Performance of Pheromone-Baited Traps to Monitor the Seasonal Abundance of Tortrix Moths in Chestnut Groves
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Monitoring Traps
2.2. Fruit Collection
2.3. Identification of Specimens
2.4. Statistical Analysis
3. Results
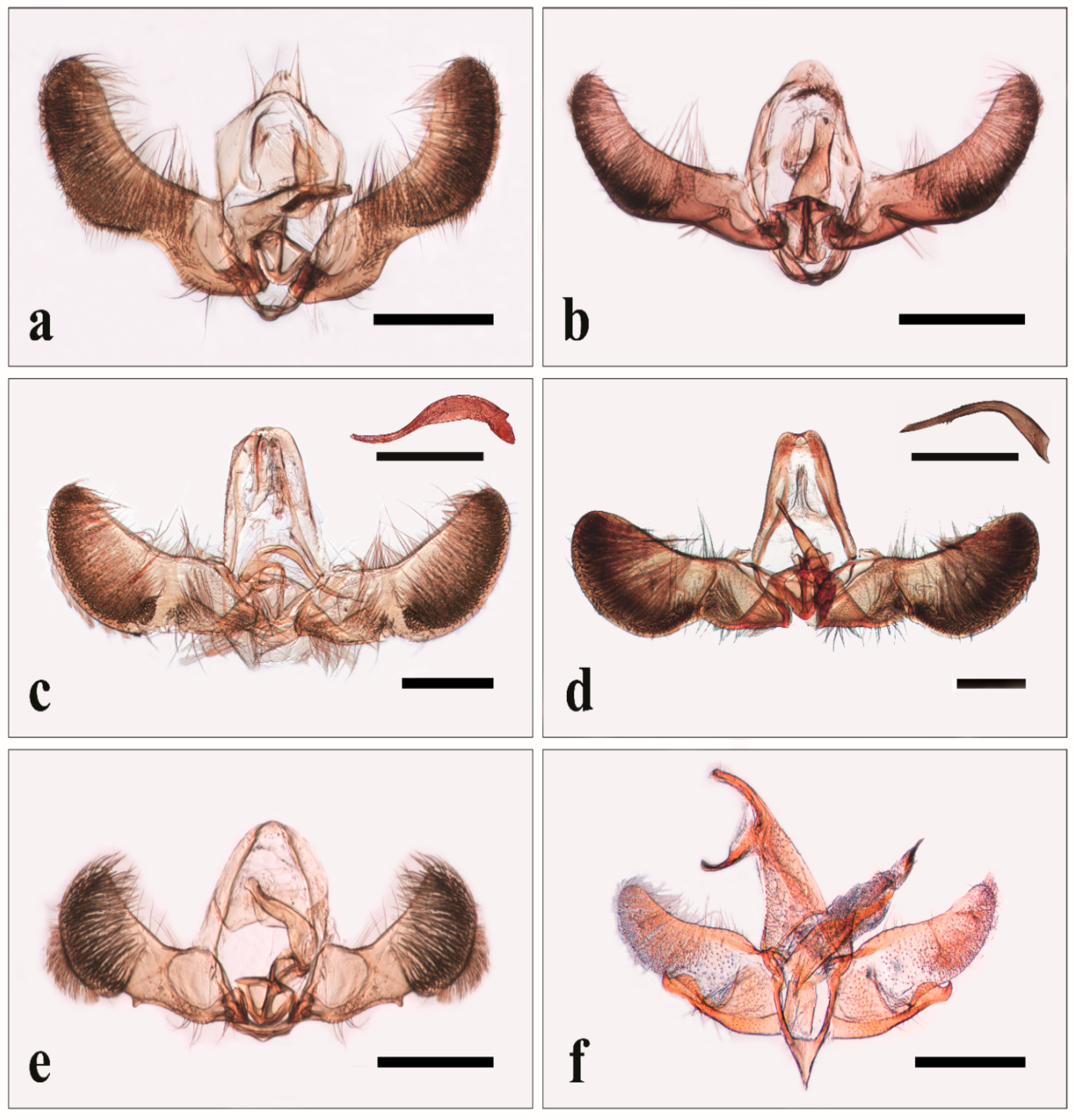
3.1. Identification of Specimens
3.2. Pheromone Experiment
3.2.1. Pammene fasciana
3.2.2. Cydia fagiglandana
3.2.3. Cydia splendana
3.2.4. Damage to Chestnut Husks and Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angeli, G.; Ioriatti, C.; Rama, F.; Witzgall, P. Pheromone traps for monitoring of chestnut Tortricidae Pammene fasciana L.—Cydia fagiglandana Zel. and Cydia splendana Hb [Castanea sativa Mill.—Trentino-Alto Adige]. Atti. Delle Giornate Fitopatol. 1998, 287–292. [Google Scholar]
- Ferracini, C. Pests. In The Chestnut Handbook: Crop & Forest Management; Bounous, G., Gomes-Laranjo, J., Beccaro, G., Alma, A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 317–342. [Google Scholar]
- Pedrazzoli, F.; Salvadori, C.; De Cristofaro, A.; Di Santo, P.; Endrizzi, E.; Sabbatini Peverieri, G.; Roversi, P.F.; Ziccardi, A.; Angeli, G. A new strategy of environmentally safe control of chestnut tortricid moths. IOBC/WPRS Bull. 2012, 74, 117–123. [Google Scholar]
- Speranza, S. Chestnut pests in central Italy. Acta Hortic. 1999, 494, 417–423. [Google Scholar] [CrossRef]
- De Cristofaro, A.; Rotundo, G. Chestnut fruit insect pests in the Campania Region (Southern Italy): Biology and damages. In Proceedings of the International Congress on Chestnut, Spoleto, Italy, 20–23 October 1993; pp. 625–630. [Google Scholar]
- Den Otter, C.J.; De Cristofaro, A.; Voskamp, K.E.; Rotundo, G. Electrophysiological and behavioural responses of chestnut moths, Cydia fagiglandana and C. splendana (Lep., Tortricidae), to sex attractants and odours of host plants. J. Appl. Entomol. 1996, 120, 413–421. [Google Scholar] [CrossRef]
- Rotundo, G.; De Cristofaro, A. Sex attractants for male chestnut tortricoid moths (Pammene fasciana L., Cydia fagiglandana Z. and Cydia splendana Hb.) found by electrophysiological and field studies. WPRS Bull. 1993, 16, 176. [Google Scholar]
