The Antennal Pathway of Dragonfly Nymphs, from Sensilla to the Brain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Scanning Electron Microscopy
2.3. Neuroanatomy
3. Results
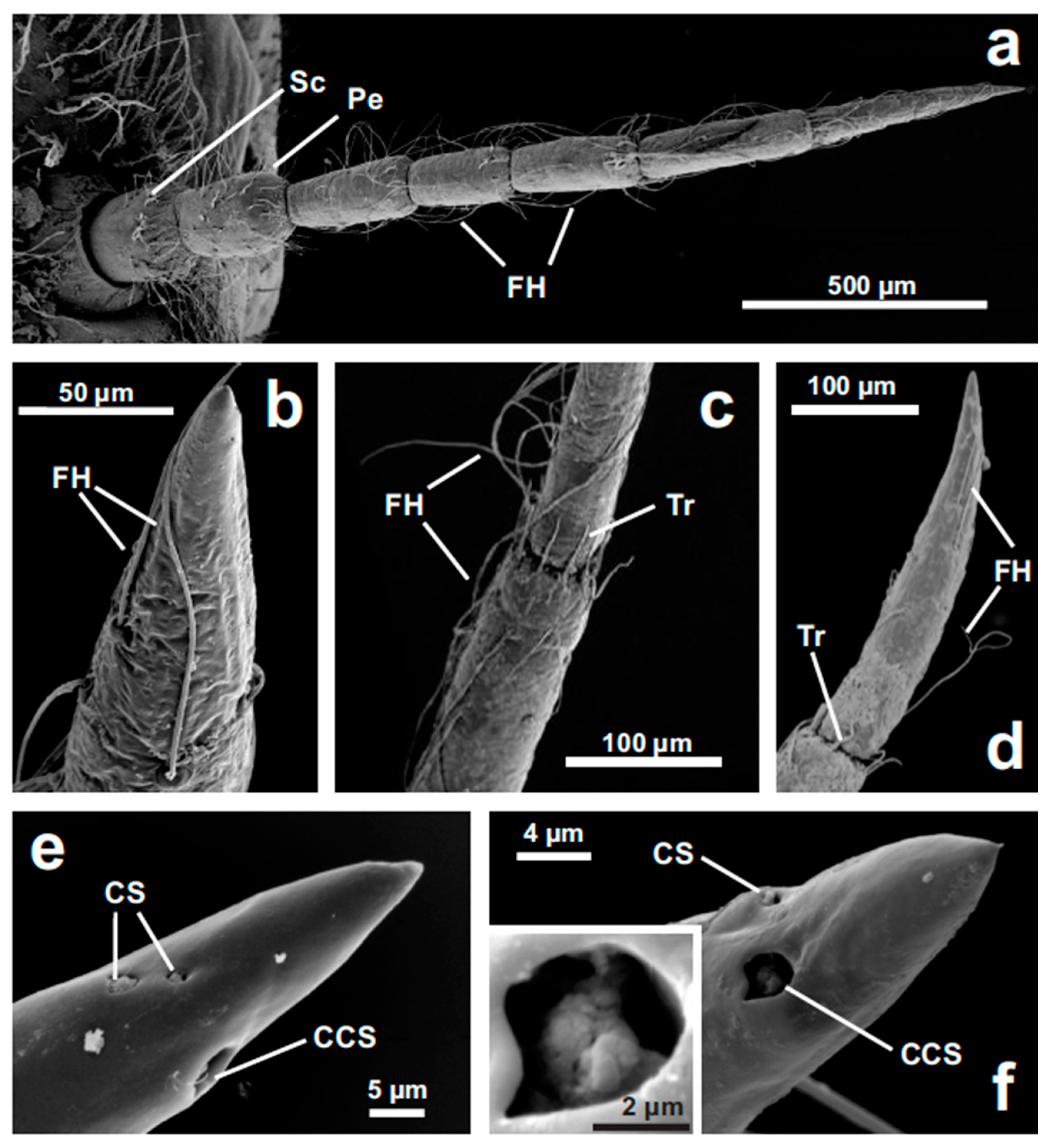
3.1. Antennal Morphology
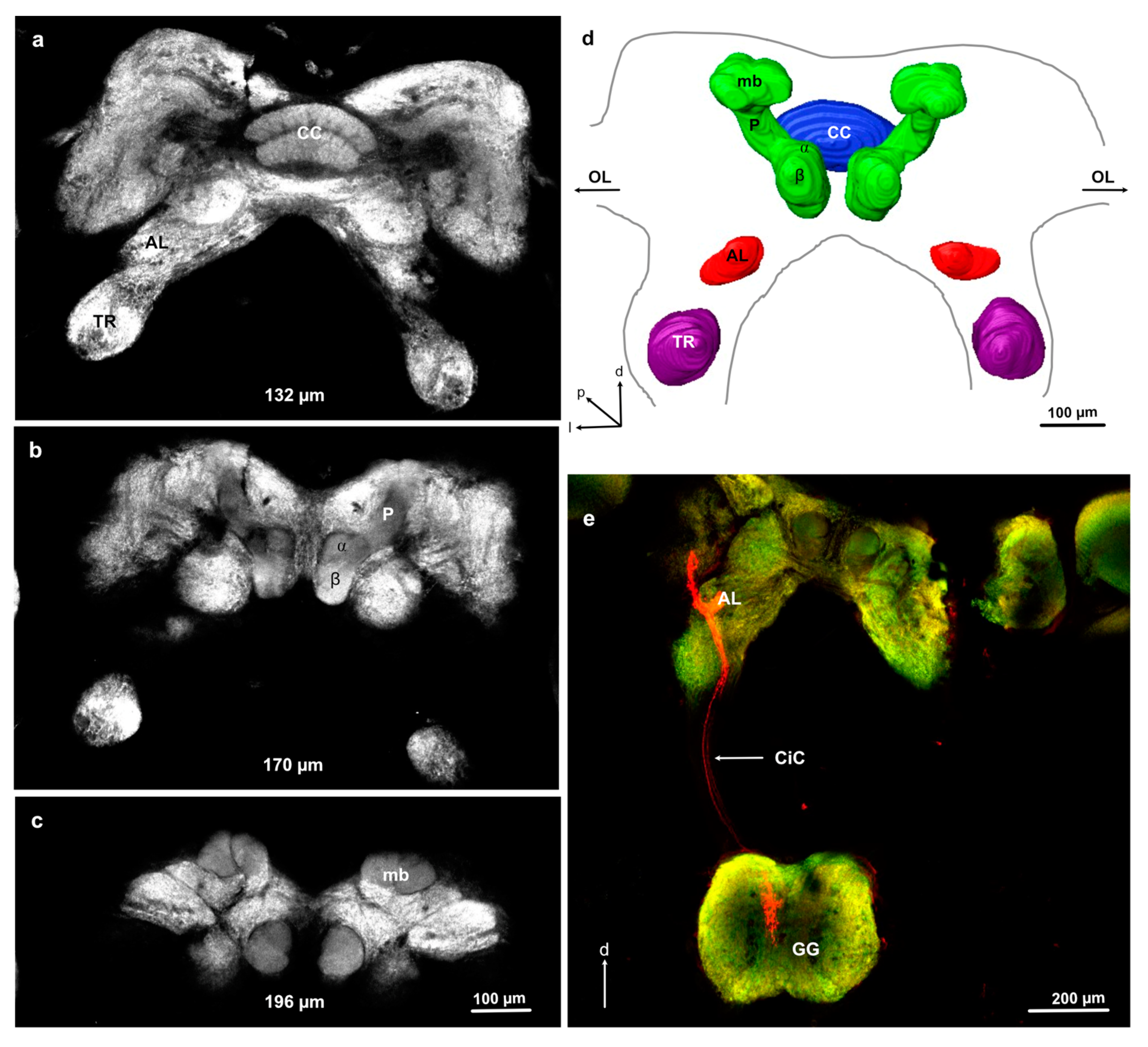
3.2. General Brain Structure
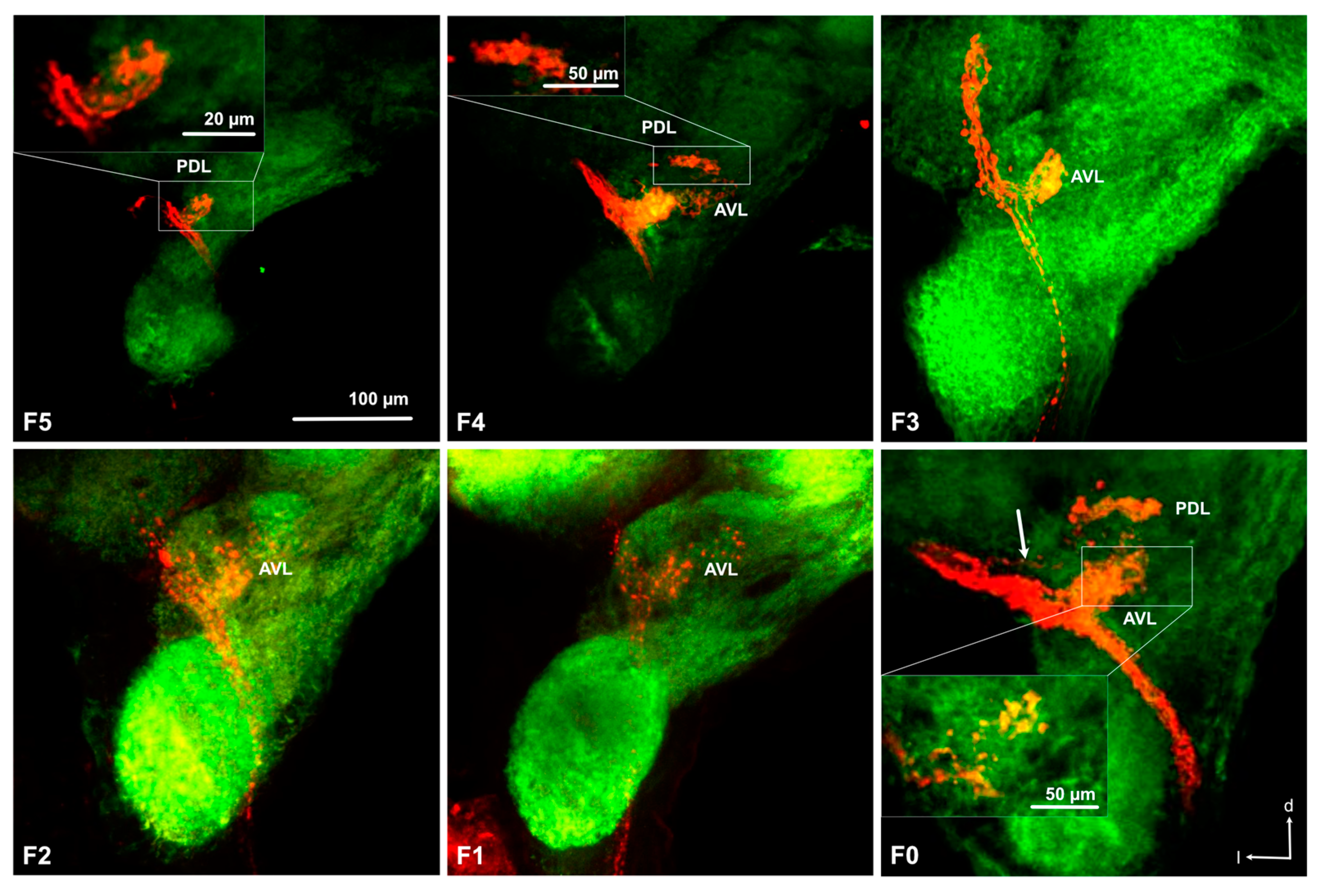
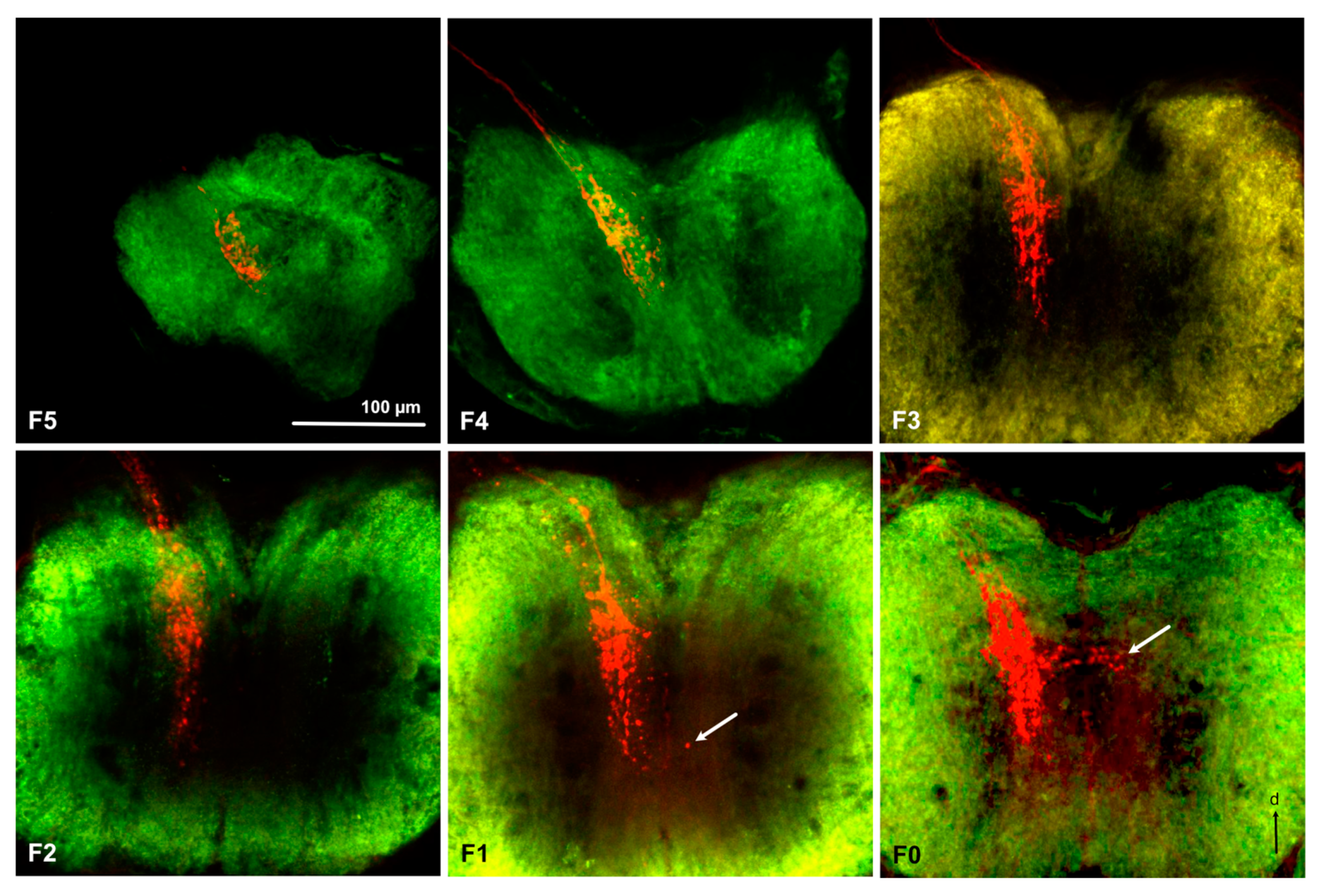
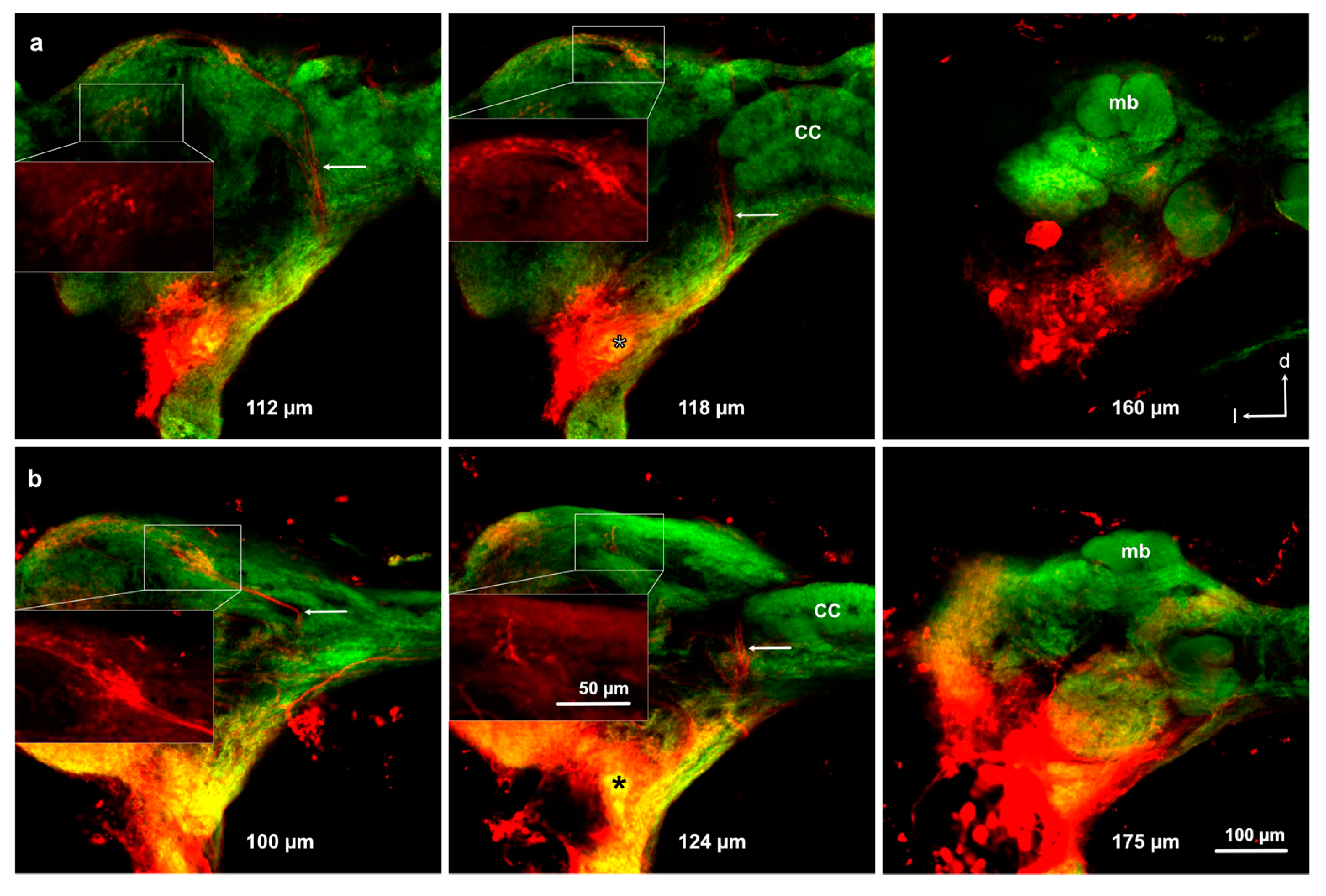
3.3. Antennal Projections to the Brain and GG
3.4. Projections from the Antennal Lobe to the Protocerebrum
4. Discussions
4.1. Sensory Equipment of L. depressa Antennae during Ontogenesis
4.2. Sensory Antennal Pathways in the Central Brain of L. depressa during Ontogenesis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dijkstra, K.D.B.; Monaghan, M.T.; Pauls, S.U. Freshwater biodiversity and aquatic insect diversification. Annu. Rev. Entomol. 2014, 59, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Schachtner, J.; Schmidt, M.; Homberg, U. Organization and evolutionary trends of primary olfactory brain centers in Tetraconata (Crustacea plus Hexapoda). Arthropod Struct. Dev. 2005, 34, 257–299. [Google Scholar] [CrossRef]
- Sombke, A.; Lipke, E.; Kenning, M.; Müller, C.H.; Hansson, B.S.; Harzsch, S. Comparative analysis of deutocerebral neuropils in Chilopoda (Myriapoda): Implications for the evolution of the arthropod olfactory system and support for the Mandibulata concept. BMC Neurosci. 2012, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.G. A review of chemosensation and related behavior in aquatic insects. J. Insect Sci. Online 2011, 11. [Google Scholar] [CrossRef]
- Del Claro, K.; Guillermo, R. Aquatic Insects; Springer: Cham, Switzerland, 2019; ISBN 978-3-030-16326-6. [Google Scholar]
- Grimaldi, D. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Kohli, M.K.; Ware, J.L.; Bechly, G. How to date a dragonfly: Fossil calibrations for odonates. Paleontol. Electron. 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Blanke, A.; Greve, C.; Wipfler, B.; Beutel, R.; Holland, B.R.; Misof, B. The identification of concerted convergence in insect heads corroborates palaeoptera. Syst. Biol. 2013, 62, 250–263. [Google Scholar] [CrossRef]
