The Role of Chrysoperla carnea (Steph.) (Neuroptera: Chrysopidae) as a Potential Dispersive Agent of Noctuid Baculoviruses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.1.1. Viruses
2.1.2. Insects
2.2. Presence of the OBs in Drop and Meconium
2.3. Infection of Healthy L2 S. exigua with Suspensions of OBs Obtained from C. carnea Excretion Products
2.4. Infection of Healthy L2 S. exigua via Direct Deposition of C. carnea Excretion Products
2.5. Chrysoperla carnea Fitness Evaluation after Prey-Mediated Ingestion of SeMNPV and AcMNPV
2.6. Chrysoperla carnea Choice Tests
2.7. Statistical Analysis
3. Results
3.1. Presence of OBs in C. carnea Excretion Products
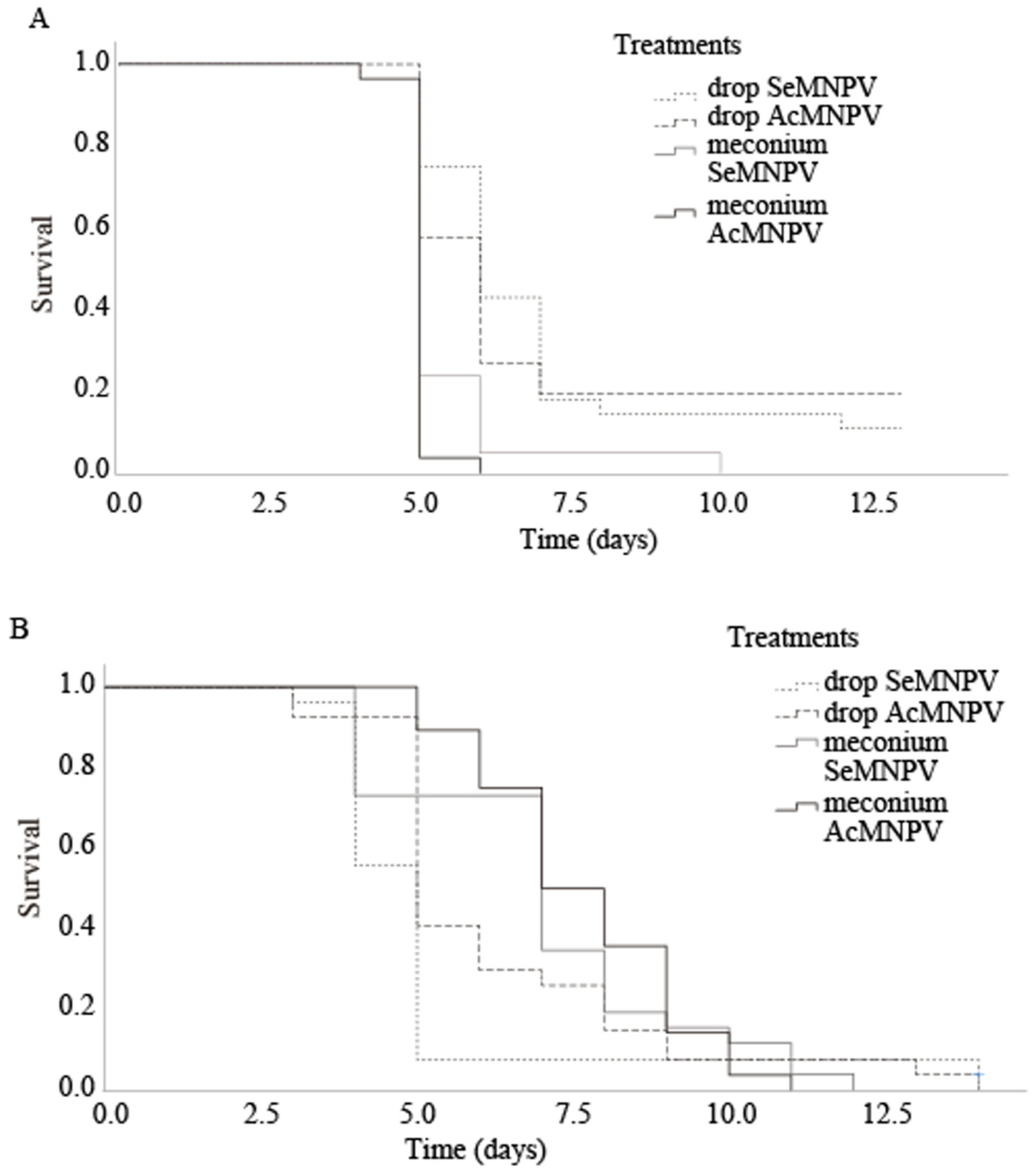
3.2. Infection of L2 S. exigua with Suspensions of OBs Obtained from C. carnea Excretion Products
3.3. Infection of L2 S. exigua via Direct Deposition of C. carnea Excretion Products
3.4. Chrysoperla carnea Fitness Evaluation after Prey-Mediated Ingestion of SeMNPV and AcMNPV
3.5. Chrysoperla carnea Choice Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szewczyk, B.; Hoyos-Carvajal, L.; Paluszek, M.; Skrzecz, I.; De Souza, M.L. Baculoviruses—Re-emerging Biopesticides. Biotechnol. Adv. 2006, 24, 143–160. [Google Scholar] [CrossRef] [Green Version]
- Eberle, K.E.; Jehle, J.A.; Huber, J.T. Microbial Control of Crop Pests Using Insect Viruses. In Integrated Pest Management: Principles and Practice; Abrol, D.P., Shankar, U., Eds.; CABI Publishing: Wallingford, UK, 2012; pp. 281–298. [Google Scholar]
- Boogaard, B.; Van Oers, M.M.; Van Lent, J.W.M. An Advanced View on Baculovirus per os Infectivity Factors. Insects 2018, 9, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, L.A. Microbial Control of Insect and Mite Pests: From Theory to Practice; Academic Press: Yakima, WA, USA, 2016; 482p. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gani, M.; Jasrotia, P.; Srivastava, K. Development of the Predator Eocanthecona furcellata on Different Proportions of Nucleopolyhedrovirus Infected Spodoptera litura Larvae and Potential for Predator Dissemination of Virus in the Field. BioControl 2013, 58, 543–552. [Google Scholar] [CrossRef]
- Vasconcelos, S.D.; Cory, J.S.; Wilson, K.R.; Sait, S.M.; Hails, R. Modified Behavior in Baculovirus-Infected Lepidopteran Larvae and Its Impact on the Spatial Distribution of Inoculum. Biol. Control 1996, 7, 299–306. [Google Scholar] [CrossRef]
