Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on the “Offensive” in Africa: Prospects for Integrated Management Initiatives
Abstract
:Simple Summary
Abstract
1. Introduction
2. Economic Impact of Tuta absoluta in Africa
3. Tuta absoluta Invasion Pathways and Distribution in Africa
4. Factors Supporting T. absoluta Invasion in Africa
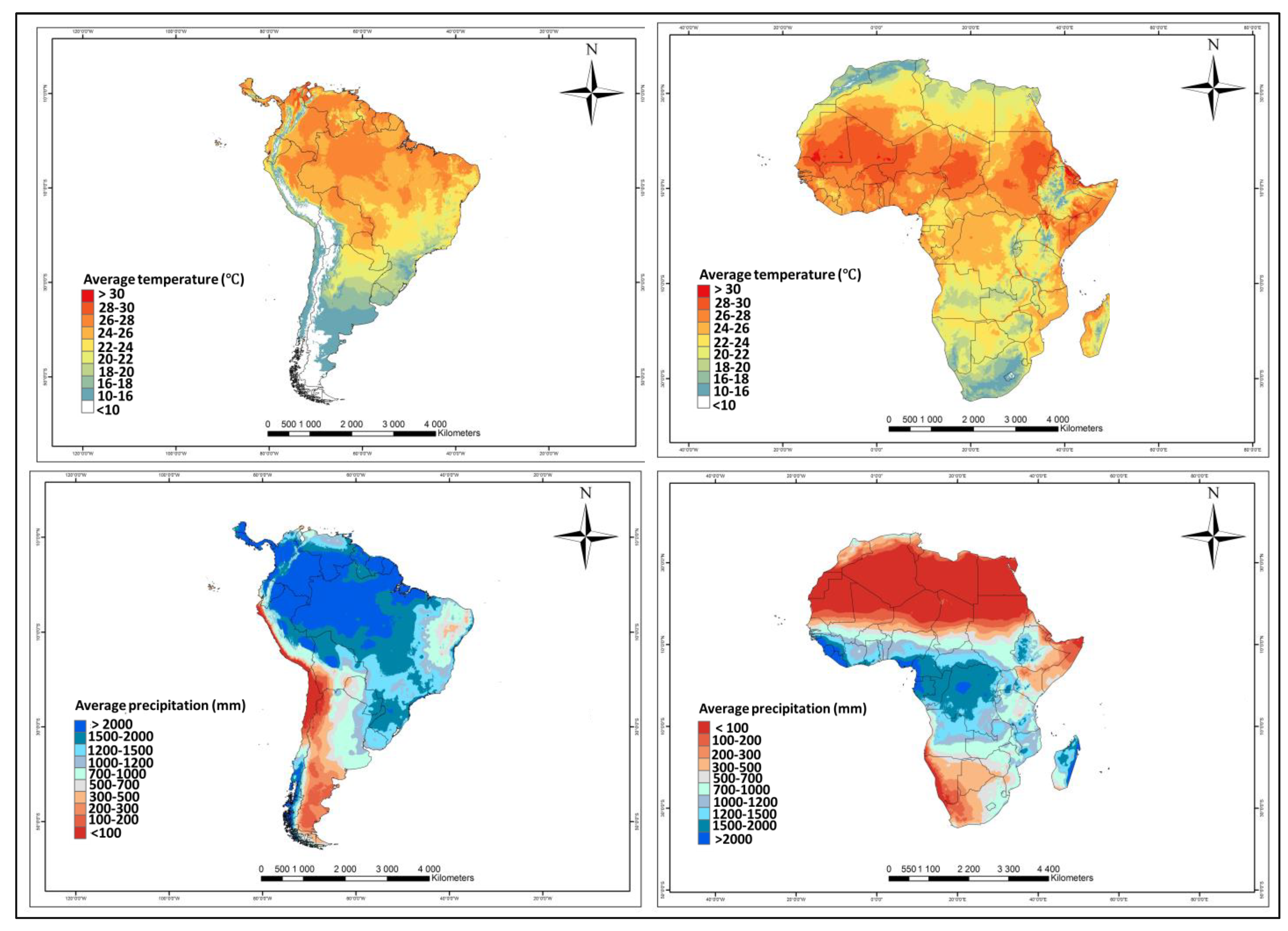
4.1. African Environments and Tuta absoluta Niche
4.2. Physiological Tolerance
4.3. Increased Number of Generations
4.4. New Niche with Limited Natural Enemies
4.5. Wide Host Range
4.6. Pesticide Resistance
5. Potential Use of Natural Substances
5.1. Botanicals
5.2. Entomopathogens
5.3. Entomopathogenic Nematodes (EPNs)
5.4. Semiochemicals
5.5. Sterile Insect Technique (SIT)
5.6. Host Plant Resistance
5.7. Use of Predators and Parasitoids
6. Use of Synthetic Pesticides and Integrated Pest Management
7. Potential for Natural Substances in Pest Control: Assets and Liabilities
7.1. Legislative and Regulatory Frameworks
7.2. Economic Dynamics
7.3. Ecological Perspectives
7.4. Farmer Perceptions and Social Dynamics
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olson, L.J. The economics of terrestrial invasive species: A review of the literature. Agric. Resour. Econ. Rev. 2006, 35, 178–194. [Google Scholar] [CrossRef] [Green Version]
- Ragsdale, D.W.; Landis, D.A.; Brodeur, J.; Heimpel, G.E.; Desneux, N. Ecology and management of the soybean aphid in North America. Annu. Rev. Entomol. 2011, 56, 375–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, M.R.; Rodrigues, A.R.S.; Silva, W.M.; Silva, T.B.M.; Silva, V.R.F.; Guedes, R.N.C.; Siqueira, H.A.A. Spinosad and the Tomato Borer Tuta absoluta: A Bioinsecticide, an Invasive Pest Threat, and High Insecticide Resistance. PLoS ONE 2014, 9, e103235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, M. Biological Invasions; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’Connell, C.; Wong, E.L.; Russel, L.; Zern, J.; Aquino, T.; et al. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosyst. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Simberloff, D.; Rejmanek, M. Encyclopedia of Biological Invasions; University of California Press: Berkeley, CA, USA, 2001. [Google Scholar]
- van Wilgen, B.W.; Measey, J.; Richardson, D.M.; Wilson, J.R.; Zengeya, T.A. Biological Invasions in South Africa: An Overview. In Biological Invasions in South Africa; Invading Nature—Springer Series in Invasion Ecology; Van Wilgen, B., Measey, J., Richardson, D., Wilson, J., Zengeya, T., Eds.; Springer: Cham, Switzerland, 2020; Volume 14. [Google Scholar]
- Tams, W.H.T. New species of African Heterocera. Entomologist 1932, 65, 1241–1249. [Google Scholar]
- Dunstan, W.R.; Magazini, I.A. Outbreaks and new records, United Republic of Tanzania. The larger grain borer on stored products. FAO Plant Prot. Bull. 1981, 29, 80–81. [Google Scholar]
- Neuenschwander, P. Biological Control of the Cassava Mealybug in Africa: A review. Biol. Control 2001, 21, 214–229. [Google Scholar] [CrossRef]
- Lux, S.A.; Copeland, R.S.; White, I.M.; Manrakhan, A.; Billah, M.K. A new invasive fruit fly species from the Bactrocera dorsalis (Hendel) group detected in East Africa. Insect Sci. Appl. 2003, 23, 355–361. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [Green Version]
- Mansour, R.; Brevault, T.; Chailleux, A.; Cherif, A.; Grissa-Lebdi, K.; Haddi, K.; Mohamed, S.A.; Nofemela, R.S.; Oke, A.; Sylla, S.; et al. Occurrence, biology, natural enemies and management of Tuta absoluta in Africa. Entomol. Gen. 2018, 38, 83–112. [Google Scholar] [CrossRef]
- FAO. Comprehensive Africa Agriculture Development Programme (CAADP); NEPAD: Rome, Italy, 2002. [Google Scholar]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.G.; Burgio, G.; Arpaia, S.; Narvaez-Vasquez, C.A.; González-Cabrera, J.; Ruescas, D.C.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest. Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Biondi, A.; Guedes, R.N.C.; Wan, F.-H.; Desneux, N. Ecology, Worldwide Spread, and Management of the Invasive South American Tomato Pinworm, Tuta absoluta: Past, Present, and Future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef]
- Campos, M.R.; Biondi, A.; Adiga, A.; Guedes, R.N.C.; Desneux, N. From the Western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest Sci. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- Han, P.; Zhang, Y.; Lu, Z.; Wang, S.; Ma, D.; Biondi, A.; Desneux, N. Are we ready for the invasion of Tuta absoluta? Unanswered key questions for elaborating an Integrated Pest Management package in Xinjiang, China. Entomol. Gen. 2018, 38, 113–125. [Google Scholar] [CrossRef]
- Ghoneim, K. Parasitic insects and mites as potential biocontrol agents for a devastative pest of tomato, Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) in the world: A review. IJRRAS 2014, 19, 3. [Google Scholar]
- Marchioro, C.A.; Krechemer, F.S.; Foerster, L.A. Estimating the development rate of the tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae), using linear and non-linear models. Pest Manag. Sci. 2016, 73, 1486–1493. [Google Scholar] [CrossRef]
- Urbaneja, A.; Vercher, R.; Navarro-Llopis, V.; Marí, F.; Porcuna, J. The tomato moth, Tuta absoluta. Phytoma Espanã 2007, 194, 16–24. (In Spanish) [Google Scholar]
- Guillemaud, T.; Blin, A.; Le Goff, I.; Desneux, N.; Reyes, M.; Tabone, E.; Tsagkarakou, A.; Niño, L.; Lombaert, E. The tomato borer, Tuta absoluta, invading the Mediterranean Basin, originates from a single introduction from Central Chile. Sci. Rep. 2015, 5, 8371. [Google Scholar] [CrossRef] [Green Version]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Guimapi, R.Y.A.; Mohamed, S.A.; Okeyo, G.O.; Ndjomatchoua, F.T.; Ekesi, S.; Tonnang, H.E.Z. Modeling the risk of invasion and spread of Tuta absoluta in Africa. Ecol. Complex. 2016, 28, 77–93. [Google Scholar] [CrossRef]
- Abbes, K.; Harbi, A.; Chermiti, B. The tomato leafminer Tuta absoluta, (Meyrick) in Tunisia: Current status and management strategies. Bull. OEPP 2012, 42, 226–233. [Google Scholar] [CrossRef]
- Shashank, P.R.; Suroshe, S.; Singh, S.; Chandrashekar, K.; Nebapure, N.M.; Meshram, N. Report of invasive tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae) from Northern India. Indian J. Agric. Sci. 2016, 86, 1635–1636. [Google Scholar]
- Bajracharya, A.; Mainali, R.P.; Bhat, B.; Bista, S.; Shashank, P.R.; Meshram, N. The first record of South American tomato leaf miner, Tuta absoluta (Meyrick 1917) (Lepidoptera: Gelechiidae) in Nepal. J. Entomol. Zool. Stud. 2016, 4, 1359–1363. [Google Scholar]
- Sankarganesh, E.; Firake, D.M.; Sharma, B.; Verma, V.K.; Behere, G.T. Invasion of the South American Tomato Pinworm, Tuta absoluta, in North-eastern India: A new challenge and biosecurity concerns. Entomol. Gen. 2017, 36, 335–345. [Google Scholar] [CrossRef]
- CABI. Tomato Leafminer (Tuta absoluta): IMPACTS and Coping Strategies for Africa; CABI: Wallingford, UK, 2019. [Google Scholar]
- Mohamed, E.S.I.; Mohamed, M.E.; Gamiel, S.A. First record of the tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Sudan. EPPO Bull. 2012, 42, 325–327. [Google Scholar] [CrossRef]
- El-Arnaouty, S.A.; Pizzol, J.; Galal, H.H.; Kortam, M.N.; Afifi, A.I.; Beyssat, V.; Desneux, N.; Biondi, A.; Heikal, I.H. Assessment of two Trichogramma species for the control of Tuta absoluta in North African tomato greenhouses. Afr. Entomol. 2014, 22, 801–809. [Google Scholar] [CrossRef]
- Pfeiffer, D.G.; Muniappan, R.; Sall, D.; Diatta, P.; Diongue, A.; Dieng, E.O. First Record of Tuta absoluta (Lepidoptera: Gelechiidae) in Senegal. Fla. Entomol. 2013, 96, 661–662. [Google Scholar] [CrossRef]
- Tumuhaise, V.; Khamis, F.M.; Agona, A.; Sseruwu, G.; Mohamed, S.A. First record of Tuta absoluta (Lepidoptera: Gelechiidae) in Uganda. Int. J. Trop. Insect Sci. 2016, 36, 135–139. [Google Scholar] [CrossRef]
- Chidege, M.; Al-zaidi, S.; Hassan, N.; Julie, A.; Kaaya, E.; Mrogoro, S. First record of tomato leaf miner Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Tanzania. Agric. Food Secur. 2016, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- IPPC. First detection of Tuta absoluta in South Africa. Report ZAF-31/1. International Plant Protection Convention. 2016. Available online: https://www.ippc.int/en/countries/south-africapestreports/2016/09/first-detection-oftuta-absoluta-in-south-africa (accessed on 30 March 2020).