- Blanke, A.; Wipfler, B.; Letsch, H.; Koch, M.; Beckmann, F.; Beutel, R.; Misof, B. Revival of Palaeoptera—Head characters support a monophyletic origin of Odonata and Ephemeroptera (Insecta). Cladistics 2012, 28, 560–581. [Google Scholar] [CrossRef]
- Rebora, M.; Salerno, G.; Piersanti, S. Aquatic insect sensilla: Morphology and function. In Aquatic Insects; Springer: Cham, Switzerland, 2019; pp. 139–166. ISBN 978-3-030-16326-6. [Google Scholar]
- Elgar, M.A.; Zhang, D.; Wang, Q.; Wittwer, B.; Thi Pham, H.; Johnson, T.L.; Freelance, C.B.; Coquilleau, M. Insect antennal morphology: The evolution of diverse solutions to odorant perception. Yale J. Biol. Med. 2018, 91, 457–469. [Google Scholar]
- Rebora, M.; Piersanti, S.; Gaino, E. A comparative investigation of the antennal sensilla in adult Anisoptera. Odonatologica 2009, 38, 329–340. [Google Scholar]
- Rebora, M.; Piersanti, S.; Gaino, E. The antennal sensilla of the adult of Libellula depressa (Odonata: Libellulidae). Arthropod Struct. Dev. 2008, 37, 504–510. [Google Scholar] [CrossRef]
- Piersanti, S.; Rebora, M.; Gaino, E. A scanning electron microscope study of the antennal sensilla in adult Zygoptera. Odonatologica 2010, 39, 235–241. [Google Scholar]
- Faucheux, M.J.; Meurgey, F. Les sensilles antennaires d’une larve fouisseuse, Ophiogomphus cecilia (Geoffroy in Fourcroy, 1785) (Odonata, Anisoptera, Gomphidae). Martinia 2009, 25, 85–92. [Google Scholar]
- Gaino, E.; Rebora, M. Apical antennal sensilla in nymphs of Libellula depressa (Odonata: Libellulidae). Invertebr. Biol. 2001, 120, 162–169. [Google Scholar] [CrossRef]
