Functional Response and Intraspecific Competition in the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
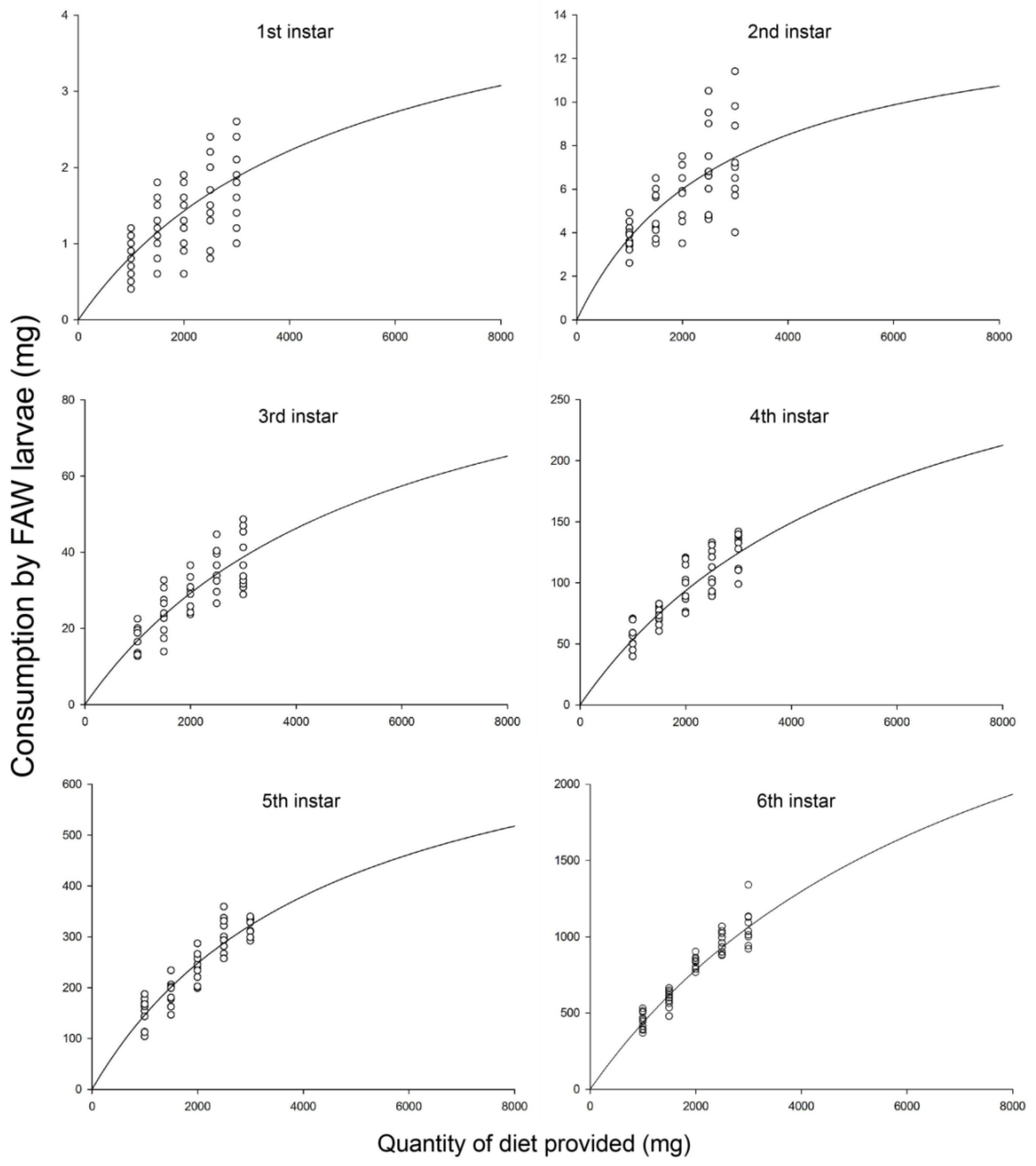
2.2. Functional Response
2.3. Intraspecific Competition
2.4. Statistical Analysis
2.4.1. Functional Response
2.4.2. Intraspecific Competition
3. Results
3.1. Functional Response
3.2. Intraspecific Competition
4. Discussion
4.1. Functional Response
4.2. Intraspecific Competition
4.3. Remaining Questions and Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.J.; Wu, M.F.; Ma, J.; Gao, B.Y.; Wu, Q.L.; Chen, A.D.; Liu, J.; Jiang, Y.Y.; Zhai, B.P.; Early, R. Prediction of migratory routes of the invasive fall armyworm in eastern China using a trajectory analytical approach. Pest Manag. Sci. 2020, 76, 454–463. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host Plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Day, R.; Early, R.; Godwin, J.; et al. Fall Armyworm: Impacts and Implications for Africa; CABI: Wallingford, Oxfordshire, UK, 2017; Evidence Note (2), September 2017, Report to DFID. [Google Scholar]
- Wyckhuys, K.A.; O’Neil, R.J. Population dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot. 2006, 25, 1180–1190. [Google Scholar] [CrossRef]
- Murúa, G.; Molina-Ochoa, J.; Coviella, C. Population dynamics of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) and its parasitoids in northwestern Argentina. Fla. Entomol. 2006, 89, 175–182. [Google Scholar] [CrossRef]
- Hruska, A.J.; Gould, F. Fall armyworm (Lepidoptera: Noctuidae) and Diatraea lineolata (Lepidoptera: Pyralidae): Impact of larval population level and temporal occurrence on maize yield in Nicaragua. J. Econ. Entomol. 1997, 90, 611–622. [Google Scholar] [CrossRef]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; Van Den Berg, J. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the Western Hemisphere. Int. J. Trop. Insect Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2016, 60, 255–267. [Google Scholar] [CrossRef]
- Jiang, X.F.; Zhang, L.; Cheng, Y.X.; Song, L.L. Advances in migration and monitoring techniques of the fall armyworm, Spodoptera frugiperda (JE Smith). Plant Prot. 2018, 45, 12–18. [Google Scholar]
- Rwomushana, I.; Bateman, M.; Beale, T.; Beseh, P.; Cameron, K.; Chiluba, M.; Clottey, V.; Davis, T.; Day, R.; Early, R.; et al. Fall Armyworm: Impacts and Implications for Africa. Evidence Note update, October 2018. Available online: https://www.invasive-species.org/wp-content/uploads/sites/2/2019/02/FAW-Evidence-Note-October-2018.pdf (accessed on 12 October 2020).
- Stokstad, E. New crop pest takes Africa at lightning speed. Science 2017, 356, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.F.; Zhao, J.Z.; He, K.L.; Zhang, F.; Wang, Z.Y. Potential invasion of the crop-devastating insect pest fall armyworm Spodoptera frugiperda to China. Plant Prot. 2018, 44, 1–10. [Google Scholar]