- Witzgall, P.; Chambon, J.P.; Bengtsson, M.; Unelius, C.R.; Appelgren, M.; Makranczy, G.; Muraleedharan, N.; Reed, D.W.; Hellrigl, K.; Buser, H.R.; et al. Sex pheromones and attractants in the Eucosmini and Grapholitini (Lepidoptera, Tortricidae). Chemoecology 1996, 7, 13–23. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. An investigation on the chemotactic responses of different entomopathogenic nematode strains to mechanically damaged maize root volatile compounds. Exp. Parasitol. 2013, 134, 349–355. [Google Scholar] [CrossRef]
- Pedrazzoli, F.; Sabbatini Peverieri, G.; Ferracini, C.; Montepaone, G.; Germinara, G.S.; Tolotti, G.; Pennacchio, F.; Caruso, S.; Endrizzi, E.; Bariselli, M.; et al. Confusione sessuale delle tortrici del castagno mediante puffer: Una storia di successi e sconfitte. In Proceedings of the VII Convegno Nazionale sul Castagno, Pergine Valsugana, Italy, 11–14 June 2019; pp. 223–227. [Google Scholar]
- Avtzis, D.N. The distribution of Pammene fasciana L. (lepidoptera: Tortricidae) in greece: An underestimated chestnut-feeding pest. Int. J. Pest Manag. 2012, 58, 115–119. [Google Scholar] [CrossRef]
- Chen, M.H.; Dorn, S. Reliable and efficient discrimination of four internal fruit-feeding Cydia and Grapholita species (Lepidoptera: Tortricidae) by polymerase chain reaction-restriction fragment length polymorphism. J. Econ. Entomol. 2009, 102, 2209–2216. [Google Scholar] [CrossRef]
- Li, T.; Cai, B.; Song, W.; Xu, W.; Zhao, Q.; Long, Q.; Bu, W.; Liu, F.; Han, Y.; Li, W.; et al. Morphological and molecular identification of larvae of Cydia pomonella (Lepidoptera, Tortricidae). Plant Quar. 2013, 4. [Google Scholar]
- Bengtsson, M.; Boutitie, A.; Jósvai, J.; Toth, M.; Andreadis, S.; Rauscher, S.; Unelius, C.R.; Witzgall, P. Pheromone races of Cydia splendana (Lepidoptera, Tortricidae) overlap in host plant association and geographic distribution. Front. Ecol. Evol. 2014, 2. [Google Scholar] [CrossRef]
- Jósvai, J.K.; Voigt, E.; Tóth, M. A pear ester-based female-targeted synthetic lure for the chestnut tortrix, Cydia splendana. Entomol. Exp. Appl. 2016, 159, 370–374. [Google Scholar] [CrossRef]
- Arraiol, A.; Aguin-Pombo, D.; Aguiar, A.M.F.; Freitas, E.; Angeli, G. Evaluation of commercial pheromone lures for monitoring Cydia splendana males (Lepidoptera: Tortricidae) in chestnuts of Madeira Island. Ii Iber. Congr. Chestnuts 2007, 250–253. [Google Scholar]