- Piersanti, S.; Rebora, M. The antennae of damselfly larvae. Arthropod Struct. Dev. 2018, 47, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Rebora, M.; Piersanti, S.; Almaas, T.J.; Gaino, E. Hygroreceptors in the larva of Libellula depressa (Odonata: Libellulidae). J. Insect Physiol. 2007, 53, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Rebora, M.; Piersanti, S.; Salerno, G.; Gorb, S. The antenna of a burrowing dragonfly larva, Onychogomphus forcipatus (Anisoptera, Gomphidae). Arthropod Struct. Dev. 2015, 44, 595–603. [Google Scholar] [CrossRef]
- Faucheux, M.F. Multiporous and aporous sensilla on the larval antennae of the relict dragonfly Epiophlebia superstes (Selys, 1889) (Odonata: Anisozygoptera: Epiophlebiidae). Bull. Inst. R. Sci. Nat. Belg. 2007, 77, 121–128. [Google Scholar]
- Rebora, M.; Piersanti, S.; Salerno, G.; Conti, E.; Gaino, E. Water deprivation tolerance and humidity response in a larval dragonfly: A possible adaptation for survival in drying ponds. Physiol. Entomol. 2007, 32, 121–126. [Google Scholar] [CrossRef]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata; Harley Books: Colchester, UK, 1999; ISBN 0-946589-64-X. [Google Scholar]
- Chivers, D.P.; Wisenden, B.D.; Smith, J.F. Damselfly larvae learn to recognize predators from chemical cues in the predator’s diet. Anim. Behav. 1996, 52, 315–320. [Google Scholar] [CrossRef]
- Wisenden, B.D.; Chivers, D.P.; Smith, R.J.F. Learned recognition of predation risk by Enallagma damselfly larvae (Odonata, Zygoptera) on the basis of chemical cues. J. Chem. Ecol. 1997, 23, 137–151. [Google Scholar] [CrossRef]
- Stoks, R. Food stress and predator-induced stress shape developmental performance in a damselfly. Oecologia 2001, 127, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Crumrine, P.W. Age specific behavioral responses of odonate larvae to chemical and visual cues from predators. J. Freshw. Ecol. 2006, 21, 9–16. [Google Scholar] [CrossRef]
- Mortensen, L.; Richardson, J.M.L. Effects of chemical cues on foraging in damselfly larvae, Enallagma antennatum. J. Insect Behav. 2008, 21, 285–295. [Google Scholar] [CrossRef]
- Siepielski, A.M.; Fallon, E.; Boersma, K. Predator olfactory cues generate a foraging–predation trade-off through prey apprehension. R. Soc. Open Sci. 2016, 3, 150537. [Google Scholar] [CrossRef]