- Mutamiswa, R.; Machekano, H.; Nyamukondiwa, C. First report of tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae), in Botswana. Agric. Food Secur. 2017, 6, 49. [Google Scholar] [CrossRef] [Green Version]
- Visser, D.; Uys, V.M.; Nieuwenhuis, R.J.; Pieterse, W. First records of the tomato leaf miner Tuta absoluta (Meyrick. (Lepidoptera: Gelechiidae) in South Africa. Biol. Invasions 2017, 6, 301–305. [Google Scholar]
- Zhang, G.; Ma, D.; Wang, Y.; Gao, Y.; Liu, W.; Zhang, R.; FU, W.; Xian, X.; Wang, J.; Kuang, M.; et al. First report of the South American tomato leafminer, Tuta absoluta (Meyrick), in China. J. Integr. Agric. 2020, 19, 1912–1917. [Google Scholar] [CrossRef]
- Tarusikirwa, V.L.; Mutamiswa, R.; English, S.; Chidawanyika, F.; Nyamukondiwa, C. Thermal plasticity in the invasive south American tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Therm. Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.S.I.; Mahmoud, M.E.E.; Elhaj, M.A.M.; Mohamed, S.A.; Ekesi, S. Host plants record for tomato leaf miner Tuta absoluta (Meyrick) in Sudan. EPPO Bull. 2015, 45, 108–111. [Google Scholar] [CrossRef]
- Özgökçe, M.S.; Bayindir, A.; Karaca, I. Temperature-dependent development of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on tomato plant Lycopersicon esculentum Mill. (Solanaceae). Turkish J. Entomol. 2016, 40, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Machekano, H.; Mutamiswa, R.; Nyamukondiwa, C. Evidence of rapid spread and establishment of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in semi-arid Botswana. Agric. Food Secur. 2018, 7, 48. [Google Scholar] [CrossRef]
- Tonnang, H.E.Z.; Mohamed, S.A.; Khamis, F.; Ekesi, S. Identification and risk assessment for worldwide invasion and spread of Tuta absoluta with a focus on Sub-Saharan Africa: Implications for phytosanitary measures and management. PLoS ONE 2015, 10, e0138319. [Google Scholar] [CrossRef]
- Biondi, A.; Desneux, N. Special issue on Tuta absoluta: Recent advances in management methods against the background of an ongoing worldwide invasion. J. Pest Sci. 2019, 92, 1313–1315. [Google Scholar] [CrossRef] [Green Version]
- Machekano, H.; Mvumi, B.M.; Nyamukondiwa, C. Plutella xylostella (L.): Pest status, control practices, perceptions and knowledge on existing and alternative management options in arid small-scale farming environments. Int. J. Pest Manag. 2020, 66, 48–64. [Google Scholar] [CrossRef]
- Silva, G.A.; Picanco, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Cocco, A.; Deliperi, S.; Delrio, G. Control of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in greenhouse tomato crops using the mating disruption technique. J. Appl. Entomol. 2013, 137, 16–28. [Google Scholar] [CrossRef]
- Bawin, T.; Dujeu, D.; De Backer, L.; Francis, F.; Verheggen, F.J. Ability of Tuta absoluta (Lepidoptera: Gelechiidae) to develop on alternative host plant species. Can. Entomol. 2016, 148, 434–442. [Google Scholar] [CrossRef]
- Margni, M.; Rossier, D.; Crettaz, P.; Jolliet, O. Life cycle impact assessment of pesticides on human health and ecosystems. Agric. Ecosyst. Environ. 2002, 93, 379–392. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, 141–147. [Google Scholar]
- Guedes, R.N.C.; Picanço, M.C. The tomato borer Tuta absoluta in South America: Pest status, management and insecticide resistance. Bull. OEPP/EPPO 2012, 42, 211–216. [Google Scholar] [CrossRef]
- Roditakis, E.; Vasakis, E.; García-Vidal, L.; del Rosario Martínez-Aguirre, M.; Rison, J.L.; Haxaire-Lutun, M.O.; Nauen, R.; Tsagkarakou, A.; Bielza, P. A four-year survey on insecticide resistance and likelihood of chemical control failure for tomato leafminer T. absoluta in the European/Asian region. J. Pest Sci. 2018, 91, 421–435. [Google Scholar]
- Lietti, M.M.M.; Botto, E.; Alzogaray, R.A. Insecticide resistance in Argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2005, 34, 113–119. [Google Scholar] [CrossRef]
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First report of T. absoluta resistance to diamide insecticides. J. Pest Sci. 2015, 88, 9–16. [Google Scholar] [CrossRef]
- Biondi, A.; Mommaerts, V.; Smagghe, G.; Viñuela, E.; Zappalà, L.; Desneux, N. The non-target impact of spinosyns on beneficial arthropods. Pest Manag. Sci. 2012, 68, 1523–1536. [Google Scholar] [CrossRef]
- van der Velden, N.; Suay, R.; Urbaneja, A.; Giorgini, M.; Ruocco, M.; Poncet, C.; Lefèvre, A. Recent Developments and Market Opportunities for IPM in Greenhouse Tomatoes in Southern Europe; Consequences for Advanced IPM Toolboxes and Greenhouse Engineering; LEI Memorandum 12-077; LEI Wageningen UR: The Hague, The Netherlands, 2012; p. 41. [Google Scholar]
- Mossa, A.T.H. Green pesticides: Essential oils as biopesticides in insect-pest management. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.A.; Campos, M.R.; Passos, L.C.; Carvalho, G.A.; Haro, M.M.; Lavoir, A.V.; Biondi, A.; Zappalà, L.; Desneux, N. Botanical insecticide and natural enemies: A potential combination for pest management against Tuta absoluta. J. Pest. Sci. 2019, 92, 1433–1443. [Google Scholar] [CrossRef] [Green Version]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex Pheromones and Their Impact on Pest Management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Tsoulnara, D.; Port, G. Efficacy of a Beauveria bassiana strain, Bacillus thuringiensis and their combination against the tomato leafminer Tuta absoluta. Entomol. Hell. 2016, 25, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Hikal, W.M.; Baeshen, R.S.; Said-Al Ahl, H.A.S. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017, 3, 1404274. [Google Scholar] [CrossRef]
- Kamali, S.; Karimi, J.; Koppenhöfer, A.M. New Insight into the Management of the Tomato Leaf Miner, Tuta absoluta (Lepidoptera: Gelechiidae) with Entomopathogenic Nematodes. J. Econ. Entomol. 2018, 111, 112–119. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Roditakis, E.; Campos, M.R.; Haddi, K.; Bielza, P.; Siqueira, H.A.A.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. J. Pest. Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Deng, A.; Ogendo, J.O.; Owuor, G.; Bett, P.K.; Omolo, E.O.; Mugisha-Kamatenesi, M.; Mihale, J.M. Factors determining the use of botanical insect pest control methods by small-holder farmers in the Lake Victoria basin, Kenya. J. Environ. Sci. Technol. 2009, 3, 108–115. [Google Scholar]
- Stevenson, P.; Isman, M.B.; Belmain, S. Pesticidal plants in Africa: A global vision of new Biol. Cntrol products from local uses. Ind. Crops Prod. 2017. [Google Scholar] [CrossRef]
- FAO. The State of Food Insecurity in the World: Economic Growth Is Necessary But Not Sufficient to Accelerate Reduction of Hunger and Malnutrition; FAO: Rome, Italy, 2012. [Google Scholar]
- Ndor, D.C. Incidence of Tomato leaf miner (Tuta absoluta: Meyrick) damage on Tomato fields in Pankshin and Kanke Local Government Areas of Plateau State. Agric. Sci. Res. J. 2018, 8, 15–19. [Google Scholar]
- Weinberger, K.; Lumpkin, T.A. Diversification into horticulture and poverty reduction: A research agenda. World Dev. 2007, 35, 1464–1480. [Google Scholar] [CrossRef]
- Bala, I.; Mukhtar, M.; Saka, H.; Abdullahi, N.; Ibrahim, S. Determination of insecticide susceptibility of field populations of tomato leaf miner (Tuta absoluta) in Northern Nigeria. Agriculture 2019, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT (Food Agric. Org. U. N.). FAOSTAT Statistics Database; FAOSTAT: Rome, Italy, 17 May 2017; Available online: http://www.fao.org/faostat (accessed on 24 April 2017).
