Meta-Analysis Suggests Differing Indirect Effects of Viral, Bacterial, and Fungal Plant Pathogens on the Natural Enemies of Insect Herbivores
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
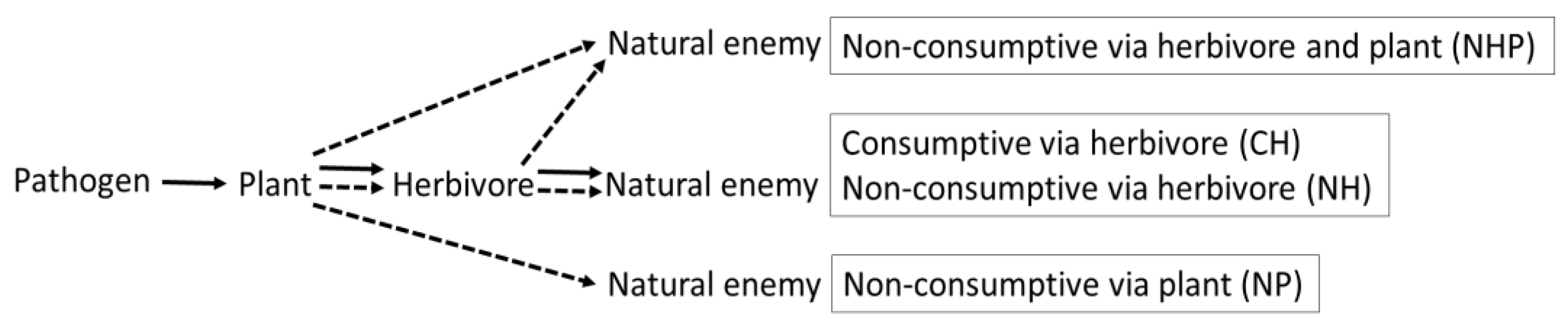
2.1. Data Collection
2.2. Moderators
2.3. Statistical Analyses
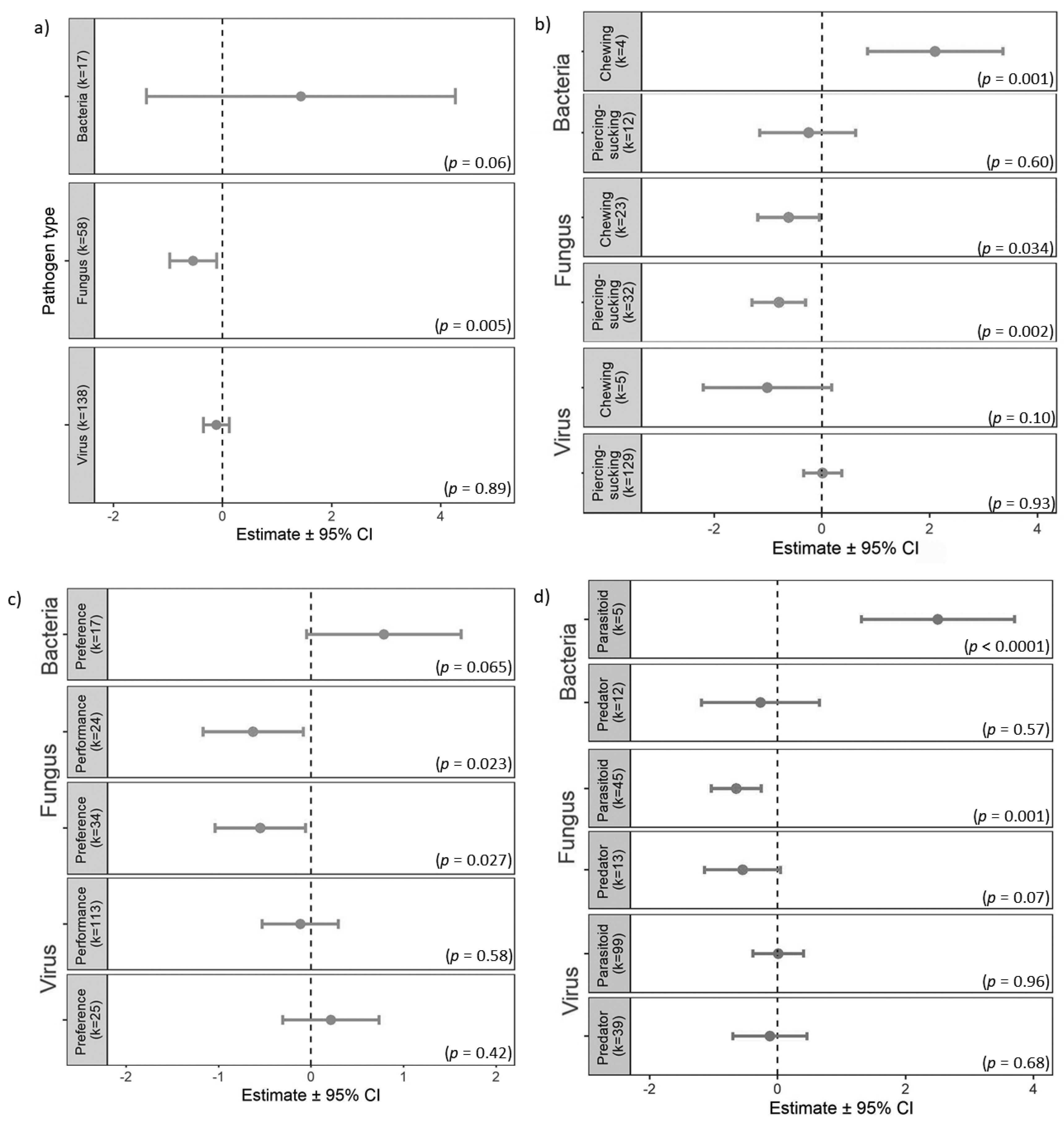
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dulermo, T.; Bligny, R.; Gout, E.; Cotton, P. Amino acid changes during sunflower infection by the necrotrophic fungus B. cinerea. Plant Signal. Behav. 2009, 4, 859–861. [Google Scholar] [CrossRef]
- Desurmont, G.A.; Xu, H.; Turlings, T.C.J. Powdery mildew suppresses herbivore-induced plant volatiles and interferes with parasitoid attraction in Brassica rapa. Plant Cell Environ. 2016, 39, 1920–1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, M.J.; Thaler, J.S.; Thomma, B.P.H.J. Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu. Rev. Entomol. 2006, 51, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; Smyers, E.; De Moraes, C.M.; Mescher, M.C. Virus infection influences host plant interactions with non-vector herbivores and predators. Funct. Ecol. 2015, 29, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Martini, X.; Pelz-Stelinski, K.S.; Stelinski, L.L. Plant pathogen-induced volatiles attract parasitoids to increase parasitism of an insect vector. Front. Ecol. Evol. 2014, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; He, Y.; Xie, W.; Wu, Q.; Zhang, Y.; Liu, Y.; Wang, S. Infection of tomato by tomato yellow leaf curl virus alters the foraging behavior and parasitism of the parasitoid Encarsia formosa on Bemisia tabaci. J. Asia. Pac. Entomol. 2018, 21, 548–552. [Google Scholar] [CrossRef]
- Ngah, N.; Thomas, R.L.; Shaw, M.W.; Fellowes, M.D.E. Asymptomatic host plant infection by the widespread pathogen Botrytis cinerea alters the life histories, behaviors, and interactions of an aphid and its natural enemies. Insects 2018, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.B.; Fellowes, M.D.E.; Godfray, H.C.J. Relative importance of fertiliser addition to plants and exclusion of predators for aphid growth in the field. Oecologia 2005, 143, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Leather, S.R.; Beare, J.A.; Cooke, R.C.A.; Fellowes, M.D.E. Are differences in life history parameters of the pine beauty moth Panolis flammea modified by host plant quality or gender? Entomol. Exp. Appl. 1998, 87, 237–243. [Google Scholar] [CrossRef]