- Lopes, D.J.H.; Macedo, N.; Figueiredo, A.; Martins, J.T.; Pimentel, R.; Ventura, L.B.; Pombo, D.A. The interfruta project and its contribution to the knowledge of chestnut moth (Cydia splendana Hubner) (Lepidoptera: Tortricidae) dispersal and infestation on Terceira Island, Azores. Acta Hortic. 2008, 784, 187–192. [Google Scholar] [CrossRef]
- Martini, A.; Baronio, P.; Baldassari, N. I lepidotteri tortricidi del castagno (Pammene fasciana (L.), Cydia fagiglandana (Zel.) e Cydia splendana (Hb.)) valutati come un’unica entità di danno. Boll. Ist. Entomol. Guido Grandi 1998, 52, 105–114. [Google Scholar]
- Rotundo, G.; De Cristofaro, A.; Parillo, R.; Germinara, G.S. Monitoring of chestnut moths by intra-and interspecific semiochemicals. Acta Hortic. 2010, 866, 435–441. [Google Scholar] [CrossRef]
- Streinz, L.; Horák, A.; Vrkoč, J.; Hrdý, I. Propheromones derived from codlemone. J. Chem. Ecol. 1993, 19, 1–9. [Google Scholar] [CrossRef]
- Razowski, J. Motyle (Lepidoptera) Polski: Grapholitini. Monogr. Fauny Pol. 1991, 19, 187. [Google Scholar]
- Bogenschütz, H. World crop pests: Tortricid pests. Their biology, natural enemies and control. Eurasian Species 1991, 5, 673–709. [Google Scholar]
- Asghar, U.; Malik, M.F.; Anwar, F.; Javed, A.; Raza, A. DNA extraction from insects by using different techniques: A review. Adv. Entomol. 2015, 3, 132–138. [Google Scholar] [CrossRef]
- Hajibabaei, M.; Janzen, D.H.; Burns, J.M.; Hallwachs, W.; Hebert, P.D.N. DNA barcodes distinguish species of tropical Lepidoptera. Proc. Natl. Acad. Sci. USA 2006, 103, 968–971. [Google Scholar] [CrossRef]
- Maresi, G.; Angeli, G.; Turchetti, T. Biological control in chestnut cultivation: Criteria for a sustainable management. IOBC/WPRS Bull. 2004, 27, 27–33. [Google Scholar]
- Pedrazzoli, F.; Tolotti, G.; Endrizzi, E.; Maresi, G.; Salvadori, C.; Angeli, G. Le tortrici del castagno in Trentino: Osservazioni fenologiche e valutazione del danno. In Proceedings of the VII Convegno Nazionale sul Castagno, Pergine Valsugana, Italy, 11–14 June 2019; Volume 25, pp. 219–222. [Google Scholar]
- Adams, C.G.; Schenker, J.H.; McGhee, P.S.; Gut, L.J.; Brunner, J.F.; Miller, J.R. Maximizing information yield from pheromone-baited monitoring traps: Estimating plume reach, trapping radius, and absolute density of Cydia pomonella (Lepidoptera: Tortricidae) in Michigan apple. J. Econ. Entomol. 2017, 110, 305–318. [Google Scholar] [CrossRef]
