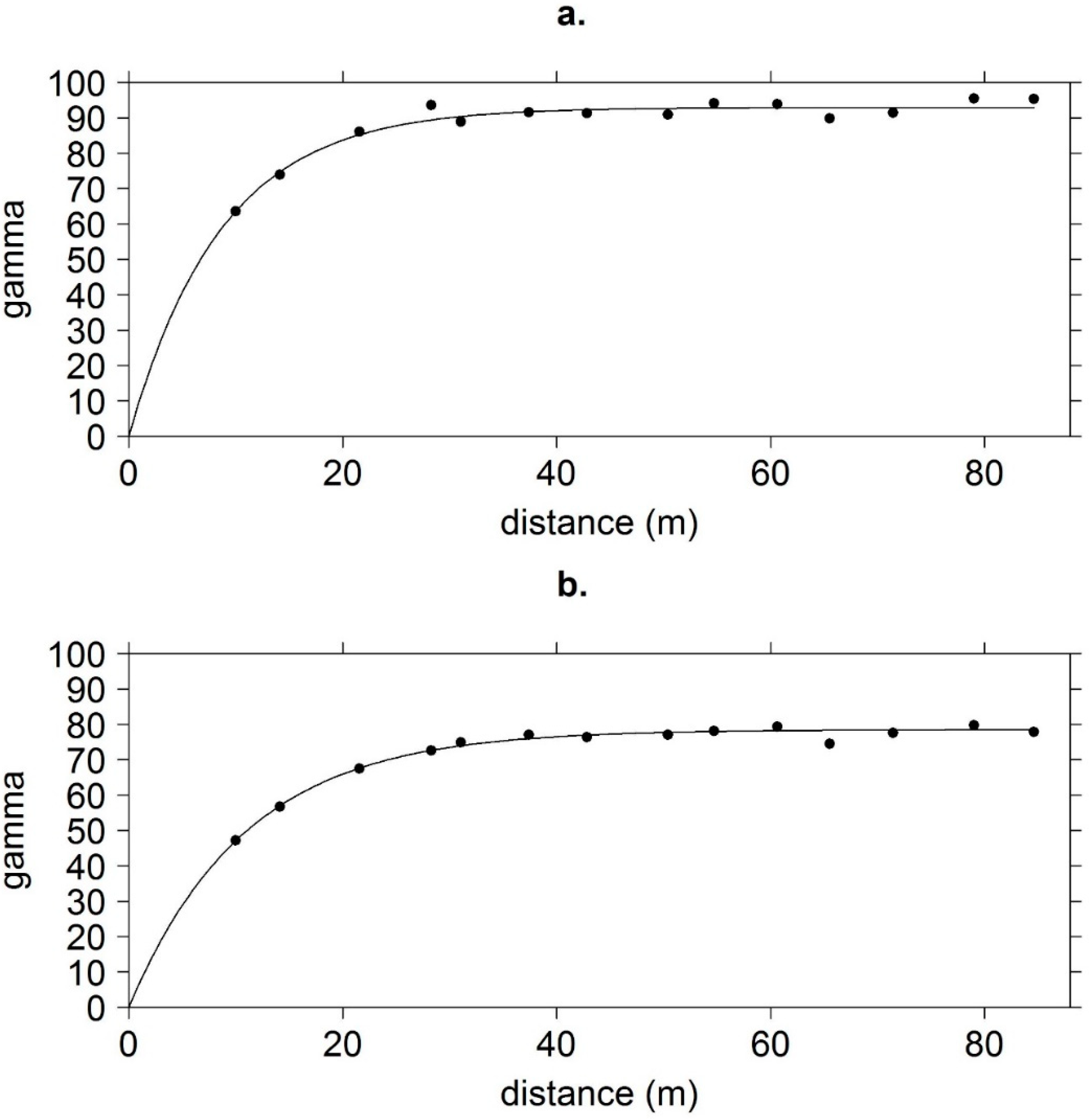
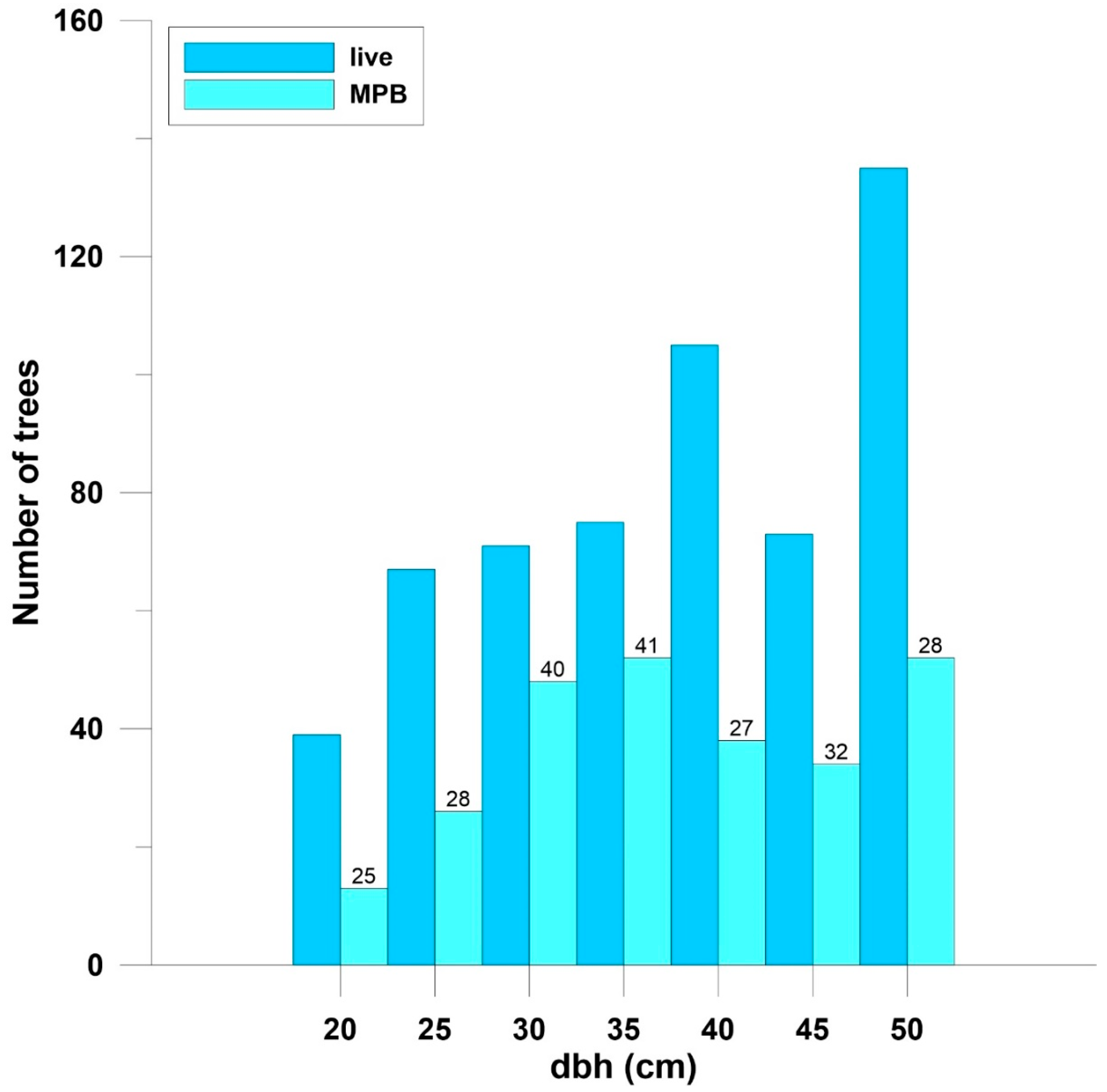
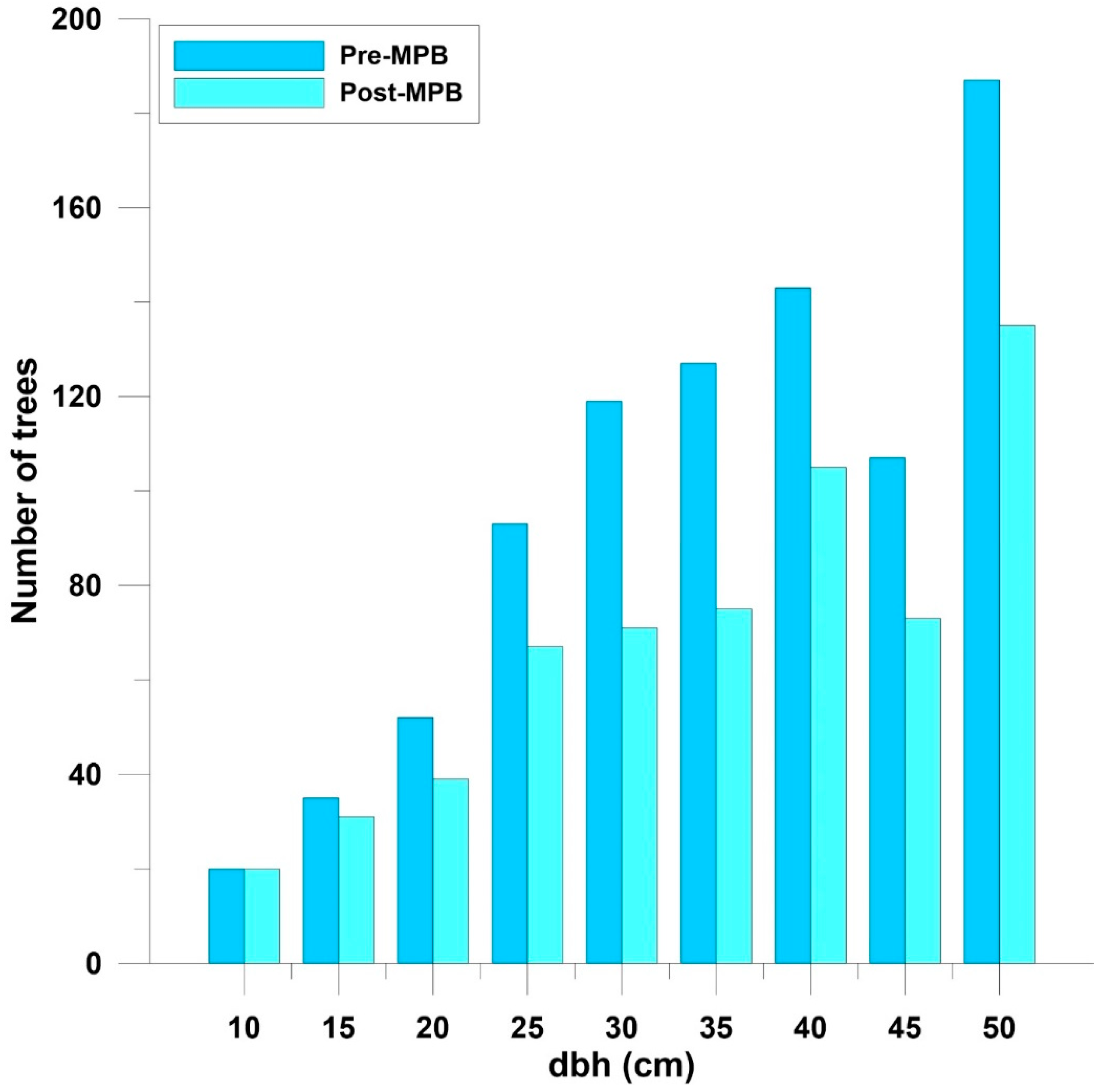
3. Results
4. Discussion
Context of the Study
5. Conclusions
Data Availability
Funding
Acknowledgments
Conflicts of Interest
References
- Negrón, J.F.; Fettig, C.J. Mountain pine beetle, a major disturbance agent in US western coniferous forests: A synthesis of the state of knowledge. Forest Sci. 2014, 60, 409–413. [Google Scholar] [CrossRef]
- Biederman, J.A.; Brooks, P.D.; Harpold, A.A.; Gochis, D.J.; Gutmann, E.; Reed, E.D.; Pendall, E.; Ewers, B.E. Multiscale observations of snow accumulation and peak snowpack following widespread, insect-induced lodgepole pine mortality. Ecohydrology 2014, 7, 150–162. [Google Scholar] [CrossRef]
- Saab, V.A.; Latif, Q.S.; Rowland, M.M.; Johnson, T.N.; Chalfoun, A.D.; Buskirk, S.W.; Heyward, J.E.; Dresser, M.A. Ecological consequences of mountain pine beetle outbreaks for wildlife in western North American forests. Forest Sci. 2014, 60, 539–559. [Google Scholar] [CrossRef]