- Ripple, W.J.; Estes, J.A.; Schmitz, O.J.; Constant, V.; Kaylor, M.J.; Lenz, A.; Motley, J.L.; Self, K.E.; Taylor, D.S.; Wolf, C. What is a trophic cascade? Trends Ecol. Evol. 2016, 31, 842–849. [Google Scholar] [CrossRef]
- de Oliveira, C.F.; Long, E.Y.; Finke, D.L. A negative effect of a pathogen on its vector? A plant pathogen increases the vulnerability of its vector to attack by natural enemies. Oecologia 2014, 174, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Belliure, B.; Janssen, A.; Sabelis, M.W. Herbivore benefits from vectoring plant virus through reduction of period of vulnerability to predation. Oecologia 2008, 156, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; Zamioudis, C.; Van der Does, D.; Van Wees, S.C.M. Signalling networks involved in induced resistance. In Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection; Walters, D.R., Newton, A.C., Lyon, G.D., Eds.; John Wiley & Sons: Chichester, UK, 2014. [Google Scholar]
- Moreira, X.; Abdala-Roberts, L.; Castagneyrol, B. Interactions between plant defence signalling pathways: Evidence from bioassays with insect herbivores and plant pathogens. J. Ecol. 2018, 106, 2353–2364. [Google Scholar] [CrossRef]
- Jaenike, J. On optimal oviposition behavior in phytophagous insects. Theor. Popul. Biol. 1978, 14, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.C.; Ng, D.; Thomas, C.D. Heritability of oviposition preference and its relationship to offspring performance within a single insect population. Evolution 1988, 42, 977–985. [Google Scholar] [CrossRef]
- Gripenberg, S.; Mayhew, P.J.; Parnell, M.; Roslin, T. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 2010, 13, 383–393. [Google Scholar] [CrossRef]
- Valladares, G.; Lawton, J.H. Host-plant selection in the holly leaf-miner: Does mother know best? J. Anim. Ecol. 1991, 60, 227–240. [Google Scholar] [CrossRef]
- Clark, K.E.; Hartley, S.E.; Johnson, S.N. Does mother know best? The preference-performance hypothesis and parent-offspring conflict in aboveground-belowground herbivore life cycles. Ecol. Entomol. 2011, 36, 117–124. [Google Scholar] [CrossRef]
- Steiner, S.; Erdmann, D.; Steidle, J.L.M.; Ruther, J. Host habitat assessment by a parasitoid using fungal volatiles. Front. Zool. 2007, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Rostás, M.; Ton, J.; Mauch-Mani, B.; Turlings, T.C.J. Fungal infection reduces herbivore-induced plant volatiles of maize but does not affect naïve parasitoids. J. Chem. Ecol. 2006, 32, 1897–1909. [Google Scholar] [CrossRef]