- Aigbedion-Atalor, P.O.; Mohameda, A.S.; Hill, M.P.; Zaluckic, M.P.; Azrag, A.G.A.; Srinivasan, R.; Ekesi, S. Host stage preference and performance of Dolichogenidea gelechiidivoris (Hymenoptera: Braconidae), a candidate for classical Biol. Cntrol of Tuta absoluta in Africa. Biol. Control 2020, 144, 104215. [Google Scholar] [CrossRef]
- Shahbaz, M.; Nouri-Ganbalani, G.; Naseri, B. Comparative damage and digestive enzyme activity of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on 12 tomato cultivars. Entomol. Res. 2017, 49, 401–408. [Google Scholar] [CrossRef]
- Kaoud, H.A. Alternative methods for the control of Tuta absoluta. Glob. J. Multidiscip. Appl. Sci. 2014, 2, 41–46. [Google Scholar]
- Sanda, N.B.; Sunusi, M.; Hamisu, H.S.; Wudil, B.S.; Sule, H.; Abdullahi, A.M. Biological invasion of tomato leafminer, Tuta absoluta (Meyrick) in Nigeria: Problems and management strategies optimization: A Review. Asian J. Agric. Horti. Res. 2018, 1, 1–14. [Google Scholar]
- Chidege, M.; Abel, J.; Afonso, Z.; Tonini, M.; Fernandez, B. Tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) detected in Namibe Province Angola. J. Appl. Life Sci. 2017, 12, 1–5. [Google Scholar] [CrossRef] [Green Version]
- European and Mediterranean Plant Protection Organization. EPPO A1 and A2 List of Pests Recommended for Regulation as Quarantine Pests. 2014. Available online: http://www.eppo.int/QUARANTINE/listA2.htm (accessed on 1 December 2014).
- Xian, X. The Potential Invasion Risk of the Tomato Leafminer Tuta absoluta in China; Institute of Plant Protection, Chinese Academy of Agricultural Science: Beijing, China, 2017. [Google Scholar]
- Karadjova, O.; Ilieva, Z.; Krumov, V.; Petrova, E.; Ventsislavov, V. Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae): Potential for entry, establishment and spread in Bulgaria. Bulg. J. Agric. Sci. 2013, 19, 563–571. [Google Scholar]
- Potting, R.P.J.; van der Gaag, D.J.; Loomans, A.; van der Straten, M.; Anderson, H.; MacLeod, A.; Castrillón, J.M.G.; Cambra, G.V. Tuta absoluta, Tomato Leaf Miner Moth or South American Tomato Moth—Pest Risk Analysis for Tuta absoluta; Ministry of Agriculture, Nature and Food Quality, Plant Protection Service of the Netherlands: Utrecht, The Netherlands, 2013.
- Illakwahhi, D.T.; Srivastava, B.B.L. Control and management of tomato leafminer—Tuta absoluta (Meyrick) (Lepidoptera, Gelechiidae). A Review. IOSR J. Appl. Chem. 2017, 10, 14–22. [Google Scholar] [CrossRef]
- Retta, A.N.; Berhe, D.H. Tomato leaf miner–Tuta absoluta (Meyrick), a devastating pest of tomatoes in the highlands of Northern Ethiopia: A call for attention and action. Res. J. Agric. Environ. Manag. 2015, 4, 264–269. [Google Scholar]
- Davies, K.F.; Chesson, P.; Harrison, S.; Inouye, B.D.; Melbourne, B.A.; Rice, K.J. Spatial heterogeneity explains the scale dependence of the native-exotic diversity relationship. Ecology 2005, 86, 1602–1610. [Google Scholar] [CrossRef]
- Richardson, D.; Pysek, P. Plant invasions: Merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geogr. 2006, 30, 409–431. [Google Scholar] [CrossRef]
- Crespo-Pérez, V.; Rebaudo, F.; Silvain, J.-F.; Dangles, O. Modeling invasive species spread in complex landscapes: The case of potato moth in Ecuador. Landsc. Ecol. 2011, 26, 1447–1461. [Google Scholar] [CrossRef]
- Osawa, T.; Mitsuhashi, H.; Niwa, H. Many alien invasive plants disperse against the direction of stream flow in riparian areas. Ecol. Complex. 2013, 15, 26–32. [Google Scholar] [CrossRef]
- Ward, N.L.; Masters, G.J. Linking climate change and species invasion: An illustration using insect herbivores. Glob. Chang. Biol. 2007, 13, 1605–1615. [Google Scholar] [CrossRef]
- Bacon, S.J.; Aebi, A.; Calanca, P.; Bacher, S. Quarantine arthropod invasions in Europe: The role of climate, hosts and propagule pressure. Divers. Distrib. 2014, 20, 84–94. [Google Scholar] [CrossRef]
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukat, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Santana, P.A.; Kumar, L.; Da Silva, R.S.; Picanço, M.C. Global geographic distribution of Tuta absoluta as affected by climate change. J. Pest. Sci. 2019, 92, 1373–1385. [Google Scholar] [CrossRef]
- Barrientos, Z.R.; Apablaza, H.J.; Norero, S.A.; Estay, P.P. Threshold temperature and thermal constant for development of the South American tomato moth, Tuta absoluta (Lepidoptera, Gelechiidae). Cienc. Investig. Agrar. 1998, 25, 133–137. [Google Scholar] [CrossRef]
- Martins, J.C.; Picanço, M.C.; Bacci, L.; Guedes, R.N.C.; Santana, P.A., Jr.; Ferreira, D.O.; Chediak, M. Life table determination of thermal requirements of the tomato borer Tuta absoluta. J. Pest Sci. 2016, 89, 897–908. [Google Scholar] [CrossRef]
- Krechemer, F.S.; Foerster, L.A. Tuta absoluta (Lepidoptera: Gelechiidae): Thermal requirements and effect of temperature on development, survival, reproduction and longevity. Eur. J. Entomol. 2015, 112, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Notz, A.P. Distribution of eggs and larvae of Scrobipalpula absoluta in potato plants. Revista de la Facultad de Agronomia (Maracay) 1992, 18, 425–432. [Google Scholar]
- Cuthbertson, A.G.; Mathers, J.J.; Blackburn, L.F.; Korycinska, A.; Luo, W.; Jacobson, R.J.; Northing, P. Population Development of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under Simulated UK Glasshouse Conditions. Insects 2013, 4, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylla, S.; Seydi, O.; Diarra, K.; Brevault, T. Seasonal decline of the tomato leafminer, Tuta absoluta, in the shifting landscape of a vegetable-growing area. Entomol. Exp. Appl. 2018, 66, 638–647. [Google Scholar] [CrossRef]
- Lee, C.E. Evolutionary genetics of invasive species. Trends Ecol. Evol. 2002, 17, 386–391. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Kleynhans, E.; Terblanche, J.S. Phenotypic plasticity of thermal tolerance contributes to the invasion potential of Mediterranean fruit flies (Ceratitis capitata). Ecol. Entomol. 2010, 35, 565–575. [Google Scholar] [CrossRef]
- Weldon, C.W.; Boardman, L.; Marlin, D.; Terblanche, J.S. Physiological mechanisms of dehydration tolerance contribute to the invasion potential of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) relative to its less widely distributed congeners. Front. Zool. 2016, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Crowl, T.A.; Crist, T.O.; Parmenter, R.R.; Belovsky, G.; Lugo, A.E. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Environ. 2008, 6, 238–246. [Google Scholar] [CrossRef]
- Olyarnik, S.V.; Bracken, M.E.; Byrnes, J.E.; Hughes, A.R.; Hultgren, K.M.; Stachowicz, J.J. Ecological Factors Affecting Community Invasibility. In Biological Invasions in Marine Ecosystems; Rilov, G., Crooks, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 215–238. [Google Scholar]
- Chown, S.L.; Terblanche, J.S. Physiological Diversity in Insects: Ecological and evolutionary contexts. Adv. Insect Physiol. 2007, 33, 50–152. [Google Scholar]
- Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Thermal plasticity potentially mediates the interaction between host Chilo partellus Swinhoe (Lepidoptera: Crambidae) and endoparasitoid Cotesia flavipes Cameron (Hymenoptera: Braconidae) under rapidly changing environments. Pest Manag. Sci. 2018, 74, 1335–1345. [Google Scholar] [CrossRef]
- Tarusikirwa, V.L.; Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Cold hardiness of the South American tomato pinworm Tuta absoluta (Lepidoptera: Gelechiidae): Both larvae and adults are chill-susceptible. Pest Manag. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pereya, P.C.; Sanchez, N.E. Effect of two Solanaceous plants on development and population parameters of the tomato leaf miner (Tuta absoluta Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2006, 35, 671–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tropea Garzia, G.; Siscaro, G.; Biondi, A.; Zappalà, L. Tuta absoluta, a South American pest of tomato now in the EPPO region: Biology, distribution and damage. Bull. OEPP/EPPO 2012, 42, 205–210. [Google Scholar] [CrossRef]
- Van Damme, V.; Berkvens, N.; Moerkens, R.; Berckmoes, E.; Wittemans, L.; De Vis, R.; Casteels, H.; Tirry, L.; De Clercq, P. Overwintering potential of the invasive leafminer Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) as a pest in greenhouse tomato production in Western Europe. J. Pest. Sci. 2015, 88, 533–541. [Google Scholar] [CrossRef]
- Uchôa-Fernandes, M.A.; Della Lucia, T.M.C.; Vilela, E.F. Mating, oviposition and pupation of Scrobipalpula absoluta (Meyrick) (Lepidoptera: Gelechiidae). An. Soc. Entomol. Bras. 1995, 24, 159–164. [Google Scholar]
- Engelbrecht, F.; Adegoke, J.; Bopape, M.J.; Naidoo, M.; Garland, R.; Thatcher, M.; Gatebe, C. Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ. Res. Lett. 2015, 10, 085004. [Google Scholar] [CrossRef]
- Middleton, B.A. Invasive Plant Species. In Encyclopedia of Ecology, 2nd ed.; Fath, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 431–440. [Google Scholar]
- Lockwood, J.L.; Cassey, P.; Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 2005, 20, 223–228. [Google Scholar] [CrossRef]
- Sax, D.F.; Stachowicz, J.J.; Brown, J.H.; Bruno, J.F.; Dawson, M.N.; Gaines, S.D.; Grosberg, R.K.; Hastings, A.; Holt, R.D.; Mayfield, M.M.; et al. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 2007, 22, 465–471. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Selvaraj, S.; Ganeshamoorthi, P.; Pandiaraj, T. Potential impacts of recent climate change on biological control agents in agro-ecosystem: A review. Int. J. Biodivers. Conserv. 2013, 5, 845–852. [Google Scholar]