- Wisenden, B.D. Olfactory assessment of predation risk in the aquatic environment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1205–1208. [Google Scholar] [CrossRef]
- Mysore, K.; Flister, S.; Müller, P.; Rodrigues, V.; Reichert, H. Brain development in the yellow fever mosquito Aedes aegypti: A comparative immunocytochemical analysis using cross-reacting antibodies from Drosophila melanogaster. Dev. Genes Evol. 2011, 221, 281–296. [Google Scholar] [CrossRef]
- Rebora, M.; Dell’Otto, A.; Rybak, J.; Piersanti, S.; Gaino, E.; Hansson, B.S. The antennal lobe of Libellula depressa (Odonata, Libellulidae). Zoology 2013, 116, 205–214. [Google Scholar] [CrossRef]
- Di Giovanni, M.V.; Goretti, E.; La Porta, G.; Ceccagnoli, D. Larval development of Libellula depressa (Odonata, Libellulidae) from pools in central Italy. Ital. J. Zool. 2000, 67, 343–347. [Google Scholar] [CrossRef]
- Guerrieri, F.; Gemeno, C.; Monsempes, C.; Anton, S.; Jacquin-Joly, E.; Lucas, P.; Devaud, J.M. Experience-dependent modulation of antennal sensitivity and input to antennal lobes in male moths (Spodoptera littoralis) pre-exposed to sex pheromone. J. Exp. Biol. 2012, 215, 2334–2341. [Google Scholar] [CrossRef]
- Tuthill, J.C.; Wilson, R.I. Mechanosensation and adaptive motor control in insects. Curr. Biol. 2016, 26, R1022–R1038. [Google Scholar] [CrossRef]
- Rebora, M.; Piersanti, S.; Gaino, E. Visual and mechanical cues used for prey detection by the larva of Libellula depressa (Odonata Libellulidae). Ethol. Ecol. Evol. 2004, 16, 133–144. [Google Scholar] [CrossRef]
- Derby, C.D.; Kozma, M.T.; Chemical, A.S. Molecular Mechanisms of Reception and Perireception in Crustacean Chemoreception: A Comparative Review. Chem. Senses 2016, 41, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Tuchina, O.; Groh, K.C.; Talarico, G.; Müller, C.H.G.; Wielsch, N.; Hupfer, Y.; Svatoš, A.; Grosse-Wilde, E.; Hansson, B.S. Morphology and histochemistry of the aesthetasc-associated epidermal glands in terrestrial hermit crabs of the genus Coenobita (Decapoda: Paguroidea). PLoS ONE 2014, 9, e96430. [Google Scholar] [CrossRef] [PubMed]
