Ontogenesis of Aldehyde Pheromones in Two Synanthropic Bed Bug Species (Heteroptera: Cimicidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Nymphs (Solvent Extraction of Exuviae)
2.3. Adults
2.4. Quantification
2.5. Statistical Analysis
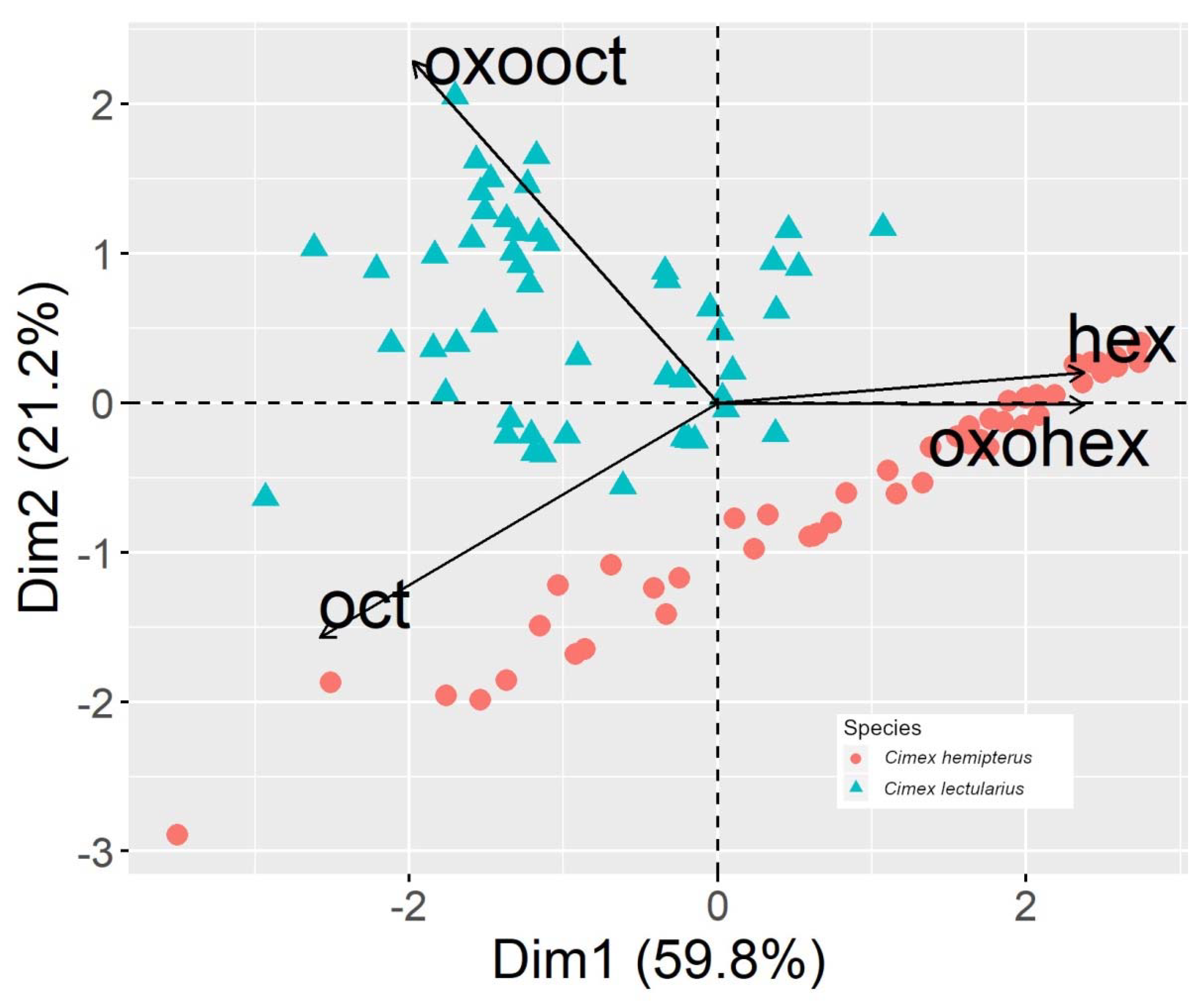
3. Results
3.1. Nymphs (Solvent Extraction of Exuviae)
3.2. Adults
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Usinger, R.L. Monograph of Cimicidae (Hemiptera-Heteroptera); Entomological Society of America: College Park, MD, USA, 1966. [Google Scholar]
- Roth, S.; Balvín, O.; Siva-Jothy, M.T.; Di Iorio, O.; Benda, P.; Calva, O.; Faundez, E.I.; Khan, F.A.A.; McFadzen, M.; Lehnert, M.P.; et al. Bedbugs evolved before their bat hosts and did not co-speciate with ancient humans. Curr. Biol. 2019, 29, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.E.; Koehler, P.G.; Buss, L.J.; Baldwin, R.W. Recent documentation of the tropical bed bug (Hemiptera: Cimicidae) in Florida since the common bed bug resurgence. Fla. Entomol. 2016, 99, 549–551. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.D.; Levine, B.A.; Vargo, E.L.; Schal, C.; Booth, W. Recent detection of multiple populations of the tropical bed bug (Hemiptera: Cimicidae) exhibiting kdr-associated mutations in Hawaii. J. Med. Entomol. 2020, 57, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Doggett, S.L.; Cains, T. The Bed Bug Resurgence in Australia. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; Wiley-Blackwell: Oxford, UK, 2018; pp. 81–86. [Google Scholar]
- Lee, C.-Y.; Hirao, M.; Wang, C.; Xu, Y. The Bed Bug Resurgence in Asia. In Advances in the Biology and Management of Modern Bed Bugs; Doggett, S.L., Miller, D.M., Lee, C.-Y., Eds.; Wiley-Blackwell: Oxford, UK, 2018; pp. 69–79. [Google Scholar]
- Newberry, K. Production of a hybrid between the bedbugs Cimex hemipterus and Cimex lectularius. Med. Vet. Entomol. 1988, 2, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Weeks, E.N.I.; Birkett, M.A.; Cameron, M.M.; Pickett, J.A.; Logan, J.G. Semiochemicals of the common bed bug, Cimex lectularius L. (Hemiptera: Cimicidae), and their potential for use in monitoring and control. Pest Manag. Sci. 2011, 67, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Choe, D.-H.; Park, H.; Vo, C.; Knyshov, A. Chemically mediated arrestment of the bed bug, Cimex lectularius, by volatiles associated with exuviae of conspecifics. PLoS ONE 2016, 11, e0159520. [Google Scholar] [CrossRef] [PubMed]