- Wolfe, L.M. Why alien invaders succeed: Support for the escape-from-enemy hypothesis. Am. Nat. 2002, 160, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Torchin, M.E.; Lafferty, K.D.; Dobson, A.P.; McKenzie, V.J.; Kuris, A.M. Introduced species and their missing parasites. Nature 2003, 421, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Colautti, R.I.; Ricciardi, A.; Grigorovich, I.A.; MacIsaac, H.J. Is invasion success explained by the enemy release hypothesis? Ecol. Lett. 2004, 7, 721–733. [Google Scholar] [CrossRef]
- Meijer, K.; Schilthuizen, M.; Beukeboom, L.; Smit, C. A review and meta-analysis of the enemy release hypothesis in plant–herbivorous insect systems. PeerJ 2016, 4, e2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zappalá, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arnò, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y.; et al. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Salas Gervassio, N.G.; Aquino, D.; Vallina, C.; Biondi, A.; Luna, M.G. A re-examination of Tuta absoluta parasitoids in South America for optimized biological control. J. Pest. Sci. 2019, 92, 1343–1357. [Google Scholar] [CrossRef]
- Idriss, G.E.A.; Mohamed, S.A.; Khamis, F.; Du Plessis, H.; Ekesi, S. Biology and performance of two indigenous larval parasitoids on Tuta absoluta (Lepidoptera: Gelechiidae) in Sudan. Biocontrol Sci. Technol. 2018, 28, 614–628. [Google Scholar] [CrossRef]
- Oke, O.A.; Kolawole, R.O.; Ogunremi, O.A.; Akinsola, O.A.; Awe, S.A. Detection of Apanteles spp (Hymenoptera: Braconidae) larval parasitoid of tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae) on greenhouse tomato in Abeokuta, Ogun state, Nigeria. In Proceedings of the 25th International Congress of Entomology–Program Book, Orlando, FL, USA, 25–30 September 2016; p. 318. [Google Scholar]
- Zlof, V.; Suffert, M. Report of the EPPO/FAO/IOBC/NEPPO Joint International Symposium on management of Tuta absoluta (tomato borer). Bull. OEPP/EPPO 2012, 42, 203–204. [Google Scholar] [CrossRef]
- Husariu, V.; Bădulescu, L.; Ciceoi, R. Tuta absoluta (Lepidoptera: Gelechiidae)—What impact for biodiversity? In Proceedings of the International Symposium ISB-Inma-Teh Agricultural and Mechanical Engineering, Bucharest, Romania, 26–28 October 2017. [Google Scholar]
- Abbes, K.; Harbi, A.; Elimem, M.; Hafsi, A.; Chermiti, B. Bioassay of three solanaceous weeds as alternative hosts for the invasive tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae) and insights on their carryover potential. Afr. Entomol. 2016, 24, 334–342. [Google Scholar] [CrossRef]
- Drouai, H.; Mimeche, F.; Zedam, A.; Mimeche, H.; Mohammed Belhamra and Mohamed Biche. New floristic records of Tuta absoluta Meyrick 1917, in Zibans’s Oasis (Biskra Algeria). J. Entomol. Zool. Stud. 2016, 4, 130–132. [Google Scholar]
- Younes, A.A.; Zohdy, Z.M.N.; Abul, F.H.; Fathy, R. Preference and Performance of the Tomato Leafminer, Tuta absoluta (Lepidoptera—Gelechiidae) Towards Three Solanaceous Host Plant Species. CPQ Microbiol. 2018, 1, 1–16. [Google Scholar]
- Gebremariam, G. Tuta absoluta: A global looming challenge in tomato production, Review paper. J. Biol. Agric. Healthc. 2015, 5, 57–62. [Google Scholar]
- Galarza, J. Evaluacion en laboratorio de Algunas Plantas Solanaceas. Posibles Hospederas de la Polilla del Tomate Scrobipapula absoluta (Meyr.) (Lepidoptera: Gelechiidae). IDIA 1984, 421–424, 30–32. [Google Scholar]
- Fernandez, S.; Montagne, A. Preferencia de oviposicion de las hembras y duracion, crecimiento y sobrevivencia de las larvas de Scrobipalpula absoluta (Meyrick). Bol. Entomol. Venez NS 1990, 5, 89–99. [Google Scholar]
- Loni, A.; Rossi, E.; Van Achterberg, C. First report of Agathis fuscipennis in Europe as parasitoid of the tomato leafminer Tuta absoluta. Bull. Insectology 2011, 64, 115–117. [Google Scholar]
- EPPO. EPPO datasheets on quarantine pests: Tuta absoluta. EPPO Bull. 2005, 35, 434–435. [Google Scholar] [CrossRef]
- Garzia, G.T. Physalis peruviana L. (Solanaceae), a host plant of Tuta absoluta in Italy. In Proceedings of the Integrated Control and Protected Crops, Mediterranean Climate. IOBC/WPRS Working Group Meeting, Crete, Greece, 7–10 September 2009. [Google Scholar]
- Portakaldali, M.; Öztemiz, S.; Kütük, H. A new host plant for Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Turkey. J. Entomol. Res. Soc. 2013, 15, 21–24. [Google Scholar]
- Nitin, K.S.; Chakravarthy, A.K.; Sridhar, V. First report of South American Tomato moth, Tuta absoluta (Meyrick) on French bean from India. J. Appl. Hort. 2017, 19, 253–254. [Google Scholar] [CrossRef]
- Borisade, O.A.; Kolawole, A.O.; Adebo, G.M.; Uwaidem, Y.I. The tomato leafminer (Tuta absoluta) (Lepidoptera: Gelechiidae) attack in Nigeria: Effect of climate change on over-sighted pest or agro-bioterrorism? J. Agric. Ext. Rural Dev. 2017, 9, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Williamson, S.; Ball, A.; Pretty, J. Trends in pesticide use and drivers for safer pest management in four African countries. Crop Prot. 2008, 27, 1327–1334. [Google Scholar] [CrossRef]
- Zekeya, N.; Chacha, M.; Ndakidemi, P.A.; Materu, C.; Chidege, M.; Mbega, E.R. Tomato Leafminer (Tuta absoluta Meyrick 1917): A Threat to Tomato Production in Africa. J. Agric. Ecol. Res. Int. 2017, 10, 1–10. [Google Scholar]
- Siqueira, H.A.A.; Guedes, R.N.C.; Picanço, M.C. Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric. Forest Entomol. 2000, 2, 147–153. [Google Scholar] [CrossRef]
- Brévault, T.; Achaleke, J.; Sougnabé, S.; Vaissayre, M. Tracking pyrethroid resistance in the polyphagous bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae), in the shifting landscape of a cotton-growing area. Bull. Entomol. Res. 2008, 98, 565–573. [Google Scholar] [CrossRef]
- Haddi, K.; Berger, M.; Bielza, P.; Cifuentes, D.; Field, L.M.; Gorman, K.; Rapisarda, C.; Williamson, M.S.; Affiliations, C.B. Identification of mutations associated with pyrethroid resistance in the voltage-gated sodium channel of the tomato leaf miner (Tuta absoluta). Insect Biochem. Mol. 2012, 42, 506–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roditakis, E.; Skarmoutsou, C.; Staurakaki, M. Toxicity of insecticides to populations of tomato borer Tuta absoluta (Meyrick) from Greece. Pest Manag. Sci. 2013, 69, 834–840. [Google Scholar] [CrossRef] [PubMed]
- El-Wakeil, N.E. Botanical Pesticides and Their Mode of Action. Gesunde Pflanz. 2013, 65, 125–149. [Google Scholar] [CrossRef]
- Bowers, M.D.; Puttick, G.M. Iridoid glycosides and insect feeding preferences: Gypsy moths (Lymantria dispar, Lymantriidae) and buckeyes (Junonia coenia, Nymphalidae). Ecol. Entomol. 1989, 14, 247–256. [Google Scholar] [CrossRef]
- Gould, K.S.; Lister, C. Flavonoid Functions in Plants. In Flavonoids: Chemistry, Biochemistry, and Applications; CRC Press LLC: Boca Raton, FL, USA, 2006; pp. 397–443. [Google Scholar]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Silva, V.C.B.; Ribeiro Neto, J.A.; Alves, S.N.; Li, L.A.R.S. Larvicidal activity of oils, fatty acids, and methyl esters from ripe and unripe fruit of Solanum lycocarpum (Solanaceae) against the vector Culex quinquefasciatus (Diptera: Culicidae). Rev. Soc. Bras. Med. 2015, 48, 610–613. [Google Scholar] [CrossRef] [Green Version]
- Velu, K.; Elumalai, D.; Hemalatha, P.; Babu, M.; Janaki, A.; Kaleena, P.K. Phytochemical screening and larvicidal activity of peel extracts of Arachis hypogaea against chikungunya and malarial vectors. Int. J. Mosq. Res. 2015, 2, 01–08. [Google Scholar]
- Rajashekar, Y.; Bakthavatsalam, N.; Shivanandappa, T. Botanicals as grain protectants. Psyche 2012, 2012, 646740. [Google Scholar] [CrossRef]
- Kona, N.E.M.; Taha, A.K.; Mahmoud, M.E.E. Effects of botanical extracts of Neem (Azadirachta indica) and jatropha (Jatropha curcus) on eggs and larvae of tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Persian Gulf Crop Prot. 2014, 3, 41–46. [Google Scholar]
- Abdel-Baky, N.F.; Al-Soqeer, A.A. Controlling the2nd instar larvae of Tuta absoluta Meyrick (Lepidoptera: Gelechiidae) by Simmondsin extracted from Jojoba seeds in KSA. J. Entomol. 2017, 14, 73–80. [Google Scholar]
- Shiberu, T.; Getu, E. Evaluation of Bio-Pesticides on Integrated Management of Tomato Leafminer, Tuta absoluta (Meyrick) (Gelechiidae: Lepidoptera) on Tomato Crops in Western Shewa of Central Ethiopia. Entomol. Ornithol. Herpetol. 2018, 7, 206. [Google Scholar] [CrossRef]
- De Brito, E.F.; Baldin, E.L.L.; de Carvalho Macedo Silva, R.; do Prado Ribeiro, L.; Vendramim, J.D. Bioactivity of Piper extracts on Tuta absoluta (Lepidoptera: Gelechiidae) in tomato. Pesq. Agropec. Bras. Brasilia. 2015, 50, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Dyer, L.A.; Palmer, A.N.D. Piper: A Model Genus for Studies of Phytochemistry, Ecology and Evolution; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004; p. 228. [Google Scholar]
- Scott, I.M.; Helson, B.V.; Strunz, G.M.; Finlay, H.; Sanchez-Vindas, P.E.; Poveda, L.; Lyons, D.B.; Philogene, B.J.R.; Arnason, J.T. Efficacy of Piper nigrum (Piperaceae) extract for control of insect defoliators of forest and ornamental trees. Can. Entomol. 2007, 139, 513–522. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M.; Abdel-Razek, A.S.; Ebadah, I.M.A.; Mahmoud, Y.A. Evaluation of some microbial agents, natural and chemical compounds for controlling tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Plant Prot. Res. 2016, 56, 372–379. [Google Scholar] [CrossRef]