- Wu, Q.L.; Jiang, Y.Y.; Wu, K.M. Analysis of migration routes of fall armyworm (Spodoptera frugiperda (JE Smith) from Myanmar to China. Plant Prot. 2019, 45, 1–9. [Google Scholar]
- Fox, L.R. Cannibalism in natural populations. Annu. Rev. Ecol. Syst. 1975, 6, 87–106. [Google Scholar] [CrossRef]
- Elgar, M.A.; Crespi, B.J. Cannibalism: Ecology and Evolution among Diverse Taxa; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Duelli, P. Is larval cannibalism in lacewings adaptive. Res. Popul. Ecol. 1981, 23, 193–209. [Google Scholar] [CrossRef]
- Joyner, K.; Gould, F. Developmental consequences of cannibalism in Heliothis zea (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1985, 78, 24–28. [Google Scholar] [CrossRef]
- Church, S.C.; Sherratt, T.N. The selective advantages of cannibalism in a Neotropical mosquito. Behav. Ecol. Sociobiol. 1996, 39, 117–123. [Google Scholar] [CrossRef]
- Gould, F.; Holtzman, G.; Rabb, R.L.; Smith, M. Genetic variation in predatory and cannibalistic tendencies of Heliothis virescens strains. Ann. Entomol. Soc. Am. 1980, 73, 243–250. [Google Scholar] [CrossRef]
- Breden, F.; Chippendale, G.M. Effect of larval density and cannibalism on growth and development of the southwestern corn borer, Diatraea grandiosella, and the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). J. Kans. Entomol. Soc. 1989, 62, 307–315. [Google Scholar]
- Pierce, N.E. Predatory and parasitic Lepidoptera: Carnivores living on plants. J. Lepid. Soc. 1995, 49, 412–453. [Google Scholar]
- Polis, G.A. The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Syst. 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Pfennig, D.W.; Reeve, H.K.; Sherman, P.W. Kin recognition and cannibalism in spadefoot toad tadpoles. Anim. Behav. 1993, 46, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations and Communities; Blackwell Science Ltd.: Oxford, UK, 1996. [Google Scholar]
- Hassell, M.P.; Varley, G.C. New inductive population model for insect parasites and its bearing on biological control. Nature 1969, 223, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Beddington, J.R. Mutual interference between parasites or predators and its effect on searching efficiency. J. Anim. Ecol. 1975, 44, 331–340. [Google Scholar] [CrossRef] [Green Version]
- DeAngelis, D.L.; Goldstein, R.A.; O’Neill, R.V. A model for tropic interaction. Ecology 1975, 56, 881–892. [Google Scholar] [CrossRef]
- Crowley, P.H.; Martin, E.K. Functional responses and interference within and between year classes of a dragonfly population. J. N. Am. Benthol. Soc. 1989, 8, 211–221. [Google Scholar] [CrossRef]
- Greene, G.L.; Leppla, N.C.; Dickerson, W.A. Velvetbean caterpillar: A rearing procedure and artificial medium. J. Econ. Entomol. 1976, 69, 487–488. [Google Scholar] [CrossRef]