- Coulter, J.P.; Bartels-Hardege, H.; Gennard, D.; Mill, P.; Cowell, A.; John, E.; Hayes, W. Aeshna grandis larvae detect chemical cues derived from carrion: Evidence of chemically-mediated food detection? (Odonata: Aeshnidae). Odonatologica 2016, 45, 191–212. [Google Scholar]
- Koperski, P. Changes in feeding behaviour of the larvae of the damselfly Enallagma cyathigerum in response to stimuli from predators. Ecol. Entomol. 1997, 22, 167–175. [Google Scholar] [CrossRef]
- Hopper, K.R. Flexible antipredator behavior in a dragonfly species that coexists with different predator types. Oikos 2001, 93, 470–476. [Google Scholar] [CrossRef]
- Yokohari, F. Hygro -and thermoreceptors. In Atlas of Arthropod Sensory Receptors: Dynamic Morphology in Relation to Function; Eguchi, E., Tominaga, Y., Eds.; Springer: Berlin, Germany, 1999. [Google Scholar]
- Piersanti, S.; Rebora, M.; Salerno, G.; Gaino, E. Behaviour of the larval dragonfly Libellula depressa (Odonata Libellulidae) in drying pools. Ethol. Ecol. Evol. 2007, 19, 127–136. [Google Scholar] [CrossRef]
- Rebora, M.; Piersanti, S.; Gaino, E. The antennal sensory function in the oldest pterygote insects: An ultrastructural overview. In Microscopy: Science, Technolology Application and Education; Méndez-Vilas, A., Diaz, J., Eds.; Formatex Research Center: Badajoz, Spain, 2010; pp. 137–145. [Google Scholar]
- Piersanti, S.; Frati, F.; Conti, E.; Rebora, M.; Salerno, G. The sense of smell in Odonata: An electrophysiological screening. J. Insect Physiol. 2014, 70, 49–58. [Google Scholar] [CrossRef]
- Gewecke, M.; Heinzel, H.G.; Philippen, J. Role of antennae of the dragonfly Orthetrum cancellatum in flight control. Nature 1974, 249, 584–585. [Google Scholar] [CrossRef]
- Gewecke, M.; Odendahl, A. Der Bewegungsapparat der Antennen des Großen Blaupfeils Orthetrum cancellatum (Odonata: Libellulidae). Entomol. Gen. 2004, 27, 73–85. [Google Scholar] [CrossRef]