- Birhan, A. Tomato leafminer [(Tuta absoluta Meyrick) (Lepidoptera: Gelechiidae)] and its current ecofriendly management strategies: A review. J. Agric. Biotech. Sustain. Dev. 2018, 10, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Zarrad, K.; Chaieb, I.; Tayeb, W.; Chraief, I.; Laarif, A.; Hammami, M.; Haouala, R. Bio-insecticidal potential of essential oils of two citrus species against two greenhouse pests Tuta absoluta Meyrick and Spodoptera littoralis Boisduval. Microbiologie et Hygiène Alimentaire 2013, 25, 84–88. [Google Scholar]
- Rodriguez, S.M.; Gerding, P.; France, A. Seleccio’n de aislamientos de hongos entomopato’genos para el control de huevos de la polilla del tomate, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Agric. Technol. 2005, 66, 151–158. [Google Scholar]
- Gonzalez-Cabrera, J.; Molla, O.; Monton, H.; Urbaneja, A. Efficacy of Bacillus thuringiensis (Berliner) in controlling the tomato borer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). BioControl 2011, 56, 71–80. [Google Scholar] [CrossRef]
- Youssef, N.A.; Hassan, G.M. Bioinsecticide activity of Bacillus thuringiensis isolates on tomato borer, Tuta absoluta (Meyrick) and their molecular identification. Afr. J. Biotechnol. 2013, 12, 3699–3709. [Google Scholar]
- Jacobson, R.J.; Chandler, D.; Fenlon, J.; Russell, K.M. Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae) to control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Sci. Technol. 2001, 11, 391–400. [Google Scholar] [CrossRef]
- Mburu, D.M.; Ochola, N.K.; Maniania, P.G.N.; Gitonga, L.M.; Ndung’u, M.W.; Wanjoya, A.K.; Hassanali, A. Relationship between virulence and repellency of entomopathogenic isolates of Metarhizium anisopliae and Beauveria bassiana to the termite Macrotermes michaelseni. J. Insect Physiol. 2009, 55, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Alsaedi, G.; Ashouri, A.; Talaei-Hassanloui, R. Evaluation of Bacillus thuringiensis to control Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under laboratory conditions. Agric. Sci. 2017, 8, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Racke, K.D. A Reduced Risk Insecticide for Organic Agriculture: Spinosad Case Study. In Crop Prot. Products for Organic Agriculture: Environmental, Health and Efficacy Assessment; Felsot, A.S., Racke, K.D., Eds.; ACS: Washington, DC, USA, 2006; pp. 92–108. [Google Scholar]
- Baniameri, V.; Cheraghian, A. The first report and control strategies of Tuta absoluta in Iran. EPPO Bull. 2012, 2, 322–324. [Google Scholar] [CrossRef]
- Biondi, A.; Zappalá, L.; Stark, J.D.; Desneux, N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef] [Green Version]
- Caparros Megido, R.; Haubruge, E.; Verheggen, F.J. First evidence of deuterotokous parthenogenesis in the tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Pest Sci. 2012, 85, 409–412. [Google Scholar] [CrossRef]
- CABI. 2013. Available online: https://www.cabi.org/isc/datasheet/49260#tonaturalEnemies (accessed on 7 April 2020).
- Contreras, J.; Mendoza, J.E.; Martínez-Aguirre, M.R.; García-Vidal, L.; Izquierdo, J.; Bielza, P. Efficacy of entomopathogenic fungus Metarhizium anisopliae against Tuta absoluta (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2014, 107, 121–124. [Google Scholar] [CrossRef]
- Gomez, J.; Barrera, G.; Lopez-Feeber, M.; Belaich, M.; Ghiringhelli, P.; Villamizar, L.F. Potential for betabaculoviruses to the control of tomato leafminer Tuta absoluta (Meyrick). J. Appl. Entomol. 2017, 142, 67–77. [Google Scholar] [CrossRef]
- Gozel, C.; Kasap, I. Efficacy of entomopathogenic nematodes against the Tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in tomato field. Turk. Entomol. Derg-Tu. 2015, 39. [Google Scholar] [CrossRef] [Green Version]
- Broekaert, W.F.; Cammue, B.P.A.; De Bolle, M.F.C.; Thevissen, K.; De Samblanx, G.W.; Osborn, R.W. Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 1997, 16, 297–323. [Google Scholar] [CrossRef]
- Adams, B.J.; Nguyen, K.B. Taxonomy and Systematics, in Entomopathogenic Nematology; Gaugler, R., Ed.; CABI: New York, NY, USA, 2002; pp. 1–34. [Google Scholar]
- Boemare, N. Biology, Taxonomy and Systematics of Photorhabdus and Xenorhabdus. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI: New York, NY, USA, 2002; pp. 35–56. [Google Scholar]
- De Waal, J.Y.; Malan, A.P.; Addison, M.F. Efficacy of entomopathogenic nematodes (Rhabditida: Heterorhabditidae and Steinernematidae) against codling moth, Cydia pomonella (Lepidoptera: Tortricidae) in temperate regions. Biocontrol Sci. Technol. 2011, 21, 1161–1176. [Google Scholar] [CrossRef]
- Van Damme, V.M.; Beck, B.K.; Berckmoes, E.; Moerkens, R.; Wittemans, L.; de Vis, R.; Nuyttens, D.; Casteels, H.F.; Maes, M.; Tirry, L.; et al. Efficacy of entomopathogenic nematodes against larvae of Tuta absoluta in the laboratory. Pest Manag. Sci. 2015, 72, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Malan, A.P.; Knoetze, R.; Moore, S.D. Isolation and identification of entomopathogenic nematodes from citrus orchards in South Africa and their biocontrol potential against false codling moth. J. Invertebr. Pathol. 2011, 108, 115–125. [Google Scholar] [CrossRef]
- Nthenga, I.; Knoetze, R.; Berry, S.; Tiedt, L.R.; Malan, A.P. Steinernema sacchari n. sp. (Rhabditida: Steinernematidae), a new entomopathogenic nematode from South Africa. Nematology 2014, 16, 475–494. [Google Scholar] [CrossRef] [Green Version]
- Caparros Megido, R.; Haubruge, E.; Verheggen, F.J. Pheromone-based management strategies to control the tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). A review. Biotechnol. Agron. Soc. Environ. 2013, 17, 475–482. [Google Scholar]
- Mashaly, A.; Ali, M.; Al-Khalifa, M. Trail Pheromones in Pest Control. In New Perspectives in Plant Protection; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Filho, M.M.; Vilelaa, E.F.; Jham, G.N.; Attygalle, A.; Svatos, A.; Meinwald, J. Initial studies of mating disruption of the tomato moth, Tuta absoluta (Lepidoptera: Gelechiidae) using synthetic sex pheromone. J. Braz. Chem. Soc. 2000, 6, 621–628. [Google Scholar] [CrossRef] [Green Version]
- El-aassar, M.R.; Soliman, M.H.A.; Elaal, A.A.A. Efficiency of sex pheromone traps and some bio and chemical insecticides against tomato borer larvae, Tuta absoluta (Meyrick) and estimate the damages of leaves and fruit tomato plant. Ann Agric Sci. 2015, 60, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Cherif, A.; Harbaoui, K.; Zappalà, L.; Grissa-Lebdi, K. Efficacy of mass trapping and insecticides to control Tuta absoluta in Tunisia. J. Plant Dis. Protect. 2018, 125, 51–61. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyck, V.A.; Hendrichs, J.; Robinson, A.S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Knipling, E.F. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 459–467. [Google Scholar] [CrossRef]
- Carabajal Paladino, L.Z.; Ferrari, M.E.; Lauría, J.P.; Cagnotti, C.L.; Šíchová, J.; López, S.N. The Effect of X-Rays on Cytological Traits of Tuta absoluta (Lepidoptera: Gelechiidae). Fla. Entomol. 2016, 99, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Suckling, D.M.; Stringer, L.D.; Mitchell, V.J.; Sullivan, T.E.S.; Sullivan, N.J.; Simmons, G.S.; Barrington, A.M.; El-Sayed, A.M. Comparative fitness of irradiated sterile light brown apple moths (Lepidoptera: Tortricidae) in a wind tunnel, hedgerow and vineyard. J. Econ. Entomol. 2011, 104, 1301–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chidawanyika, F.; Terblanche, J.S. Costs and benefits of thermal acclimation for codling moth, Cydia pomonella (Lepidoptera: Tortricidae): Implications for pest control and the sterile insect release programme. Evol. Appl. 2011, 4, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Cagnotti, C.L.; Andorno, A.V.; Hernández, C.M.; Carabajal Paladino, L.Z.; Botto, E.; López, S. Inherited Sterility in Tuta absoluta (Lepidoptera: Gelechiidae): Pest Population Suppression and Potential for Combined Use with a Generalist Predator. Fla. Entomol. 2016, 99, 87–94. [Google Scholar] [CrossRef]
- Nepgen, E.S. A Study on the Application Technology of the Sterile Insect Technique, with Focus on False Codling Moth, Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae), a Pest of Citrus in South Africa. Master’s Thesis, Rhodes University, Grahamstown, South Africa, 2014; p. 106. [Google Scholar]
- Bloem, K.A.; Bloem, S.; Carpenter, J.E. Impact of Moth Suppression/eradication Programmes Using the Sterile Insect Technique or Inherited Sterility. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 677–700. [Google Scholar]
- Carpenter, J.E. Area-wide Integration of Lepidopteran F1 Sterility and Augmentative Biological Control. In Area-Wide Control of Fruit Flies and Other Insect Pests, Proceedings of the International Conference on Area-Wide Control of Insect Pests, and the 5th International Symposium on Fruit Flies of Economic Importance, Penang, Malaysia, 28 May–5 June 1998; Tan, K.H., Ed.; Penerbit Universiti Sains Malaysia: Pulau Pinang, Malaysia, 2000; pp. 193–200. [Google Scholar]
- Dyck, V.A.; Reyes Flores, J.; Vreysen, M.J.B.; Regidor, E.E.; Barnes, T.T.B.; Riera, P.G.; Lindquist, D.; Loosjes, M. Management of Area-wide Integrated Pest Management Programmes that Integrate the Sterile Insect Technique. In Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 525–538. [Google Scholar]
- Giustolin, T.A.; Vendramim, J.D.; Alves, S.B.; Vieira, S.A.; Pereira, R.M. Susceptibility of Tuta absoluta (Meyrick) (Lepidoptera, Gelechiidae) reared on two species of Lycopersicon to Bacillus thuringiensis var. kurstaki. J. Appl. Entomol. 2001, 125, 551–556. [Google Scholar] [CrossRef]
- Gharekhani, G.H.; Salek-Ebrahimi, H. Evaluating the damage of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on some cultivars of tomato under greenhouse condition. Arch. Phytopathol. Pflanzenschutz. 2014, 47, 429–436. [Google Scholar] [CrossRef]