- Juliano, S.A. Nonlinear curve fitting: Predation and functional response curves. In Design and Analysis of Ecological Experiments; Oxford University Press: New York, NY, USA, 2001; pp. 178–196. [Google Scholar]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Livdahl, T.P.; Stiven, A.E. Statistical difficulties in the analysis of predator functional response data. Can. Entomol. 1983, 115, 1365–1370. [Google Scholar] [CrossRef]
- Watson, D.M.; Du, T.Y.; Li, M.; Xiong, J.J.; Liu, D.G.; Huang, M.D.; Rae, D.J.; Beattie, G. Functional responses of, and mutual interference in Aleurodothrips fasciapennis (Franklin)(Thysanoptera: Phlaeothripidae) and implications for its use as a biocontrol agent. Gen. Appl. Entomol. J. Entomol. Soc. N. S. W. 2000, 29, 31–37. [Google Scholar]
- Wu, H.; Cheng, X.; Zou, Y.; Wei, C.; Lu, F.; Ma, F. Predatism of Harmonia axyridis adults on different ranges of starvation to Myzus persicae. J. Anhui Agric. Coll. 2000, 27, 348–351. [Google Scholar]
- Deng, J.; Tan, Z.; Shan, Q.; Wu, X.; Liu, J. Functional Responses and Density Interference Effect in Harmonis axyridis Pallas A Predator to Myzus nicotianae (Blackman). J. Southwest Agric. Univ. 2002, 24, 433–435. [Google Scholar]
- Ge, Y.; Wan, L.; Zhao, S. Predation of Harmonia axyridis Pallas on Rhopalosiphum nymphaeae Linné (Homoptera: Aphididae). Acta Agri. Univ. Jiangxiensis Nat. Sci. 2006, 28, 208–212. [Google Scholar]
- Koch, R.L.; Hutchison, W.D.; Venette, R.C.; Heimpel, G.E. Susceptibility of immature monarch butterfly, Danaus plexippus (Lepidoptera: Nymphalidae: Danainae), to predation by Harmonia axyridis (Coleoptera: Coccinellidae). Biol. Control 2003, 28, 265–270. [Google Scholar] [CrossRef]
- Fan, Y.; Petitt, F.L. Parameter estimation of the functional response. Environ. Entomol. 1994, 23, 785–794. [Google Scholar] [CrossRef]
- Fan, Y.Q.; Petitt, F.L. Functional response, variance, and regression analysis: A reply to Williams and Juliano. Environ. Entomol. 1997, 26, 1–3. [Google Scholar] [CrossRef]
- Shu, C.; Lai, Y.; Yang, E.; Chen, S.Y.; Xiang, M.C.; Liu, X.Z. Functional response of the fungus Hirsutella rhossiliensis to the nematode, Heterodera glycines. Sci. China Life Sci. 2015, 58, 704–712. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.; Xu, M.; Ricciardi, A.; Dick, J.T.; Luo, D.; Wei, H.; Hu, Y.; Wei, Q. The influence of warming on the biogeographic and phylogenetic dependence of herbivore–plant interactions. Ecol. Evol. 2019, 9, 2231–2241. [Google Scholar] [CrossRef]
- Wu, P.; Haseeb, M.; Zhang, R.; Kanga, L.H.; Legaspi, J.C. In vitro consumption patterns of pepper weevil, Anthonomus eugenii (Coleoptera: Curculionidae) on two commercial pepper cultivars in Florida. Appl. Entomol. Zool. 2019, 54, 473–479. [Google Scholar] [CrossRef]