- Lee, J.-H. Fuxa Transport of Wild-Type and Recombinant Nucleopolyhedroviruses by Scavenging and Predatory Arthropods. Microb. Ecol. 2000, 39, 301–313. [Google Scholar]
- Smith, C.R.; Heinz, K.M.; Sansone, C.G.; Flexner, J.L. Impact of Recombinant Baculovirus Field Applications on a Nontarget Heliothine Parasitoid, Microplitis croceipes (Hymenoptera: Braconidae). J. Econ. Entomol. 2000, 93, 1109–1117. [Google Scholar] [CrossRef]
- Medina, P.; Budia, F.; Estal, P.; Viñuela, E. Effects of Three Modern Insecticides, Pyriproxyfen, Spinosad and Tebufenozide, on Survival and Reproduction of Chrysoperla carnea Adults. Ann. Appl. Biol. 2003, 142, 55–61. [Google Scholar] [CrossRef]
- Canard, M. Natural Food and Feeding Habits of Lacewings. In Lacewings in the Crop Environment; McEwen, P., Nwe, T., Whittington, A., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 116–129. [Google Scholar] [CrossRef]
- Huang, N.; Enkegaard, A. Predation Capacity and Prey Preference of Chrysoperla carnea on Pieris brassicae. BioControl 2009, 55, 379–385. [Google Scholar] [CrossRef]
- Hassanpour, M.; Mohaghegh, J.; Iranipour, S.; Nouri-Ganbalani, G.; Enkegaard, A. Functional Response of Chrysoperla carnea (Neuroptera: Chrysopidae) to Helicoverpa armigera (Lepidoptera: Noctuidae): Effect of Prey and Predator Stages. Insect Sci. 2010, 18, 217–224. [Google Scholar] [CrossRef]
- Ismoilov, K.; Wang, M.; Jalilov, A.; Zhang, X.; Lu, Z.; Saidov, A.; Sun, X.; Han, P. First Report Using a Native Lacewing Species to Control Tuta absoluta: From Laboratory Trials to Field Assessment. Insects 2020, 11, 286. [Google Scholar] [CrossRef]
- Boughton, A.J.; Obrycki, J.J.; Bonning, B.C. Effects of a Protease-Expressing Recombinant Baculovirus on Nontarget Insect Predators of Heliothis virescens. Biol. Control 2003, 28, 101–110. [Google Scholar] [CrossRef]
- Castillejos, V.; Cisneros, J.; Goulson, D.; Cave, R.D.; García, L.; Caballero, P.; Williams, T. The Potential of Chrysoperla rufilabris and Doru taeniatum as Agents for Dispersal of Spodoptera frugiperda Nucleopolyhedrovirus in Maize. Entomol. Exp. Appl. 2001, 98, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.S.T. Interactions between Nuclear Polyhedrosis Virus, Host and Predators. J. Plant Dis. Prot. 1988, 95, 606–610. [Google Scholar]