- Xu, H.; He, X.; Zheng, X.; Yang, Y.; Tian, J.; Lu, Z. Infection of rice plants by rice black streaked dwarf virus improves an egg parasitoid, Anagrus nilaparvatae (Hymenoptera: Mymaridae), of rice planthoppers. Environ. Entomol. 2014, 43, 1235–1239. [Google Scholar] [CrossRef]
- de Oliveira, R.L.; Moscardini, V.F.; Gontijo, P.C.; Sâmia, R.R.; Marucci, R.C.; Budia, F.; Carvalho, G.A. Life history parameters and feeding preference of the green lacewing Ceraeochrysa cubana fed with virus-free and potato leafroll virus-infected Myzus persicae. BioControl 2016, 61, 671–679. [Google Scholar] [CrossRef]
- Joffrey, M.; Chesnais, Q.; Spicher, F.; Verrier, E.; Ameline, A.; Couty, A. Plant virus infection influences bottom-up regulation of a plant-aphid-parasitoid system. J. Pest Sci. 2018, 91, 361–372. [Google Scholar] [CrossRef]
- Ngah, N. Asymptomatic Pathogen Infection Alters Interactions at Higher Trophic Levels. Ph.D. Thesis, University of Reading, Reading, UK, 2018. [Google Scholar]
- Fernandez-Conradi, P.; Jactel, H.; Robin, C.; Tack, A.J.M.; Castagneyrol, B. Fungi reduce preference and performance of insect herbivores on challenged plants. Ecology 2018, 99, 300–311. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Behav. Stat. 1981, 6, 107–128. [Google Scholar]
- Biere, A.; Elzinga, J.A.; Honders, S.C.; Harvey, J.A. A plant pathogen reduces the enemy-free space of an insect herbivore on a shared host plant. Proc. R. Soc. B Biol. Sci. 2002, 269, 2197–2204. [Google Scholar] [CrossRef] [Green Version]
- Leimu, R.; Koricheva, J. Cumulative meta-analysis: A new tool for detection of temporal trends and publication bias in ecology. Proc. R. Soc. B Biol. Sci. 2004, 271, 1961–1966. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, M.S. The file-drawer problem revisited: A general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 2005, 59, 464–468. [Google Scholar]
- Murtaugh, P.A. Journal quality, effect size, and publication bias in meta-analysis. Ecology 2002, 83, 1162–1166. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.-L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Christiansen-Weniger, P.; Powell, G.; Hardie, J. Plant virus and parasitoid interactions in a shared insect vector/host. Entomol. Exp. Appl. 1998, 86, 205–213. [Google Scholar] [CrossRef]