- He, L.M.; Ge, S.S.; Chen, Y.C.; Wu, Q.L.; Jiang, Y.Y.; Wu, K.M. The developmental threshold temperature, effective accumulated temperature and prediction model of developmental duration of fall armyworm, Spodoptera frugiperda. Plant Prot. 2019, 5, 18–26. [Google Scholar]
- Booth, E.; Alyokhin, A.; Pinatti, S. Adult cannibalism in an oligophagous herbivore, the Colorado potato beetle. Insect Sci. 2017, 24, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.D.; Shrewsbury, P.M.; Denno, R.F. Effects of alternative food on cannibalism and herbivore suppression by carabid larvae. Ecol. Entomol. 2010, 35, 61–68. [Google Scholar] [CrossRef]
- Fang, Y.H.; Tao, M.; Ma, J.; Cao, K.Q.; Chen, G.H.; Li, Q. Study of the predation functional responses of Leis axyridis Pallas to Aphis citricol Vander Goot. J. Yunnan Agric. Univ. Nat. Sci. 2013, 28, 306–309. [Google Scholar]
- Shen, P.; Chang, C.; Zhu, H.; Wang, X.; Tian, W.; Zhang, Y.; Han, S. Predation of Cyamophila willieti by the adults of Harmonia axyridis. Plant Prot. 2009, 35, 66–69. [Google Scholar]
- Wiseman, B.R.; McMillian, W.W. Competition and survival among the corn earworm, the tobacco budworm, and the fall armyworm. J. Econ. Entomol. 1969, 62, 734–735. [Google Scholar] [CrossRef]
- Chapman, J.W.; Williams, T.; Escribano, A.; Caballero, P.; Cave, R.D.; Goulson, D. Fitness consequences of cannibalism in the fall armyworm, Spodoptera frugiperda. Behav. Ecol. 1999, 10, 298–303. [Google Scholar] [CrossRef] [Green Version]
- Morrill, W.L.; Greene, G.L. Distribution of fall armyworm larvae. 1. Regions of field corn plants infested by larvae. Environ. Entomol. 1973, 2, 195–198. [Google Scholar] [CrossRef]
- Labatte, J.M. Within-plant distribution of fall armyworm (Lepidoptera: Noctuidae) larvae on corn during whorl-stage infestation. Fla. Entomol. 1993, 76, 437–447. [Google Scholar] [CrossRef]
- Vickery, R.A. Studies of the Fall Armyworm in the Gulf Coast Region of Texas; USDA Technical Bulletin: Washington, DC, USA, 1929. [Google Scholar]
- Carvalho, R.P.L.; Silveira, N.S. Observacoes do comportamento de Spodoptera frugiperda (JE Smith, 1797) (Lepidoptera, Noctuidae) ao atacar milho em condicoes de campo. In Resúmen de los trabajos presentados al Prima Congreso Latinoamericano de Entomología; Congreso Latinoamericano de Entomología: Cuzco, Peru, 1971; pp. 92–93. [Google Scholar]
- Wang, D.T.; Zhang, L.; Cheng, Y.X.; Jiang, X.F. Larval stage related cannibalism in the fall armyworm, Spodoptera frugiperda. Plant Prot. 2019, 3, 94–98. [Google Scholar]
- Yang, H.; Rong, R.; Song, F.P.; Sun, C.P.; Wei, J.; Zhang, J.; Huang, D.F. In vivo fluorescence observation of parasporal inclusion formation in Bacillus thuringiensis. Sci. China Life Sci. 2010, 53, 1106–1111. [Google Scholar] [CrossRef]
- Dhandapani, N.; Jayaraj, S.; Rabindra, R.J. Cannibalism on nuclear polyhedrosis virus infected larvae by Heliothis armigera (Hubn.) and its effect on viral infection. Int. J. Trop. Insect Sci. 1993, 14, 427–430. [Google Scholar] [CrossRef]