- Biederman, J.A.; Meixner, T.; Harpold, A.A.; Reed, D.E.; Gutmann, E.D.; Gaun, J.A.; Brooks, P.D. Riparian zones attenuate nitrogen loss following bark beetle-induced lodgepole pine mortality. J. Geophys. Res.-Biogeo 2016, 121, 933–948. [Google Scholar] [CrossRef] [Green Version]
- Negrón, J.F.; Cain, B. Mountain pine beetle in Colorado: A story of changing forests. J. Forest. 2019, 117, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Klutsch, J.G.; Negrón, J.F.; Costello, S.L.; Rhoads, C.C.; West, D.R.; Popp, J.; Caissie, R. Stand characteristics and downed woody debris accumulations associated with a mountain pine beetle (Dendroctonus ponderosae Hopkins) outbreak in Colorado. For. Ecol. Manag. 2009, 258, 641–649. [Google Scholar] [CrossRef]
- Hynum, B.G.; Berryman, A.A. Dendroctonus ponderosae (Coleoptera: Scolytidae): Pre-aggregation landing and gallery initiation on lodgepole pine. Can. Entomol. 1980, 112, 185–191. [Google Scholar] [CrossRef]
- Raffa, K.F.; Berrymn, A.A. Gustatory cues in the orientation of Dendroctonus ponderosae Coleoptera: Scolytidae) to host trees. Can. Entomol. 1982, 114, 97–104. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Borden, J.H. Primary attraction and kairomonal host discrimination in three species of Dendroctonus (Coleoptera: Scolytidae). Agric. For. Entomol. 2005, 7, 219–230. [Google Scholar] [CrossRef]
- Campbell, S.A.; Borden, J.H. Integration of visual and olfactory cues of hosts and non-hosts by three bark beetles (Coleoptera: Scolytidae). Ecol. Entomol. 2006, 31, 437–449. [Google Scholar] [CrossRef]
- Geiszler, D.R.; Gara, R.W. Mountain pine beetle attack dynamics in lodgepole pine. In Theory and Practice of Mountain Pine Beetle Management in Lodgepole Pine Forests, Symposium Proceedings, Pullman, WA, USA, 25–27 April 1978; Berryman, A.A., Amman, G.D., Stark, R.W., Eds.; University of Idaho Forest, Wildlife and Range Experiment Station: Moscow, ID, USA; U.S. Department of Agriculture, Forest Service, Forest Insect and Disease Research: Washington, DC, USA, 1978; pp. 182–187. [Google Scholar]
- Progar, R.A.; Gillette, N.; Fettig, C.J.; Hrinkevich, K. Applied chemical ecology of the mountain pine beetle. Forest Sci. 2014, 60, 414–433. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Preisler, H.K. Analysis of spatial patterns of lodgepole pine attacked by outbreak populations of the mountain pine beetle. Forest Sci. 1991, 37, 1390–1408. [Google Scholar]
- McCambridge, W.F.; Hawksworth, F.G.; Edminster, C.B.; Laut, J.G. Ponderosa Pine Mortality Resulting from a Mountain Pine Beetle Outbreak; Research Paper, RM-RP-235; US Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1982; p. 7.
- Negrón, J.F.; Popp, J.B. Probability of ponderosa pine infestation by mountain pine beetle in the Colorado Front Range. For. Ecol. Manag. 2004, 191, 17–27. [Google Scholar] [CrossRef]
- Graham, R.T.; Asherin, L.A.; Battaglia, M.A.; Jain, T.B.; Mata, S.A. Mountain Pine Beetles: A Century of Knowledge, Control Attempts, and Impacts Central to the Black Hills; General Technical Report, RMRS-GTR-353; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2016; p. 193.