- Rebora, M.; Salerno, G.; Piersanti, S.; Dell’Otto, A.; Gaino, E. Olfaction in dragonflies: Electrophysiological evidence. J. Insect Physiol. 2012, 58, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Piersanti, S.; Frati, F.; Rebora, M.; Salerno, G. Carbon dioxide detection in adult Odonata. Zoology 2016, 119, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Piersanti, S.; Frati, F.; Conti, E.; Gaino, E.; Rebora, M.; Salerno, G. First evidence of the use of olfaction in Odonata behaviour. J. Insect Physiol. 2014, 62, 26–31. [Google Scholar] [CrossRef]
- Frati, F.; Piersanti, S.; Conti, E.; Rebora, M.; Salerno, G. Scent of a dragonfly: Sex recognition in a polymorphic coenagrionid. PLoS ONE 2015, 10, e0136697. [Google Scholar] [CrossRef]
- Frati, F.; Piersanti, S.; Rebora, M.; Salerno, G. Volatile cues can drive the oviposition behavior in Odonata. J. Insect Physiol. 2016, 91, 34–38. [Google Scholar] [CrossRef]
- Piersanti, S.; Rebora, M.; Almaas, T.J.; Salerno, G.; Gaino, E. Electrophysiological identification of thermo- and hygro-sensitive receptor neurons on the antennae of the dragonfly Libellula depressa. J. Insect Physiol. 2011, 57, 1391–1398. [Google Scholar] [CrossRef]
- Rebora, M.; Frati, F.; Piersanti, S.; Salerno, G.; Selvaggini, R.; Fincke, O.M. Field tests of multiple sensory cues in sex recognition and harassment of a colour polymorphic damselfly. Anim. Behav. 2018, 136, 127–136. [Google Scholar] [CrossRef]
- Gaino, E.; Piersanti, S.; Rebora, M. Ultrastructural organization of the larval spiracles in Libellula depressa L. (Anisoptera: Libellulidae). Odonatologica 2007, 36, 373–379. [Google Scholar]
- Rospars, J. Structure and development of the insect antennodeutocerbral system. Int. J. Insect Morphol. Embryol. 1988, 17, 243–294. [Google Scholar] [CrossRef]
- Strausfeld, N.J. Atlas of an Insect Brain; Springer: Berlin, Germany, 1976. [Google Scholar]
- Missbach, C.; Harzsch, S.; Hansson, B.S. New insights into an ancient insect nose: The olfactory pathway of Lepismachilis y-signata (Archaeognatha: Machilidae). Arthropod Struct. Dev. 2011, 40, 317–333. [Google Scholar] [CrossRef]