- Dial, C.I.; Adler, P.H. Larval behavior and cannibalism in Heliothis zea (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1990, 83, 258–263. [Google Scholar] [CrossRef]
- Hassell, M.P. The Dynamics of Arthropod Predator-Prey Systems; Princeton University Press: Princeton, NJ, USA, 1978; p. 237. [Google Scholar]
- Gitonga, L.M.; Overholt, W.A.; Löhr, B.; Magambo, J.K.; Mueke, J.M. Functional response of Orius albidipennis (Hemiptera: Anthocoridae) to Megalurothrips sjostedti (Thysanoptera: Thripidae). Biol. Control 2002, 24, 1–6. [Google Scholar] [CrossRef]
- Lee, J.; Kang, T. Functional response of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) to Aphis gossypii Glover (Homoptera: Aphididae) in the laboratory. Biol. Control 2004, 31, 306–310. [Google Scholar] [CrossRef]
- Wiedenmann, R.N.; O’Neil, R.J. Searching behavior and time budgets of the predator Podisus maculiventris. Entomol. Exp. Appl. 1991, 60, 83–93. [Google Scholar] [CrossRef]
- Wiedenmann, R.N.; O’Neil, R.J. Laboratory measurement of the functional response of Podisus maculiventris (Say) (Heteroptera: Pentatomidae). Environ. Entomol. 1991, 20, 610–614. [Google Scholar] [CrossRef]
- Wiedenmann, R.N.; O’Neil, R.J. Searching strategy of the predator Podisus maculiventris (Say) (Heteroptera: Pentatomidae). Environ. Entomol. 1992, 21, 1–9. [Google Scholar] [CrossRef]
- Murdoch, W.W. The functional response of predators. J. Appl. Ecol. 1973, 10, 335–342. [Google Scholar]
- Kareiva, P. The spatial dimension in pest-enemy interactions. In Critical Issues in Biological Control; Intercept: Andover, UK, 1990; pp. 213–227. [Google Scholar]
- Sun, G.; Wang, S.; Ren, Q.; Jin, Z.; Wu, Y. Effects of time delay and space on herbivore dynamics: Linking inducible defenses of plants to herbivore outbreak. Sci. Rep. 2015, 5, 11246. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Demiris, N.; Milonas, P.G.; Preston, S.; Kypraios, T. Does mutual interference affect the feeding rate of aphidophagous coccinellids? A modeling perspective. PLoS ONE 2016, 11, e146168. [Google Scholar] [CrossRef] [Green Version]
- Hodek, I.; Honek, A. Ecology of Coccinellidae; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; p. 464. [Google Scholar]
- Garratt, J.; Kennedy, A. Use of models to assess the reduction in contamination of water bodies by agricultural pesticides through the implementation of policy instruments: A case study of the Voluntary Initiative in the UK. Pest Manag. Sci. Former. Pestic. Sci. 2006, 62, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.; Seo, M.J.; Shin, J.G.; Jang, C.; Yu, Y.M. Toxicity of greenhouse pesticides to multicolored Asian lady beetles, Harmonia axyridis (Coleoptera: Coccinellidae). Biol. Control 2003, 28, 164–170. [Google Scholar] [CrossRef]