- Han, P.; Bayram, Y.; Shaltiel-Harpaz, L.; Sohrabi, F.; Saji, A.; Esenali, U.T.; Jalilov, A.; Ali, A.; Shashank, P.R.; Ismoilov, K.; et al. Tuta absoluta continues to disperse in Asia: Damage, ongoing management and future challenges. J. Pest Sci. 2019, 92, 1317–1327. [Google Scholar] [CrossRef]
- Fernandes, M.E.F.; Fernandes, F.L.; Silva, D.J.H.; Picanço, M.; Jhamc, G.N.; Carneiro, P.; Queiroz, R.B. Trichomes and hydrocarbons associated with the tomato plant antixenosis to the leafminer. An. Acad. Bras. Ciências 2012, 84, 201–210. [Google Scholar] [CrossRef]
- Oliveira, F.A.; da Silva, D.J.H.; Leite, G.L.D.; Jham, G.N.; Picanço, M. Resistance of 57 greenhouse-grown accessions of Lycopersicon esculentum and three cultivars to Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Sci. Hortic. 2009, 119, 182–187. [Google Scholar] [CrossRef]
- De Oliveira, C.M.; de Andrade, V.C., Jr.; Maluf, W.R.; Neiva, I.P.; Maciel, G.M. Resistance of tomato strains to the moth Tuta absoluta imparted by allelochemicals and trichome density. Ciênc. Agrotec. 2012, 36, 45–52. [Google Scholar] [CrossRef]
- Sohrabi, F.; Nooryazdan, H.R.; Gharati, B.; Saeidi, Z. Plant resistance to the moth Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in tomato cultivars. Neotrop. Entomol. 2016, 46, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, S.; Fathipour, Y.; Asgari, S. Susceptibility of seven selected tomato cultivars to Tuta absoluta (Lepidoptera: Gelechiidae): Implications for its management. J. Econ. Entomol. 2017, 110, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Proffit, M.; Birgersson, G.; Bengtsson, M.; Reis, R.; Witzgall, P.; Lima, E. Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J. Chem. Ecol. 2011, 37, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.R.; Ament, K.; Sabelis, M.W.; Harin, M.A.; Schuurink, R.C. Differential timing of spider mite-induced direct and indirect defences in tomato plants. Plant Physiol. 2004, 135, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbaneja, A.; González-Cabrera, J.; Arnó, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest Manag. Sci. 2012, 68, 1215–1222. [Google Scholar] [CrossRef]
- Luna, M.G.; Pereyra, P.C.; Coviella, C.E.; Junior, R.R.; Witzgall, P.; Lim, E. Potential of Biological Control agents against Tuta absoluta (Lepidoptera: Gelechiidae): Current knowledge in Argentina. Fla. Entomol. 2015, 98, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Jaworski, C.C.; Chailleux, A.; Bearez, P.; Desneux, N. Apparent competition between major pests reduces pest population densities on tomato crop, but not yield loss. J. Pest Sci. 2015, 88, 793–803. [Google Scholar] [CrossRef] [Green Version]
- Mollá, O.; González-Cabrera, J.; Urbaneja, A. The combined use of Bacillus thuringiensis and Nesidiocoris tenuis against the tomato borer Tuta absoluta. Biol. Control 2011, 56, 883–891. [Google Scholar] [CrossRef]
- Arnó, J.; Sorribas, R.; Prat, M.; Matas, M.; Pozo, C.; Rodríguez, D.; Garreta, A.; Gómez, A.; Gabarra, R. Tuta absoluta, a new pest in IPM tomatoes in the northeast of Spain. IOBC/WPRS Bull. 2009, 49, 203–208. [Google Scholar]
- Parra, J.R.P.; Zucchi, R.A. Trichogramma in Brazil: Feasibility of use after twenty years of research. Neotrop. Entomol. 2004, 33, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Chailleux, A.; Desneux, N.; Seguret, J.; Do Thi Khanh, H.; Maignet, P.; Tabone, E. Assessing European Egg Parasitoids as a Mean of Controlling the Invasive South American Tomato Pinworm Tuta absoluta. PLoS ONE 2012, 7, e48068. [Google Scholar] [CrossRef]
- Pratissoli, D.; Thuler, R.T.; Andrade, G.S.; Zanotti, L.C.M.; da Silva, A.F. Estimate of Trichogramma pretiosum to control Tuta absoluta in stalked tomato. Pesqui. Agropecu. Bras. 2005, 40, 715–718. [Google Scholar] [CrossRef]
- Cabello, T.; Gallego, J.R.; Fernandez, F.J.; Gamez, M.; Vila, E.; Del Pino, M.; Hernandez-Suarez, E. Biological Control strategies for the South American tomato moth (Lepidoptera: Gelechiidae) in greenhouse tomatoes. J. Econ. Entomol. 2012, 105, 2085–2096. [Google Scholar] [CrossRef] [PubMed]
- Mansour, R.; Cherif, A.; Attia-Barhoumi, S.; Zappalà, L.; Grissa-Lebdi, K. Tuta absoluta in Tunisia: Ten years of invasion and pest management. Phytoparasitica 2019, 47, 461–474. [Google Scholar] [CrossRef]
- Colomo, M.V.; Berta, D.C.; Chocobar, M.J. El complejo de himenópteros parasitoides que atacan a la “polilla del tomate” Tuta absoluta (Lepidoptera: Gelechiidae) en la Argentina. Acta Zool. Lilloana. 2002, 46, 81–92. [Google Scholar]
- Luna, M.G.; Sánchez, N.E.; Pereyra, P.C. Parasitism of Tuta absoluta (Lepidoptera: Gelechiidae) by Pseudapanteles dignus (Hymenoptera: Braconidae) under laboratory conditions. Environ. Entomol. 2007, 36, 887–893. [Google Scholar] [CrossRef] [Green Version]
- Mollá, O.; Monton, H.; Beitia Crespo, F.J.; Urbaneja, A. La polilla del tomate Tuta absoluta (Meyrick), una nueva plaga invasora. Terralia 2008, 69, 36–42. [Google Scholar]
- Marchiori, C.H.; Silva, C.G.; Lobo, A.P. Parasitoids of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) collected on tomato plants in larvae, State of Minas Gerais, Brazil. Braz. J. Biol. 2004, 64, 551–552. [Google Scholar] [CrossRef]
- Doğanlar, M.; Yiğit, A. Parasitoid complex of the tomato leaf miner, Tuta absoluta (Meyrick 1917), (Lepidoptera: Gelechiidae) in Hatay, Turkey. Tarim ve Doga Dergisi. 2011, 14, 28–37. [Google Scholar]
- Gabarra, R.; Arnó, J. Resultados de las experiencias de control biológico de la polilla del tomate en cultivo de invernadero y aire libre en Cataluña. Phytoma España. 2010, 217, 65–68. [Google Scholar]
- Zappalá, L.; Bernardo, U.; Biondi, A.; Cocco, A.; Deliperi, S.; Delrio, G.; Giorgini, M.; Pedata, P.C.; Rapisarda, C.; Tropea Garzia, G.; et al. Recruitment of native parasitoids by the exotic pest Tuta absoluta (Meyrick) in Southern Italy. Bull. Insectology 2012, 65, 51–61. [Google Scholar]
- Cáceres, S.; Aguirre, A.; Miño, V.; Almonacid, R. Líneas de trabajo para el manejo integrado de la polilla del tomate en Corrientes. Libro de Resúmenes del Taller: La polilla del tomate en la Argentina: Estado actual del conocimiento y prospectiva para un manejo integrado de plagas; Facultad De Ciencias Naturales Museo, Univesidad Nacional De La Plata: Buenes Aires, Argentina, 2011; p. 7. [Google Scholar]
- Biondi, A.; Chailleux, A.; Lambion, J.; Zappalá, L.; Desneux, N. Indigenous natural enemies attacking Tuta absoluta (Lepidoptera: Gelechiidae) in Southern France. Egypt J. Biol. Pest Control 2013, 23, 117–121. [Google Scholar]
- Gabarra, R.; Arnó, J.; Lara, L.; Verdú, M.J.; Ribes, A.; Beitia, F.; Urbaneja, A.; Téllez, M.M.; Mollá, O.; Riudavets, J. Native parasitoids associated with Tuta absoluta in the tomato production areas of the Spanish Mediterranean Coast. Biocontrol 2013. [Google Scholar] [CrossRef]
- Boualem, M.; Allaoui, H.; Hamadi, R.; Medjahed, M. Biologie et complexe des ennemis naturels de Tuta absoluta á Mostaganem (Algérie). EPPO Bull. 2012, 42, 268–274. [Google Scholar] [CrossRef]
- Riciputi, C. Pomodoro, contro la Tuta tre nuovi predatori naturali. Colt. Protette. 2011, 40, 32–34. [Google Scholar]
- Zouba, A.; Chermiti, B.; Kadri, K.; Fattouch, S. Molecular characterization of Trichogramma bourarachae strains (Hymenoptera: Trichogrammatidae) from open field tomato crops in the South West of Tunisia. Biomirror 2013, 4, 13–19. [Google Scholar]
- Mollá, O.; Alonso, M.; Monton, H.; Beitia, F.; Verdú, M.J.; González-Cabrera, J.; Urbaneja, A. Control Biologico de Tuta absoluta. Catalogacion de enemigos naturales y potencial de los mıridos depredadores como agentes de control. Phytoma Spain 2010, 217, 42–46. [Google Scholar]
- Ferracini, C.; Ingegno, B.L.; Mosti, M.; Navone, P.; Tavella, L.; Alma, A. Promising native candidates for biological control of Tuta absoluta in Italy. IOBC/WPRS Bull. 2012, 80, 51–55. [Google Scholar]
- Pérez-Aguilar, D.A.; Soares, M.A.; Passos, L.C.; Martínez, A.M.; Pineda, S.; Carvalho, G.A. Lethal and sublethal effects of insecticides on Engytatus varians (Heteroptera: Miridae), a predator of Tuta absoluta (Lepidoptera: Gelechiidae). Ecotoxicology 2018, 27, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Cabello, T.; Gallego, J.R.; Fernandez-Maldonado, F.J.; Soler, A.; Beltran, D.; Parra, A.; Vila, E. The damsel bug Nabis pseudoferus (Hem.: Nabidae) as a new biological control agent of the South American Tomato Pinworm, Tuta absoluta (Lep.: Gelechiidae), in tomato crops of Spain. IOBC/WPRS Bull. 2009, 49, 219–223. [Google Scholar]
- Al-Jboory, I.J.; Katbeh-Bader, A.; Al-Zaidi, S. First observation and identification of some natural enemies collected from heavily infested tomato by Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in Jordan. Middle East J. Sci. Res. 2012, 11, 435–438. [Google Scholar]
- Braham, M.; Hajji, L. Management of Tuta absoluta (Lepidoptera, Gelechiidae) with Insecticides on Tomatoes; Insecticides.—Pest Engineering; Perveen, F., Ed.; InTech: Rijeka, Croitia, 2012; pp. 1–23. ISBN 978-953-307-895-3. [Google Scholar]
- Radwan, E.; Taha, H. Toxic and biochemical effects of different insecticides on the tomato leafminer, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Egypt Acad. J. Biol. Sci. 2012, 4, 1–10. [Google Scholar] [CrossRef]
- Deleva, E.A.; Harizanova, V.B. Efficacy Evaluation of Insecticides on Larvae of the Tomato Borer Tuta absoluta, Meyrick (Lepidoptera: Gelechiidae) under Laboratory Conditions. J. Int. Sci. Publ. Agric. Food. 2014, 2. Available online: http://www.scientificpublications.net (accessed on 13 May 2020).