- Domingue, M.J.; Kramer, M.; Feldlaufer, M.F. Sexual dimorphism of arrestment and gregariousness in the bed bug (Cimex lectularius) in response to cuticular extracts from nymphal exuviae. Physiol. Entomol. 2010, 35, 203–213. [Google Scholar] [CrossRef]
- Siljander, E.; Gries, R.; Khaskin, G.; Gries, G. Identification of the airborne aggregation pheromone of the common bed bug, Cimex lectularius. J. Chem. Ecol. 2008, 34, 708–718. [Google Scholar] [CrossRef]
- Weeks, E.N.I.; Logan, J.G.; Gezan, S.A.; Woodcock, C.M.; Birkett, M.A.; Pickett, J.A.; Cameron, M.M. A bioassay for studying behavioural responses of the common bed bug, Cimex lectularius (Hemiptera: Cimicidae) to bed bug-derived volatiles. Bull. Entomol. Res. 2011, 101, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Schildknecht, H.; Holoubek, K.; Weis, K.H.; Krämer, H. Defensive substances of the arthropods, their isolation and identification. Angew. Chem. Int. 1964, 3, 73–82. [Google Scholar] [CrossRef]
- Feldlaufer, M.F.; Domingue, M.J.; Chauhan, K.R.; Aldrich, J.R. 4-oxo-aldehydes from the dorsal abdominal glands of the bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 2010, 47, 140–143. [Google Scholar] [CrossRef]
- Künckel, M.J. The bed-bug and its odoriferous apparatus. Ann. Mag. Nat. Hist. 1886, 18, 167–168. [Google Scholar] [CrossRef] [Green Version]
- Staddon, B.W. The scent glands of Heteroptera. Adv. Insect Physiol. 1979, 14, 351–418. [Google Scholar] [CrossRef]
- Harraca, V.; Ryne, C.; Ignell, R. Nymphs of the common bed bug (Cimex lectularius) produce anti-aphrodisiac defence against conspecific males. BMC Biol. 2010, 8, 121:1–121:7. [Google Scholar] [CrossRef] [Green Version]
- Kilpinen, O.; Liu, D.; Adamsen, A.P.S. Real-time measurement of volatile chemicals released by bed bugs during mating activities. PLoS ONE 2012, 7, e50981. [Google Scholar] [CrossRef] [PubMed]
- Levinson, H.Z.; Levinson, A.R.; Maschwitz, U. Action and composition of the alarm pheromone of the bedbug Cimex lectularius L. Naturwissenschaften 1974, 61, 684–685. [Google Scholar] [CrossRef] [PubMed]
- Gries, R.; Britton, R.; Holmes, M.; Zhai, H.; Draper, J.; Gries, G. Bed bug aggregation pheromone finally identified. Angew. Chem. Int. 2015, 127, 1135–1138. [Google Scholar] [CrossRef]
- Ulrich, K.R.; Kramer, M.; Feldlaufer, M.F. Ability of bed bug (Hemiptera: Cimicidae) defensive secretions (E)-2-hexenal and (E)-2-octenal to attract adults of the common bed bug Cimex lectularius. Physiol. Entomol. 2016, 41, 103–110. [Google Scholar] [CrossRef]