- Sharanabasappa, D.; Kalleshwaraswamy, C.M.; Asokan, R.; Swamy, H.M.; Maruthi, M.S.; Pavithra, H.B.; Hegde, K.; Navi, S.; Prabhu, S.T.; Goergen, G. First report of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hortic. Ecosyst. 2018, 24, 23–29. [Google Scholar]

| Larval Instar | P0 | P1 | P2 | P3 |
|---|---|---|---|---|
| 1st | −2.12654 ** | −0.04317 ** | 0.00022 * | −4.67219 |
| (SE) | 0.122 | 0.006 | 0.00000042 | 0.21 |
| 2nd | −1.4321 ** | −0.00531 * | 0.000023 | −0.00000012 |
| (SE) | 0.128 | 0.009 | 0.0000002 | 0.000000031 |
| 3rd | −0.20345 | −0.00711 * | 0.000034 | −0.000000082 |
| (SE) | 0.091 | 0.006 | 0.0000084 | 0.000000007 |
| 4th | 2.14786 * | −0.004253 * | −0.000027 | 0.000000077 |
| (SE) | 0.523 | 0.037 | 0.0000063 | 0.00000003 |
| 5th | 2.53204 ** | −0.054678 ** | 0.000375 | −0.00000063 |
| (SE) | 0.311 | 0.024 | 0.0000694 | 0.00000011 |
| 6th | 2.24428 ** | −0.0132424 * | 0.0000362 | −0.00000003 |
| (SE) | 0.104 | 0.008 | 0.00000627 | 0.000000017 |
| Larval Instar | R2 | F1,3 | p | Holling’s Disc Equation | a | Th (min) |
|---|---|---|---|---|---|---|
| 1st | 0.976 | 122.977 | 0.002 | Na = 0.0010N/(1 + 0.00020N) | 0.001 | 296.47 |
| 2nd | 0.991 | 330.687 | <0.001 | Na = 0.0051N/(1 + 0.00035N) | 0.005 | 100.98 |
| 3rd | 0.996 | 709.639 | <0.001 | Na = 0.0199N/(1 + 0.00018N) | 0.02 | 12.88 |
| 4th | 0.997 | 934.697 | <0.001 | Na = 0.0627N/(1 + 0.00017N) | 0.063 | 3.8 |
| 5th | 0.986 | 208.166 | 0.001 | Na = 0.1786N/(1 + 0.00022N) | 0.179 | 1.81 |
| 6th | 0.983 | 171.244 | 0.001 | Na = 0.4932N/(1 + 0.00013N) | 0.493 | 0.37 |
| Larval Instar | R2 | F1,3 | p | Intraspecific Competition Equation | Q (mg) | m |
|---|---|---|---|---|---|---|
| 4th | 0.856 | 17.903 | 0.024 | E = 54.6709P−0.4185 | 54.7 | 0.42 |
| 5th | 0.804 | 12.289 | 0.039 | E = 160.4898P−0.4799 | 160.5 | 0.48 |
| 6th | 0.805 | 12.403 | 0.039 | E = 445.7573P−0.3325 | 445.8 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Q.; Haseeb, M.; Fan, J.; Wu, P.; Tian, T.; Zhang, R. Functional Response and Intraspecific Competition in the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects 2020, 11, 806. https://doi.org/10.3390/insects11110806
Ren Q, Haseeb M, Fan J, Wu P, Tian T, Zhang R. Functional Response and Intraspecific Competition in the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects. 2020; 11(11):806. https://doi.org/10.3390/insects11110806
Chicago/Turabian StyleRen, Qilin, Muhammad Haseeb, Jingyu Fan, Pengxiang Wu, Tianqi Tian, and Runzhi Zhang. 2020. "Functional Response and Intraspecific Competition in the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae)" Insects 11, no. 11: 806. https://doi.org/10.3390/insects11110806
APA StyleRen, Q., Haseeb, M., Fan, J., Wu, P., Tian, T., & Zhang, R. (2020). Functional Response and Intraspecific Competition in the Fall Armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Insects, 11(11), 806. https://doi.org/10.3390/insects11110806