- Kollmann, M.; Minoli, S.; Bonhomme, J.; Homberg, U.; Schachtner, J.; Tagu, D.; Anton, S. Revisiting the anatomy of the central nervous system of a hemimetabolous model insect species: The pea aphid Acyrthosiphon pisum. Cell Tissue Res. 2010, 343, 343–355. [Google Scholar] [CrossRef]
- Bräunig, P.; Krumpholz, K. Internal receptors in insect appendages project directly into a special brain neuropile. Front. Zool. 2013, 10, 54. [Google Scholar] [CrossRef]
- Strausfeld, N.J.; Sinakevitch, I.; Brown, S.M.; Farris, S.M. Ground plan of the insect mushroom body: Functional and evolutionary implications. J. Comp. Neurol. 2009, 513, 265–291. [Google Scholar] [CrossRef]
- Fahrbach, S.E. Structure of the mushroom bodies of the insect brain. Annu. Rev. Entomol. 2006, 51, 209–232. [Google Scholar] [CrossRef]
- Farris, S.M.; Strausfeld, N.J. Development of laminar organization in the mushroom bodies of the cockroach: Kenyon cell proliferation, outgrowth, and maturation. J. Comp. Neurol. 2001, 439, 331–351. [Google Scholar] [CrossRef]
- Kristoffersen, L.; Hansson, B.S.; Anderbrant, O.; Larsson, M.C. Aglomerular hemipteran antennal lobes-basic neuroanatomy of a small nose. Chem. Senses 2008, 33, 771–778. [Google Scholar] [CrossRef]
- Bouchebti, S.; Arganda, S. Insect lifestyle and evolution of brain morphology. Curr. Opin. Insect Sci. 2020. [Google Scholar] [CrossRef]
- Gaino, E.; Rebora, M. Ultrastructure of the antennal sensilla of the mayfly Baetis rhodani (Pictet) (Ephemeroptera: Baetidae). Int. J. Insect Morphol. Embryol. 1998, 27, 143–149. [Google Scholar] [CrossRef]
- Piersanti, S.; Rebora, M.; López-Rodríguez, M.J.; de Figueroa, J.M.T. A comparison between the adult antennal sensilla of the cavernicolous stonefly Protonemura gevi and other epigean Protonemura species (Plecoptera: Nemouridae) in a biological context. Ann. Société Entomol. Fr. 2017, 53, 47–54. [Google Scholar] [CrossRef]