- Liedtke, H.C.; Åbjörnsson, K.; Harraca, V.; Knudsen, J.T.; Wallin, E.A.; Hedenström, E.; Ryne, C. Alarm pheromones and chemical communication in nymphs of the tropical bed bug Cimex hemipterus (Hemiptera: Cimicidae). PLoS ONE 2011, 6, e18156. [Google Scholar] [CrossRef]
- Mendki, M.J.; Ganesan, K.; Parashar, B.D.; Sukumaran, D.; Prakash, S. Aggregation responses of Cimex hemipterus F. to semiochemicals identified from their excreta. J. Vector Borne Dis. 2014, 51, 224–229. [Google Scholar] [PubMed]
- Moreira, J.A.; Millar, J.G. Short and simple syntheses of 4-Oxo-(E)-2-hexenal and homologs: Pheromone components and defensive compounds of Hemiptera. J. Chem. Ecol. 2005, 31, 965–968. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons using rank sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. R Package Version 0.8.30. 2019. Available online: https://github.com/droglenc/FSA (accessed on 24 January 2020).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 31 October 2019).
- Feldlaufer, M.F.; Blomquist, G.J. Cuticular hydrocarbons from the bed bug Cimex lectularius L. Biochem. Syst. Ecol. 2011, 39, 283–285. [Google Scholar] [CrossRef]
| Cimex lectularius | ||||||||||
| Compound | Instar | |||||||||
| First | Second | Third | Fourth | Fifth | ||||||
| Mean ± SE | % abundance | Mean ± SE | % abundance | Mean ± SE | % abundance | Mean ± SE | % abundance | Mean ± SE | % abundance | |
| (E)-2-hexenal | 0.018 ± 0.002 a | 5.6 ± 0.3 | 0.074 ± 0.007 ab | 7.6 ± 0.8 | 0.158 ± 0.012 bc | 8.7 ± 0.8 | 0.315 ± 0.049 c | 11.6 ± 1.2 | 1.326 ± 0.292 c | 17.5 ± 1.8 |
| 4-oxo-(E)-2-hexenal | 0.083 ± 0.005 a | 26.9 ± 0.4 | 0.219 ± 0.020 a | 21.5 ± 1.3 | 0.628 ± 0.040 b | 33.2 ± 1.3 | 0.690 ± 0.101 b | 24.7 ± 1.7 | 1.472 ± 0.293 b | 21.1 ± 0.9 |
| (E)-2-octenal | 0.159 ± 0.010 a | 51.7 ± 0.4 | 0.586 ± 0.043 ab | 57.6 ± 0.9 | 0.951 ± 0.103 bc | 48.2 ± 1.4 | 1.496 ± 0.154 cd | 55.9 ± 1.6 | 3.624 ± 0.646 d | 56.2 ± 1.7 |
| 4-oxo-(E)-2-octenal | 0.048 ± 0.003 a | 15.8 ± 0.5 | 0.137 ± 0.013 b | 13.3 ± 0.6 | 0.198 ± 0.026 bc | 9.9 ± 0.6 | 0.217 ± 0.031 bc | 7.8 ± 0.5 | 0.311 ± 0.053 c | 5.3 ± 0.6 |
| Cimex hemipterus | ||||||||||
| Compound | Instar | |||||||||
| First | Second | Third | Fourth | Fifth | ||||||
| Mean ± SE | % abundance | Mean ± SE | % abundance | Mean ± SE | % abundance | Mean ± SE | % abundance | Mean ± SE | % abundance | |
| (E)-2-hexenal | 0.006 ± 0.002 a | 4.0 ± 0.6 | 0.022 ± 0.007 ab | 7.7 ± 1.2 | 0.255 ± 0.054 bc | 15.7 ± 1.2 | 0.854 ± 0.148 cd | 24.3 ± 1.4 | 1.460 ± 0.174 d | 20.6 ± 1.4 |