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M. Plant Resistance to Arthropods: Molecular and Conventional Approaches; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Fusire, M. Integrated Pest Management: Cost-Saving Techniques for Small Holder Farmers; Community Technology Development Trust: Harare, Zimbabwe, 2008. [Google Scholar]
- Bale, J.S. Harmonization of regulations for invertebrate biocontrol agents in Europe: Progress, problems and solutions. J. Appl. Entomol. 2011, 135, 503–513. [Google Scholar] [CrossRef]
- Cherry, A.J.; Gwynn, R.L. Perspectives on the development of Biological Control agents in Africa. Biocontrol Sci. Technol. 2007, 17, 665–676. [Google Scholar] [CrossRef]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. Biol. Control 2012, 57, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mason, P.J.; Everatt, M.J.; Loomans, A.J.M.; Collatz, J. Harmonizing the regulation of invertebrate biological control agents in the EPPO region: Using the NAPPO region as a model. Bull. OEPP/EPPO 2017, 47, 79–90. [Google Scholar] [CrossRef]
- Benjamin, E.O.; Wesseler, J.H.H. A socioeconomic analysis of biocontrol in integrated pest management: A review of the effects of uncertainty, irreversibility and flexibility. NJAS—Wagen. J. Life Sci. 2016, 77, 53–60. [Google Scholar] [CrossRef]
- Gwynn, R.; Maniania, J.K. Africa with special reference to Kenya. In Use and Regulation of Microbial Pesticides in Representative Jurisdictions Worldwide; Kabuluk, T., Svircev, A., Goettel, M., Woo, S.G., Eds.; IOBC Global: St. Paul, MN, USA, 2010; pp. 12–17. [Google Scholar]
- Grzywacz, D.; Stevenson, P.C.; Mushobozi, W.L.; Belmain, S.; Wilson, K. The use of indigenous ecological resources for pest control in Africa. Food Secur. 2014, 6, 71–86. [Google Scholar] [CrossRef] [Green Version]
- Sola, P.; Mvumi, B.M.; Ogendo, J.O.; Mponda, O.; Kamanula, J.F.; Nyirenda, S.P.; Belmain, S.R.; Stevenson, P.C. Botanical pesticide production, trade and regulatory mechanisms in sub-Saharan Africa: Making a case for plant based pesticidal products. Food Secur. 2014, 6, 369–384. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic Value of Biological Control in Integrated Pest Management of Managed Plant Systems. Annu. Rev. Entomol. 2015, 60, 621–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoabeng, B.W.; Gurr, G.M.; Gitau, C.W.; Stevenson, P.C. Cost: Benefit analysis of botanical insecticide use in cabbage: Implications for smallholder farmers in developing countries. Crop Prot. 2014, 57, 71–76. [Google Scholar] [CrossRef]
- Belmain, S.; Stevenson, P. Ethnobotanicals in Ghana: Reviving and Modernizing Age-Old Farmer, Practice. Pestic. Outlook 2001, 12, 233–238. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). International Code of Conduct on Pesticide Management: Guidelines for the Registration of Microbial, Botanical and Semiochemical Pest Control Agents for Plant Protection and Public Health Uses. Rome, Italy. 2017. Available online: www.fao.org/publications (accessed on 23 April 2020).
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2345283. [Google Scholar] [CrossRef] [Green Version]
- Cannell, E. European farmers plough ahead: Pesticide use reduction. Pestic. News 2007, 78. Available online: http://www.pan-Europe.info (accessed on 16 March 2011).
- De Groote, H.; Douro-Kpindou, O.K.; Ouambama, Z.; Gbongboui, C.; Müller, D.; Attignon, S.; Lomer, C. Assessing the feasibility of biological control of locusts and grasshoppers in West Africa: Incorporating the farmers’ perspective. Agric. Hum. Values 2001, 18, 413–428. [Google Scholar] [CrossRef]
- Jussaume, R.A.; Glenna, L. Considering structural, individual and social network explanations for ecologically sustainable agriculture: An example drawn from Washington State wheat growers. Sustainability 2009, 1, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Grogan, K.A. When ignorance is bliss: Pest control decisions involving beneficial insects. Ecol. Econ. 2014, 107, 104–113. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Heong, K.L.; Sanchez-Bayo, F.; Bianchi, F.J.J.A.; Lundgren, J.G.; Bentley, J.W. Ecological illiteracy can deepen farmers’ pesticide dependency. Environ. Res. Lett. 2019, 14, 093004. [Google Scholar] [CrossRef]
- Grzywacz, D.; Cherry, A.C.; Gwynn, R. Biological pesticides for Africa: Why has so little of the research undertaken to date led to new products to help Africa’s poor? Pestic. Outlook 2009, 20, 77–81. [Google Scholar] [CrossRef]
- Leng, P.; Zhang, Z.; Pan, G.; Zhao, M. Applications and development trends in biopesticides. Afr. J. Biotechnol. 2011, 10, 19864–19873. [Google Scholar]
- McConnachie, A.J.; de Wit, M.P.; Hill, M.P.; Byrne, M.J. Economic evaluation of the successful biological control of Azolla filiculoides in South Africa. Biol. Control 2003, 28, 25–32. [Google Scholar] [CrossRef]
- TEEB (The Economics of Ecosystems and Biodiversity). Ecological and Economic Foundation; Earthscan: Cambridge, UK, 2020. [Google Scholar]
- Pimentel, D. Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Rahaman, P.F.; Sharma, S.B.; Wightman, J.A. A review of insect parasitic nematodes research in India: 1927-1997. Int. J. Pest Manag. 2000, 46, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Lacey, L.A.; Georgis, R. Entomopathogenic Nematodes for Control of Insect Pests Above and Below Ground with Comments on Commercial Production. J. Nematol. 2012, 44, 218–225. [Google Scholar]
- Knox, C.; Moore, S.D.; Luke, G.A.; Hill, M.P. Baculovirus-based strategies for the management of insect pests: A focus on development and application in South Africa. Biocontrol Sci. Technol. 2015, 25, 1–20. [Google Scholar] [CrossRef]
- Copping, L.G.; Menn, J.J. Biopesticides: A review of their action, applications and efficacy. Pest Manag. Sci. 2000, 56, 651–676. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; van Lenteren, J.C. The status of Biological Control and recommendations for improving uptake for the future. Biol. Control 2018, 63, 155–167. [Google Scholar] [CrossRef]
- Pimentel, D.; Goodman, N. Ecological basis for the management of insect populations. OIKOS 1978, 30, 422–437. [Google Scholar] [CrossRef]
- Tena, A.; Wäckers, F.L.; Heimpel, G.E.; Urbaneja, A.; Pekas, A. Parasitoid nutritional ecology in a community context: The importance of honeydew and implications for biological control. Curr. Opin. Insect Sci. 2016, 14, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Chidawanyika, F.; Mudavanhu, P.; Nyamukondiwa, C. Global Climate Change as a Driver of Bottom-Up and Top-Down Factors in Agricultural Landscapes and the Fate of Host-Parasitoid Interactions. Front. Ecol. Evol. 2019, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Bélair, G.; Fournier, Y.; Dauphinais, N. Efficacy of steinernematid nematodes against three insect pests of crucifers in Quebec. J. Nematol. 2003, 35, 259–265. [Google Scholar]
- Muniappan, R.; Bamba, J. Biological control of Chromolaena odorata: Successes and Failures. In Proceedings of the X International Symposium on Biological Control of Weeds, 4–14 July 1999; Spencer, N.R., Ed.; Montana State University: Bozeman, MT, USA, 2000; pp. 151–154. [Google Scholar]
- Myers, J.H. What Can We Learn from biological control Failures? In Proceedings of the X International Symposium on Biological Control of Weeds, Bozeman, MT, USA, 4–14 July 1999; Spencer, N.R., Ed.; Montana State University: Bozeman, MT, USA, 2000; pp. 81–85. [Google Scholar]
- Georgis, R.; Koppenhöfer, A.M.; Lacey, L.A.; Bélair, G.; Duncan, L.W.; Grewal, P.S.; Samish, M.; Tan, L.; Torr, P.; van Tol, R.W.H.M. Successes and failures in the use of parasitic nematodes for pest control. Biol. Control 2006, 38, 103–123. [Google Scholar] [CrossRef]
- Ngowi, A.V.F.; Mbise, T.J.; Ijani, A.S.M.; London, L.; Ajayi, O.C. Knowledge, attitudes and practices (KAP) among agricultural extension workers concerning the reduction of the adverse impact in agricultural areas in Tanzania. Crop Prot. 2007, 26, 1617–1624. [Google Scholar] [CrossRef] [Green Version]
- Goldberger, J.R.; Lehrer, N. Biological control adoption in western U.S. orchard systems: Results from grower surveys. Biol. Control 2016, 102, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Obopile, M.; Munthali, D.C.; Matilo, B. Farmers’ knowledge, perceptions and management of vegetable pests and diseases in Botswana. Crop Prot. 2008, 27, 1220–1224. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Hughes, A.C.; Buamas, C.; Johnson, A.C.; Vasseur, L.; Raymondin, L.; Deguine, J.P.; Sheil, D. Biological control of an agricultural pest protects tropical forests. Commun. Biol. 2019. [Google Scholar] [CrossRef] [Green Version]

| Family | Host Plant | Reference |
|---|---|---|