- Fettig, C.J.; Klepzig, K.D.; Billings, R.F.; Munson, A.S.; Nebeker, T.E.; Negrón, J.F.; Nowak, J.T. The effectiveness of vegetation management practices for prevention and control of bark beetle infestations in coniferous forests of the western and southern United States. For. Ecol. Manag. 2007, 238, 24–53. [Google Scholar] [CrossRef]
- Negrón, J.F.; Klutsch, J.G. Probability of Infestation and Extent of Mortality Models for Mountain Pine Beetle in Lodgepole Pine Forests in Colorado; Research Note RMRS-RN-77; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2017; p. 13.
- Olsen, W.K.; Schmid, J.M.; Mata, S.A. Stand characteristics associated with mountain pine beetle infestations in ponderosa pine. Forest Sci. 1996, 42, 310–327. [Google Scholar]
- Negrón, J.F.; Anhold, J.A.; Munson, S. Within-stand spatial distribution of tree mortality caused by the Douglas-fir beetle (Coleoptera: Scolytidae). Environ. Entomol. 2001, 30, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Schmid, J.M.; Mata, S.A. Natural Variability of Specific Forest Insect Populations and Their Associated Effects in Colorado; General Technical Report RM-GTR-275; USDA Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1996; p. 14.
- Gillette, N.; Wood, D.L.; Hines, S.J.; Runyon, J.B.; Negrón, J. The once and future forest: Consequences of mountain pine beetle treatment decisions. Forest Sci. 2014, 60, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Negrón, J.F.; Allen, K.K.; Ambourne, A.; Cook, B.; Marchan, K. Large-scale thinnings, ponderosa pine, and mountain pine beetle in the Black Hills, USA. Forest Sci. 2017, 63, 529–536. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.M.; Battaglia, M.A.; Fornwalt, P.J.; Gannon, B.; Huckaby, L.S.; Julian, C.; Cheng, A.S. Historical (1860) forest structure in ponderosa pine forests of the northern Front Range, Colorado. Can. J. For. Res. 2015, 45, 1462–1473. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.M.; Kaufmann, M.R.; Shepperd, W.D. Long-term, landscape patterns of past fire events in a montane ponderosa pine forest of central Colorado. Landscape Ecol. 1999, 14, 513–532. [Google Scholar] [CrossRef]
- Noss, R.F.; Franklin, J.F.; Baker, W.L.; Schoennagel, T.; Moyle, P.B. Managing fire-prone forests in the western United States. Front. Ecol. Environ. 2006, 4, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, Y. Landscape restoration of a forest with a historically mixed severity fire regime: What was the historical landscape pattern of forest and openings? For. Ecol. Manag. 2014, 331, 264–271. [Google Scholar] [CrossRef]
- Underhill, J.L.; Dickinson, Y.; Rudney, A.; Thinnes, J. Silviculture of the Colorado Front Range landscape restoration initiative. J. Forest. 2014, 112, 484–493. [Google Scholar] [CrossRef]
- Chambers, M.E.; Fornwalt, P.J.; Malone, S.L.; Battaglia, M.A. Patterns of conifer regeneration following high severity wildfire in ponderosa pine–dominated forests of the Colorado Front Range. For. Ecol. Manag. 2016, 378, 57–67. [Google Scholar] [CrossRef]
- Johnston, B.C. Plant. Associations of Region Two: Potential Plant Communities of Wyoming, South. Dakota, Nebraska, Colorado and Kansas; R2-Ecol-87-2; US Department of Agriculture, Forest Service, Rocky Mountain Region: Lakewood, CO, USA, 1987; p. 429.
- Isaaks, E.H.; Srivastava, R.M. An introduction to applied geostatistics, 1st ed.; Oxford University Press: New York, NY, USA, 1989; p. 561. [Google Scholar]
- Oliver, M.A.; Webster, R. Kriging: A method of interpolation for geographical information systems. Int. J. Geogr. Inf. Syst. 1990, 4, 313–332. [Google Scholar] [CrossRef]
- Lundquist, J.E.; Reich, R.M. Landscape dynamics of mountain pine beetles. Forest Sci. 2014, 60, 464–475. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Gurevitch, J. Integrating the statistical analysis of spatial data in ecology. Ecography 2002, 25, 553–557. [Google Scholar] [CrossRef]
- Sciarrettaa, A.; Trematerra, P. Geostatistical tools for the study of insect spatial distribution: Practical implications in the integrated management of orchard and vineyard pests. Plant. Prot. Sci. 2014, 50, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 2nd ed.; Dover Publocations Inc.: Mineola, NY, USA, 2009; p. 384. [Google Scholar]
- Colorado State Forest Service. 2015 Report on the Health of Colorado’s Forests: 15 Years of Change; Colorado State Forest Service: Fort Collins, CO, USA; Available online: http://csfs.colostate.edu/media/sites/22/2016/02/ForestHealthReport-2015.pdf (accessed on 15 June 2018).