- Rebora, M.; Tierno de Figueroa, J.M.; Piersanti, S. Antennal sensilla of the stonefly Dinocras cephalotes (Plecoptera: Perlidae). Arthropod Struct. Dev. 2016, 45, 552–561. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, G.; Buscariollo, D.; Pitts, R.J.; Wenger, H.; Zwiebel, L.J. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 6433–6438. [Google Scholar] [CrossRef] [PubMed]
- Zacharuk, R.Y.; Yin, L.R.; Blue, S.G. Fine structure of the antenna and its sensory cone in larvae of Aedes aegypti (L.). J. Morphol. 1971, 135, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Galizia, C.G.; Rössler, W. Parallel Olfactory Systems in Insects: Anatomy and Function. Annu. Rev. Entomol. 2010, 55, 399–420. [Google Scholar] [CrossRef]
- Martin, J.P.J.; Beyerlein, A.A.; Dacks, A.M.A.; Reisenman, C.E.C.; Riffell, J.A.J.; Lei, H.H.; Hildebrand, J.G.J. The neurobiology of insect olfaction: Sensory processing in a comparative context. Prog. Neurobiol. 2011, 95, 427–447. [Google Scholar] [CrossRef]
- Bridi, J.C.; Ludlow, Z.N.; Kottler, B.; Hartmann, B.; Broeck, L.V.; Dearlove, J.; Göker, M.; Strausfeld, N.J.; Callaerts, P.; Hirth, F. Ancestral regulatory mechanisms specify conserved midbrain circuitry in arthropods and vertebrates. Proc. Natl. Acad. Sci. USA 2020, 117, 19544–19555. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piersanti, S.; Rebora, M.; Salerno, G.; Anton, S. The Antennal Pathway of Dragonfly Nymphs, from Sensilla to the Brain. Insects 2020, 11, 886. https://doi.org/10.3390/insects11120886
Piersanti S, Rebora M, Salerno G, Anton S. The Antennal Pathway of Dragonfly Nymphs, from Sensilla to the Brain. Insects. 2020; 11(12):886. https://doi.org/10.3390/insects11120886
Chicago/Turabian StylePiersanti, Silvana, Manuela Rebora, Gianandrea Salerno, and Sylvia Anton. 2020. "The Antennal Pathway of Dragonfly Nymphs, from Sensilla to the Brain" Insects 11, no. 12: 886. https://doi.org/10.3390/insects11120886
APA StylePiersanti, S., Rebora, M., Salerno, G., & Anton, S. (2020). The Antennal Pathway of Dragonfly Nymphs, from Sensilla to the Brain. Insects, 11(12), 886. https://doi.org/10.3390/insects11120886