| 4-oxo-(E)-2-hexenal | 0.038 ± 0.008 a | 30.0 ± 1.4 | 0.079 ± 0.019 a | 32.6 ± 2.7 | 0.565 ± 0.083 b | 37.9 ± 1.1 | 1.178 ± 0.133 bc | 35.6 ± 1.1 | 2.639 ± 0.269 c | 37.1 ± 1.1 |
| (E)-2-octenal | 0.075 ± 0.013 a | 64.8 ± 1.8 | 0.119 ± 0.022 a | 59.4 ± 3.4 | 0.658 ± 0.075 b | 46.2 ± 1.6 | 1.297 ± 0.135 bc | 39.8 ± 1.0 | 2.922 ± 0.285 c | 41.6 ± 0.9 |
| 4-oxo-(E)-2-octenal | 0.001 ± 0.0003 ab | 1.2 ± 0.3 | 0.001 ± 0.0005 b | 0.3 ± 0.2 | 0.004 ± 0.002 ab | 0.3 ± 0.1 | 0.010 ± 0.002 bc | 0.3 ± 0.1 | 0.049 ± 0.009 c | 0.7 ± 0.1 |
| Instar | Compound | Cimex lectularius (Mean ± SE; µg/exuvia) | Cimex hemipterus (Mean ± SE; µg/exuvia) |
|---|---|---|---|
| First | (E)-2-hexenal | 0.018 ± 0.002 | 0.006 ± 0.002 *** |
| 4-oxo-(E)-2-hexenal | 0.083 ± 0.005 | 0.038 ± 0.008 ** | |
| (E)-2-octenal | 0.159 ± 0.010 | 0.075 ± 0.013 *** | |
| 4-oxo-(E)-2-octenal | 0.048 ± 0.003 | 0.001 ± 0.0003 *** | |
| Second | (E)-2-hexenal | 0.074 ± 0.007 | 0.022 ± 0.007 ** |
| 4-oxo-(E)-2-hexenal | 0.219 ± 0.020 | 0.079 ± 0.019 *** | |
| (E)-2-octenal | 0.586 ± 0.043 | 0.119 ± 0.022 *** | |
| 4-oxo-(E)-2-octenal | 0.137 ± 0.013 | 0.001 ± 0.0005 *** | |
| Third | (E)-2-hexenal | 0.158 ± 0.012 | 0.255 ± 0.054 |
| 4-oxo-(E)-2-hexenal | 0.628 ± 0.040 | 0.565 ± 0.083 | |
| (E)-2-octenal | 0.951 ± 0.103 | 0.658 ± 0.075 | |
| 4-oxo-(E)-2-octenal | 0.198 ± 0.026 | 0.004 ± 0.002 *** | |
| Fourth | (E)-2-hexenal | 0.315 ± 0.049 | 0.854 ± 0.148 ** |
| 4-oxo-(E)-2-hexenal | 0.690 ± 0.101 | 1.178 ± 0.133 * | |
| (E)-2-octenal | 1.496 ± 0.154 | 1.297 ± 0.135 | |
| 4-oxo-(E)-2-octenal | 0.217 ± 0.031 | 0.010 ± 0.002 *** | |
| Fifth | (E)-2-hexenal | 1.326 ± 0.292 | 1.460 ± 0.174 |
| 4-oxo-(E)-2-hexenal | 1.472 ± 0.293 | 2.639 ± 0.269 * | |
| (E)-2-octenal | 3.624 ± 0.646 | 2.922 ± 0.285 | |
| 4-oxo-(E)-2-octenal | 0.311 ± 0.053 | 0.049 ± 0.009 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dery, M.; Arriola, K.; Lee, C.-Y.; Choe, D.-H. Ontogenesis of Aldehyde Pheromones in Two Synanthropic Bed Bug Species (Heteroptera: Cimicidae). Insects 2020, 11, 759. https://doi.org/10.3390/insects11110759
Dery M, Arriola K, Lee C-Y, Choe D-H. Ontogenesis of Aldehyde Pheromones in Two Synanthropic Bed Bug Species (Heteroptera: Cimicidae). Insects. 2020; 11(11):759. https://doi.org/10.3390/insects11110759
Chicago/Turabian StyleDery, Mark, Kyle Arriola, Chow-Yang Lee, and Dong-Hwan Choe. 2020. "Ontogenesis of Aldehyde Pheromones in Two Synanthropic Bed Bug Species (Heteroptera: Cimicidae)" Insects 11, no. 11: 759. https://doi.org/10.3390/insects11110759