- Blomefield, T.; Knight, A. Codling moth management: Monitoring methods, control guidelines and predictive models. In Proceedings of the XXI-International Congress of Entomology, Foz Do Iguassu, Brazil, 20–26 August 2000; p. 664. [Google Scholar]
- Riedl, H.; Hoying, S.A.; Barnett, W.W.; DeTar, J.E. Relationship of within-tree placement of the pheromone trap to codling moth catches. Environ. Entomol. 1979, 8, 765–769. [Google Scholar] [CrossRef]
- Sieber, T.N.; Jermini, M.; Conedera, M. Effects of the harvest method on the infestation of chestnuts (Castanea sativa) by insects and moulds. J. Phytopathol. 2007, 155, 497–504. [Google Scholar] [CrossRef]






| Species | Composition | Loading | Notes |
|---|---|---|---|
| P. fasciana | Z8-12:Ac + Z8-12:OH | 0.75 mg + 0.25 mg | Commercial |
| C. fagiglandana | E8,E10-12Ac | 0.01 mg | Commercial |
| C. fagiglandana | E8,E10-12Ac | 1.0 mg | Experimental |
| C. splendana | E8,Z10-12:Ac | 1.0 mg | Commercial |
| C. splendana | Tricarbonyl-[(8,9,10,11-η)-8,10-dodecadien-1-yl acetate]-iron (1) | 2.5 mg | Experimental |
| 2018 | ||||||||||||
| Species | Pheromone | Average No. Males | Total No. Males Trapped | % | Peveragno | Villarfocchiardo | Carro | Montese | Badia del Borgo | Molazzana | ||
| per Site (± SE) | ||||||||||||
| PF | CP | 503.17 | ±154.57 | 3019 | 84.14 | 86 | 1088 | 602 | 471 | 671 | 101 | |
| CF | CP | 72.50 | ±27.83 | a | 435 | 12.12 | 2 | 38 | 108 | 128 | 159 | 0 |
| CF | EP | 6.00 | ±2.86 | b | 36 | 1.00 | 2 | 17 | 0 | 12 | 5 | 0 |
| CS | CP | 3.67 | ±2.54 | A | 22 | 0.61 | 0 | 3 | 0 | 3 | 16 | 0 |
| CS | EP | 12.67 | ±8.31 | B | 76 | 2.12 | 1 | 23 | 0 | 50 | 2 | 0 |
| Total | 3.588 | |||||||||||
| 2019 | ||||||||||||
| Species | Pheromone | Average No. Males | Total No. Males Trapped | % | Peveragno | Villarfocchiardo | Carro | Montese | Badia del Borgo | Molazzana | ||
| per Site (± SE) | ||||||||||||
| PF | CP | 86.11 | ±20.73 | 5298 | 76.96 | 158 | 1132 | 2.397 | 0 | 793 | 818 | |
| CF | CP | 64.83 | ±12.39 | a | 389 | 5.65 | 1 | 16 | 94 | 0 | 234 | 44 |
| CF | EP | 61.00 | ±8.77 | a | 366 | 5.32 | 16 | 26 | 124 | 1 | 169 | 30 |
| CS | CP | 26.33 | ±6.75 | A | 158 | 2.30 | 8 | 15 | 108 | 0 | 17 | 10 |
| CS | EP | 112.17 | ±47.41 | B | 673 | 9.78 | 11 | 90 | 469 | 4 | 97 | 2 |
| Total | 6.884 | |||||||||||
| Site | Region | 2018 | 2019 | ||||
|---|---|---|---|---|---|---|---|
| Total No. Chestnut Husks | Healthy (%) | Damaged (%) | Total No. Chestnut Husks | Healthy (%) | Damaged (%) | ||