- Al-Naemi, F.; Hatcher, P.E. Contrasting effects of necrotrophic and biotrophic plant pathogens on the aphid Aphis fabae. Entomol. Exp. Appl. 2013, 148, 234–245. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Castella, G.; Kostecki, M.; Golinski, P.; Ritieni, A.; Chelkowski, J. Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 1998, 64, 3084–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponzio, C.; Weldegergis, B.T.; Dicke, M.; Gols, R. Compatible and incompatible pathogen–plant interactions differentially affect plant volatile emissions and the attraction of parasitoid wasps. Funct. Ecol. 2016, 30, 1779–1789. [Google Scholar] [CrossRef]
- Eberl, F.; Uhe, C.; Unsicker, S.B. Friend or foe? The role of leaf-inhabiting fungal pathogens and endophytes in tree-insect interactions. Fungal Ecol. 2019, 38, 104–112. [Google Scholar] [CrossRef]
- Ponzio, C.; Gols, R.; Pieterse, C.M.J.; Dicke, M. Ecological and phytohormonal aspects of plant volatile emission in response to single and dual infestations with herbivores and phytopathogens. Funct. Ecol. 2013, 27, 587–598. [Google Scholar] [CrossRef]
- Mumm, R.; Dicke, M. Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Can. J. Zool. 2010, 88, 628–667. [Google Scholar] [CrossRef]
- Dáder, B.; Then, C.; Berthelot, E.; Ducousso, M.; Ng, J.C.K.; Drucker, M. Insect transmission of plant viruses: Multilayered interactions optimize viral propagation. Insect Sci. 2017, 24, 929–946. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisakrapikoop, U.; Pirie, T.J.; Fellowes, M.D.E. Meta-Analysis Suggests Differing Indirect Effects of Viral, Bacterial, and Fungal Plant Pathogens on the Natural Enemies of Insect Herbivores. Insects 2020, 11, 765. https://doi.org/10.3390/insects11110765
Srisakrapikoop U, Pirie TJ, Fellowes MDE. Meta-Analysis Suggests Differing Indirect Effects of Viral, Bacterial, and Fungal Plant Pathogens on the Natural Enemies of Insect Herbivores. Insects. 2020; 11(11):765. https://doi.org/10.3390/insects11110765
Chicago/Turabian StyleSrisakrapikoop, Ussawit, Tara J. Pirie, and Mark D. E. Fellowes. 2020. "Meta-Analysis Suggests Differing Indirect Effects of Viral, Bacterial, and Fungal Plant Pathogens on the Natural Enemies of Insect Herbivores" Insects 11, no. 11: 765. https://doi.org/10.3390/insects11110765
APA StyleSrisakrapikoop, U., Pirie, T. J., & Fellowes, M. D. E. (2020). Meta-Analysis Suggests Differing Indirect Effects of Viral, Bacterial, and Fungal Plant Pathogens on the Natural Enemies of Insect Herbivores. Insects, 11(11), 765. https://doi.org/10.3390/insects11110765