- Cáceres, C.E.; Knight, C.J.; Hall, S.R. Predator–Spreaders: Predation Can Enhance Parasite Success in a Planktonic Host–Parasite System. Ecology 2009, 90, 2850–2858. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.S.T.; Boucias, D.G. Interaction Between Nuclear Polyhedrosis Virus-Infected Anticarsia gemmatalis (Lepidoptera: Noctuidae) Larvae and Predator Podisus maculiventris (Say) (Hemiptera: Pentatomidae). Environ. Entomol. 1984, 13, 599–602. [Google Scholar] [CrossRef]
- Bianchi, F.J.; Snoeijing, I.; Van Der Werf, W.; Mans, R.M.; Smits, P.H.; Vlak, J.M. Biological Activity of SeMNPV, AcMNPV, and Three AcMNPV Deletion Mutants against Spodoptera exigua Larvae (Lepidoptera: Noctuidae). J. Invertebr. Pathol. 2000, 75, 28–35. [Google Scholar] [CrossRef]
- Elvira, S.; Gorría, N.; Muñoz, D.; Williams, T.; Caballero, P. A Simplified Low-Cost Diet for Rearing Spodoptera exigua (Lepidoptera: Noctuidae) and Its Effect on S. exigua Nucleopolyhedrovirus Production. J. Econ. Entomol. 2010, 103, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Medina, P.; Budia, F.; Tirry, L.; Smagghe, G.; Viñuela, E. Compatibility of Spinosad, Tebufenozide and Azadirachtin with Eggs and Pupae of the Predator Chrysoperla carnea (Stephens) Under Laboratory Conditions. Biocontrol Sci. Technol. 2001, 11, 597–610. [Google Scholar] [CrossRef]
- Hughes, P.; Wood, H. A Synchronous Peroral Technique for the Bioassay of Insect Viruses. J. Invertebr. Pathol. 1981, 37, 154–159. [Google Scholar] [CrossRef]
- Vogt, H.; Bigler, F.; Brown, K.; Candolfi, M.P.; Kemmeter, F.; Kuhner, C.; Moll, M.; Travis, A.; Ufer, A.; Viñuela, E.; et al. Laboratory Method to Test Effects of Plant Protection Products on Larvae of Chrysoperla carnea (Neuroptera: Chrysopidae). In Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods; Blumel, S., Forster, R., Bakker, F.M., Grimm, C., Hassan, S.A., Heimbach, U., Mead-Briggs, M.A., Reber, B., Schmuck, R., Vogt, H., et al., Eds.; IOBC-OILB: Gent, Belgium, 2000; pp. 107–119. [Google Scholar]
- Garzón, A.; Freire, B.C.; Carvalho, G.A.; Oliveira, R.L.; Medina, P.; Budia, F. Development and Reproduction of Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae) Fed on Myzus persicae (Sulzer) (Hemiptera: Aphididae) Vectoring Potato leafroll virus (PLRV). Neotrop. Entomol. 2015, 44, 604–609. [Google Scholar] [CrossRef]
- SPSS Software; Version 24.0.0.0; SPSS Inc.: Chicago, IL, USA, 2018.