| Peveragno | Piedmont | - | - | - | 538 | 51.5 | 48.5 a |
| Villar Focchiardo | 100 | 92.0 | 8.0 a | 585 | 26.2 | 73.8 b | |
| Carro | Liguria | - | - | - | 190 | 18.4 | 81.6 b |
| Montese | Emilia Romagna | 161 | 80.7 | 19.3 b | - | - | - |
| Badia del Borgo | Tuscany | 120 | 66.7 | 33.3 c | - | - | - |
| Molazzana | - | - | - | - | - | - | |
| Site | Region | 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total No. Chestnut Fruit | Damage (%) at Collection | Damage (%) after Rearing | Average No. Larvae Recorded (± SE) | Total No. Chestnut Fruit | Damage (%) at Collection | Damage (%) after Rearing | Average No. Larvae Recorded (± SE) | ||
| Peveragno | Piedmont | 1000 | 1.00 | 1.70 a | 2.14 ± 0.98 a | 1657 | 2.66 | 21.12 a | 6.25 ± 1.61 a |
| Villar Focchiardo | 1000 | 10.20 | 18.70 b | 1.40 ± 0.26 a | 1593 | 4.71 | 9.42 b | 16.11 ± 3.68 b | |
| Carro | Liguria | 1169 | 19.80 | 22.90 b | 5.29 ± 1.33 b | 830 | 8.67 | 18.43 a | 7.36 ± 1.74 a |
| Montese | Emilia Romagna | 1012 | 4.50 | 4.60 a | 2.44 ± 1.06 a | 898 | 6.68 | 10.90 b | 6.42 ± 1.37 a |
| Badia del Borgo | Tuscany | 2975 | 2.80 | 4.60 a | 1.10 ± 0.16 a | 1156 | 2.42 | 9.08 b | 3.45 ± 0.89 a |
| Molazzana | 995 | 8.20 | 8.70 a | 2.27 ± 0.83 a | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferracini, C.; Pogolotti, C.; Lentini, G.; Saitta, V.; Busato, E.; Rama, F.; Alma, A. Performance of Pheromone-Baited Traps to Monitor the Seasonal Abundance of Tortrix Moths in Chestnut Groves. Insects 2020, 11, 807. https://doi.org/10.3390/insects11110807
Ferracini C, Pogolotti C, Lentini G, Saitta V, Busato E, Rama F, Alma A. Performance of Pheromone-Baited Traps to Monitor the Seasonal Abundance of Tortrix Moths in Chestnut Groves. Insects. 2020; 11(11):807. https://doi.org/10.3390/insects11110807
Chicago/Turabian StyleFerracini, Chiara, Cristina Pogolotti, Giada Lentini, Valerio Saitta, Enrico Busato, Franco Rama, and Alberto Alma. 2020. "Performance of Pheromone-Baited Traps to Monitor the Seasonal Abundance of Tortrix Moths in Chestnut Groves" Insects 11, no. 11: 807. https://doi.org/10.3390/insects11110807
APA StyleFerracini, C., Pogolotti, C., Lentini, G., Saitta, V., Busato, E., Rama, F., & Alma, A. (2020). Performance of Pheromone-Baited Traps to Monitor the Seasonal Abundance of Tortrix Moths in Chestnut Groves. Insects, 11(11), 807. https://doi.org/10.3390/insects11110807