| Solanaceae | Solanum tuberosum L. | [15] |
| Solanum nigrum L. | [125] | |
| Solanum melongena L. | [128] | |
| Solanum aethiopicum L. | [96] | |
| Solanum anguivi Lam. | [82] | |
| Solanum macrocarpon L. | [82] | |
| Solanum scabrum Mill. | [82] | |
| Solanum villosum Mill. | [82] | |
| Solanum aculeatissimum (Jacq.) | [42] | |
| Solanum coccineum (Jacq.) | [42] | |
| Solanum supinum Dunal | [42] | |
| Solanum americanum Mill. | [128] | |
| Solanum bonariense L. | [129] | |
| Solanum elaeagnifolium Cav. | [129] | |
| Solanum gracilius Herter | [129] | |
| Solanum hirtum Vahl | [130] | |
| Solanum pseudo-capsicum L. | [129] | |
| Solanum sisymbrifolium Lamb | [129] | |
| Solanum dulcamara Linnaeus | [48] | |
| Solanum lyratum Thunb. | [131] | |
| Solanum puberulum Nuttal ex Seemann | [131] | |
| Nicotiana longiflora Cav. | [124] | |
| Nicotiana tabacum L. | [15] | |
| Nicotiana rustica L. | [48] | |
| Nicotiana glauca (Graham) | [82] | |
| Datura stramonium L. | [124] | |
| Datura quercifolia Kunth | [128] | |
| Datura ferox L. | [132] | |
| Xanthium brasilicum Vell. | [124] | |
| Capsicum annum L. | [41] | |
| Capsicum frutescens L. | [96] | |
| Nicandra physalodes (L.) Gaertner | [48] | |
| Lycium halimifolium Mill. | [48] | |
| Lycium chilense (Coralillo) | [131] | |
| Lycium hirsutum L. | [82] | |
| Physalis peruviana L. | [133] | |
| Physalis angulata L. | [130] | |
| Amaranthaceae | Amaranthus spinosus L. | [124] |
| Amaranthus viridis L. | [78] | |
| Spinacia oleracea L. | [126] | |
| Beta vulgaris vulgaris L. | [126,134] | |
| Chenopodium bonus-henricus (L.) Rchb. | [126] | |
| Chenopodium rubrum (L.) S. Fuentes, Uotila & Borsch | [126] | |
| Chenopodium album L. | [134] | |
| Fabaceae | Phaseolus vulgaris L. | [135] |
| Medicago sativa L. | [124] | |
| Vicia faba L. | [124] | |
| Cucurbitaceae | Citrullus lanatus (Thunb.) Matsum. & Nakai | [124] |
| Convolvulaceae | Convolvulus arvensis L. | [134] |
| Calystegia sepium (L.) Brown | [134] | |
| Malvaceae | Malva sylvestris L. | [48] |
| Asteraceae | Sonchus oleraceus L. | [78] |
| Xanthium strumarium L. | [136] | |
| Poaceae | Sorghum halepense (L.) Pers. | [78] |
| Brassicaceae | Sinapis arvensis L. | [136] |
| Natural Substance | Species | Host Developmental Stage | Reference |
|---|---|---|---|
| Botanicals | Azadirachtin spp. | E, L, P | [150] |
| Petroleum ether extract | E, L | [150] | |
| Jatropha curcus | E, L | [150] | |
| Allium sativum | L | [156,157] | |
| Ocimum basilicum | L | [156,157] | |
| Thymus vulgaris | L | [156,157] | |
| Ricinus communis | L | [156,157] | |
| Eucalyptus spp. | L | [156,157] | |
| Melia azedarach | L | [156,157] | |
| Geranium spp. | L | [156,157] | |
| Allium cepa | L | [156,157] | |
| Citrus aurantium | L | [158] | |
| Piper amalago var. medium | L | [153] | |
| Piper glabratum | L | [153] | |
| Piper mikanianum | L | [153] | |
| Simmondsia chinensis | L | [151] |
| Type of Microbial | Species | Host | Reference |
|---|---|---|---|
| Bacillus thuringiensis | L | [160,169] | |
| Entomopathogens | Bacillus thuringiensis kurstaki | L | [169] |
| Beauveria bassiana | L | [169] | |
| Metarhizium beauveria | L | [170] | |
| Metarhizium anisopliae | L | [170] | |
| Baculoviruses (NPVs) | L | [171] | |
| Saccharopolyspora spinosa | L | [172] | |
| Entomopathogenic nematodes | Steinernema affine | L | [172] |
| Steinernema carpocapsae | L | [172] | |
| Steinernema feltiae | L | [62] | |
| Heterorhabditis bacteriophora | L | [62] | |
| Other NSs | Pheromones | A | [36,59] |
| Antimicrobial peptides (AMPs) | E, L, P | [173] |
| Natural Enemy | Species | Host | Reference |
|---|---|---|---|
| Parasitoids | Agathis fuscipennis | L | [131] |
| Apanteles dignus | L, P | [15,16] | |
| Apanteles gelechiidivoris | L | [15,16] | |
| Baryscapus bruchophagi | L | [220] | |
| Brachymeria secundaria | L | [220] | |
| Bracon lucileae | L | [15,16] | |
| Bracon lulensis | L | [15,16] | |
| Bracon spp. | P | [15,16,119] | |
| Bracon tutus | L | [15,16] | |
| Campoplex haywardi | L | [15,16] | |
| Capidosoma desantis | E | [15,16] | |
| Capidosoma koehleri | E | [15,16] | |
| Cheolras semele | - | [221] | |
| Closterocerus clarus | L | [220] | |
| Clostrocerus formosus | L | [15,16] | |
| Copidosoma desantisi | E | [15,16] | |
| Copidosoma koehleri | E | [15,16] | |
| Diadegma spp., D. ledicola and D. pulchripes | P | [15,16,119] | |
| Diglyphus crassinervis | L | [221] | |
| Diglyphus isaea | L | [221] | |
| Dineulophus phthormiaeae | L | [15,16] | |
| Dolichogenidea litae | - | [221] | |
| Elachertus inunctus | L | [222] | |
| Elasmus phthorimaeae | L | [221] | |
| Encarsia porteri | E | [223] | |
| Goniozus nigrifemur | L | [15] | |
| Habrobracon didemie | L | [220] | |
| Habrobracon hebetor | L | [220] | |
| Habrobracon nigricans | L | [16,224] | |
| Habrobracon osculator | L | [119] | |
| Halticoptera aenea | L | [222] | |
| Hemiptarsenus zilahisebessi | L | [225] | |
| Hockeria unicolor | L | [220,221] | |
| Horismenus sp | P | [15,16,119] | |
| Hyposoter didymator | - | [226] | |
| Necremnus artynes | L | [218,225] | |
| Necremnus metalarus | L | [206] | |
| Necremnus tidius | L | [227] | |
| Neochrysocharis formosa | L | [119] | |
| Neochrysocharis formosa | L | [222,224] | |
| Neochrysocharis formosa | L | [15,16] | |
| Pnigalio cristatus | L | [220] | |
| Pnigalio incompletus | - | [220] | |
| Pnigalio soemius | L | [221] | |
| Pnigalio sp. soemius complex | L | [222] | |
| Pseudapanteles dignus | L | [15,16] | |
| Pteromalus intermedius | L | [220] | |
| Pteromalus semotus | - | [221] | |
| Retisympiesis phthorimaea | L | [15,16] | |
| Retisympiesis phthorimaea | L | [15,16] | |
| Temelucha anatolica | - | [221] | |
| Trichogramma achaeae | E | [214] | |
| Trichogramma achaeae | E | [224] | |
| Trichogramma bactrae | E | [15,16] | |
| Trichogramma bourarachae | E | [228] | |
| Trichogramma dendrolimi | E | [15,16] | |
| Trichogramma exiguum | E | [15,16] | |
| Trichogramma fasciatum | E | [15,16] | |
| Trichogramma lopezandinensis | E | [15,16] | |
| Trichogramma minutum | E | [15,16] | |
| Trichogramma nerudai | E | [15,16] | |
| Trichogramma pintoi | E | [15,16] | |
| Trichogramma pretiosum | E | [15,16,213] | |
| Trichogramma rojasi | E | [15,16] | |
| Trichogramma telengai | E | [15,16] | |
| Zoophthorus macrops | - | [221] | |
| Predators | Amblyseius cucumeris | E, L | [229] |
| Amblyseius swirskii | E, L | [229] | |
| Brachygastra lecheguana | L | [15] | |
| Calosoma granulatum | L, P | [15] | |
| Coleomegilla maculata | E, L | [15] | |
| Cycloneda sanguinea | E | [15] | |
| Dicyphus errans | E, L | [230] | |
| Dicyphus maroccanus | E, L | [230] | |
| Dicyphus. tamaninii | E, L | [206,229] | |
| Doru lineare | E | [15] | |
| Engytatus varians | E | [229] | |
| Eriopsis conexa | E | [15] | |
| Franklinothrips vespiformis | L | [15] | |
| Labidura riparia | P | [15] | |
| Lebia concina | L, P | [15] | |
| Macrolophus pygmaeus | E, L | [231] | |
| Nabis ibericus | L | [229,232] | |
| Nesidiocoris tenuis | E | [231] | |
| Orius albidipennis | - | [230,233] | |
| Orius insidiosus | E, L | [15] | |
| Podisus nigrispinus | L | [15] | |
| Polistes carnifex | L | [15] | |
| Polistes melanosoma | L | [15] | |
| Polistes versicolor | L | [15] | |
| Polybia ignobilis | L | [15] | |
| Polybia scutellaris | L | [15] | |
| Protonectarina sylveirae | L | [15] | |
| Protopolybia exigua | L | [15] | |
| Scolothrips sexmaculatus | L | [15] | |
| Solenopsis geminata | L, P | [15] | |
| Solenopsis saevissima | L, P | [15] | |
| Synoeca cyanea | L | [15] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarusikirwa, V.L.; Machekano, H.; Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on the “Offensive” in Africa: Prospects for Integrated Management Initiatives. Insects 2020, 11, 764. https://doi.org/10.3390/insects11110764
Tarusikirwa VL, Machekano H, Mutamiswa R, Chidawanyika F, Nyamukondiwa C. Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on the “Offensive” in Africa: Prospects for Integrated Management Initiatives. Insects. 2020; 11(11):764. https://doi.org/10.3390/insects11110764
Chicago/Turabian StyleTarusikirwa, Vimbai L., Honest Machekano, Reyard Mutamiswa, Frank Chidawanyika, and Casper Nyamukondiwa. 2020. "Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on the “Offensive” in Africa: Prospects for Integrated Management Initiatives" Insects 11, no. 11: 764. https://doi.org/10.3390/insects11110764
APA StyleTarusikirwa, V. L., Machekano, H., Mutamiswa, R., Chidawanyika, F., & Nyamukondiwa, C. (2020). Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on the “Offensive” in Africa: Prospects for Integrated Management Initiatives. Insects, 11(11), 764. https://doi.org/10.3390/insects11110764