- Goulson, D. Can Host Susceptibility to Baculovirus Infection be Predicted from Host Taxonomy or Life History? Environ. Entomol. 2003, 32, 61–70. [Google Scholar] [CrossRef]
- Johnson, P.T.; Dobson, A.; Lafferty, K.D.; Marcogliese, D.J.; Memmott, J.; Orlofske, S.A.; Poulin, R.; Thieltges, D.W. When Parasites Become Prey: Ecological and Epidemiological Significance of Eating Parasites. Trends Ecol. Evol. 2010, 25, 362–371. [Google Scholar] [CrossRef]
- Flick, A.J.; Acevedo, M.A.; Elderd, B.D. The Negative Effects of Pathogen-Infected Prey on Predators: A Meta-Analysis. Oikos 2016, 125, 1554–1560. [Google Scholar] [CrossRef]
- Flick, A.J. Intraguild Predation: Interactions Between Predators, Pathogens, and Their Shared Resource in Crop Pest Communities. Ph.D. Thesis, Louisiana State University, Baton Rouge, LA, USA, 2018; 116p. Available online: https://digitalcommons.lsu.edu/gradschool_dissertations/4786 (accessed on 30 August 2019).
- Thieltges, D.W.; Amundsen, P.-A.; Hechinger, R.F.; Johnson, P.T.J.; Lafferty, K.D.; Mouritsen, K.N.; Preston, D.L.; Reise, K.; Zander, C.D.; Poulin, R. Parasites as Prey in Aquatic Food Webs: Implications for Predator Infection and Parasite Transmission. Oikos 2013, 122, 1473–1482. [Google Scholar] [CrossRef] [Green Version]
| Drop | Meconium | |
|---|---|---|
| SeMNPV | 1.06 × 106 ± 1.38 × 105 | 5.11 × 106 ± 6.06 × 105 |
| AcMNPV | 8.58 × 105 ± 5.99 × 104 | 5.14 × 106 ± 2.62 × 105 |
| Treatment | Infected with Suspension of OBs | Infected Directly with OBs | ||||
|---|---|---|---|---|---|---|
| MTD 1 (Days) | 95% Confidence Limits | MTD 1 (Days) | 95% Confidence Limits | |||
| SeMNPV drops | 6 a | 5.4 | 6.6 | 5 a | 4.8 | 5.2 |
| AcMNPV drops | 6 a | 5.4 | 6.6 | 5 ab | 4.3 | 5.7 |
| SeMNPV meconia | 5 b | 4.7 | 5.3 | 7 b | 5.6 | 8.4 |
| AcMNPV meconia | 5 b | 4.9 | 5.1 | 7 b | 6.1 | 7.9 |
| Treatment | L3 Instar | Pupal Stage | |||
|---|---|---|---|---|---|
| Consumption Rate 1 | Developmental Time (Days) 2 | Pupation n (%) 3 | Developmental Time (Days) 4 | Pupal Weight (mg) 5 | |
| HL | 8.70 ± 0.10 a | 4.59 ± 0.15 a | 44 a (78.60) | 9.62 ± 0.10 a | 10.56 ± 0.21 a |
| SeMNPV-IL | 8.52 ± 0.09 a | 5.08 ± 0.16 b | 50 a (89.30) | 9.70 ± 0.11 a | 9.65 ± 0.22 b |
| AcMNPV-IL | 8.69 ± 0.10 a | 5.96 ± 0.17 c | 50 a (89.30) | 11.09 ± 0.11 b | 9.45 ± 0.24 b |
| Treatment | Emergence 1 n (%) | Females/Males (n) 2 | Preoviposition (Days) 3 | Fecundity (Eggs/Day−1) 4 | Fertility (%) 5 | Adult Weight (mg) | |