- Sibold, J.S.; Veblen, T.T.; Chipko, K.; Lawson, L.; Mathis, E.; Scott, J. Influences of secondary disturbances on lodgepole pine stand development in Rocky Mountain National Park. Ecol. Appl. 2007, 17, 1638–1655. [Google Scholar] [CrossRef]
- Flannigan, M.D.; Amiro, B.D.; Logan, K.A.; Stocks, B.J.; Wotton, B.B. Forest Fires and Climate Change in the 21ST Century. Mitig. Adap. Strat. Glob. Change 2006, 11, 847–859. [Google Scholar] [CrossRef]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewis, K.J.; Worrall, J.J.; Woods, A.J. Climate change and forests diseases. Plant. Pathol. 2011, 60, 133–149. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidl, R.; Fernandes, P.M.; Fonseca, T.F.; Gillet, F.; Jönsson, A.M.; Merganičová, K.; Netherer, S.; Arpaci, A.; Bontemps, J.; Bugmann, H.; et al. Modelling natural disturbances in forest ecosystems: A review. Ecol. Model. 2011, 222, 903–924. [Google Scholar] [CrossRef] [Green Version]
- Vose, J.M.; Peterson, D.L.; Domke, G.M.; Fettig, C.J.; Joyce, L.A.; Keane, R.E.; Luce, C.H.; Prestemon, J.P.; Band, L.E.; Clark, J.S.; et al. Forests. In Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II; Reidmiller, D.R., Avery, C.W., Easterling, D.R., Kunkel, K.E., Lewis, K.L.M., Maycock, T.K., Stewart, B.C., Eds.; U.S. Global Change Research Program: Washington, DC, USA, 2018; pp. 223–258. [Google Scholar]
- Jarvis, D.S.; Kulakowski, D. Long-term history and synchrony of mountain pine beetle outbreaks in lodgepole pine forests. J. Biogeogr. 2015, 42, 1029–1039. [Google Scholar] [CrossRef]
- Fettig, C.J.; Gibson, K.E.; Munson, A.F.; Negrón, J.F. Cultural practices for prevention and mitigation of mountain pine beetle infestations. Forest Sci. 2014, 60, 450–463. [Google Scholar] [CrossRef] [Green Version]

| Variable | Pre-MPB | Post-MPB | Difference | p |
|---|---|---|---|---|
| Ponderosa pine basal area | 11.8 (0.5) | 8.2 (0.5) | 3.6 (0.4) | <0.0001 |
| Percent Ponderosa pine basal area | 77.4 (2.2) | 56.2 (2.4) | 21.1 (1.9) | <0.0001 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrón, J.F. Within-Stand Distribution of Tree Mortality Caused by Mountain Pine Beetle, Dendroctonus ponderosae Hopkins. Insects 2020, 11, 112. https://doi.org/10.3390/insects11020112
Negrón JF. Within-Stand Distribution of Tree Mortality Caused by Mountain Pine Beetle, Dendroctonus ponderosae Hopkins. Insects. 2020; 11(2):112. https://doi.org/10.3390/insects11020112
Chicago/Turabian StyleNegrón, José F. 2020. "Within-Stand Distribution of Tree Mortality Caused by Mountain Pine Beetle, Dendroctonus ponderosae Hopkins" Insects 11, no. 2: 112. https://doi.org/10.3390/insects11020112
APA StyleNegrón, J. F. (2020). Within-Stand Distribution of Tree Mortality Caused by Mountain Pine Beetle, Dendroctonus ponderosae Hopkins. Insects, 11(2), 112. https://doi.org/10.3390/insects11020112