|---|---|---|---|---|---|---|---|
| ♀ 6 | ♂ 7 | ||||||
| HL | 42 a (95.50) | 22/20 a | 5.25 ± 0.13 a | 18.51 ± 3.43 a | 85.56 ± 2.00 a | 21.41 ± 0.65 a | 8.57 ± 0.25 a |
| SeMNPV-IL | 46 a (92.00) | 16/30 a | 5.50 ± 0.15 a | 10.75 ± 1.44 a | 82.52 ± 6.77 a | 21.88 ± 0.69 a | 8.50 ± 0.28 a |
| AcMNPV-IL | 46 a (92.00) | 17/29 a | 6.67 ± 0.31 b | 14.36 ± 3.20 a | 83.26 ± 3.25 a | 22.69 ± 0.63 a | 8.33 ± 0.27 a |
| Infected vs. Healthy S. exigua Larvae | ||||
| SeMNPV | AcMNPV | |||
| First Attack | Searching Time | First Attack | Searching Time | |
| Infected larvae | 20 a | 9.96 ± 2.14 a | 21 a | 6.70 ± 1.93 a |
| Healthy larvae | 22 a | 8.75 ± 1.41 a | 19 a | 8.24 ± 1.62 a |
| X2 1 = 0.95; p = 0.758 | F1,40 = 0.23; p = 0.635 | X2 1 = 0.10; p = 0.752 | F1,37 = 0.38; p = 0.543 | |
| Infected S. exigua Larvae vs. M. euphorbiae | ||||
| SeMNPV | AcMNPV | |||
| First Attack | Searching Time | First Attack | Searching Time | |
| Infected larvae | 33 a | 7.63 ± 1.41 a | 28 a | 8.55 ± 1.43 a |
| M. euphorbiae | 7 b | 11.66 ± 2.98 a | 12 b | 9.86 ± 1.92 a |
| X2 1 = 16.90; p < 0.001 | F1,38 = 1.44; p = 0.238 | X2 1 = 6.40; p = 0.011 | F1,37 = 0.27; p = 0.609 | |
| Healthy S. exigua Larvae vs. M. euphorbiae | ||||
| First Attack | Searching Time | |||
| Healthy larvae | 34 a | 14.01 ± 9.35 a | ||
| M. euphorbiae | 12 b | 11.01 ± 6.86 a | ||
| X2 1 = 5.58; p = 0.018 | F1,45 = 1.02; p = 0.317 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Cárdenas, O.G.; Adán, Á.; Beperet, I.; Medina, P.; Caballero, P.; Garzón, A. The Role of Chrysoperla carnea (Steph.) (Neuroptera: Chrysopidae) as a Potential Dispersive Agent of Noctuid Baculoviruses. Insects 2020, 11, 760. https://doi.org/10.3390/insects11110760
Gutiérrez-Cárdenas OG, Adán Á, Beperet I, Medina P, Caballero P, Garzón A. The Role of Chrysoperla carnea (Steph.) (Neuroptera: Chrysopidae) as a Potential Dispersive Agent of Noctuid Baculoviruses. Insects. 2020; 11(11):760. https://doi.org/10.3390/insects11110760
Chicago/Turabian StyleGutiérrez-Cárdenas, Oscar Giovanni, Ángeles Adán, Inés Beperet, Pilar Medina, Primitivo Caballero, and Agustín Garzón. 2020. "The Role of Chrysoperla carnea (Steph.) (Neuroptera: Chrysopidae) as a Potential Dispersive Agent of Noctuid Baculoviruses" Insects 11, no. 11: 760. https://doi.org/10.3390/insects11110760







