Changes in Honey Bee Head Proteome in Response to Dietary 24-Methylenecholesterol
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Dietary Supplementation
2.2. Proteomics of Honey Bee Heads
2.2.1. Sample Preparation
2.2.2. Mass Spectrometry
2.3. Data Analysis
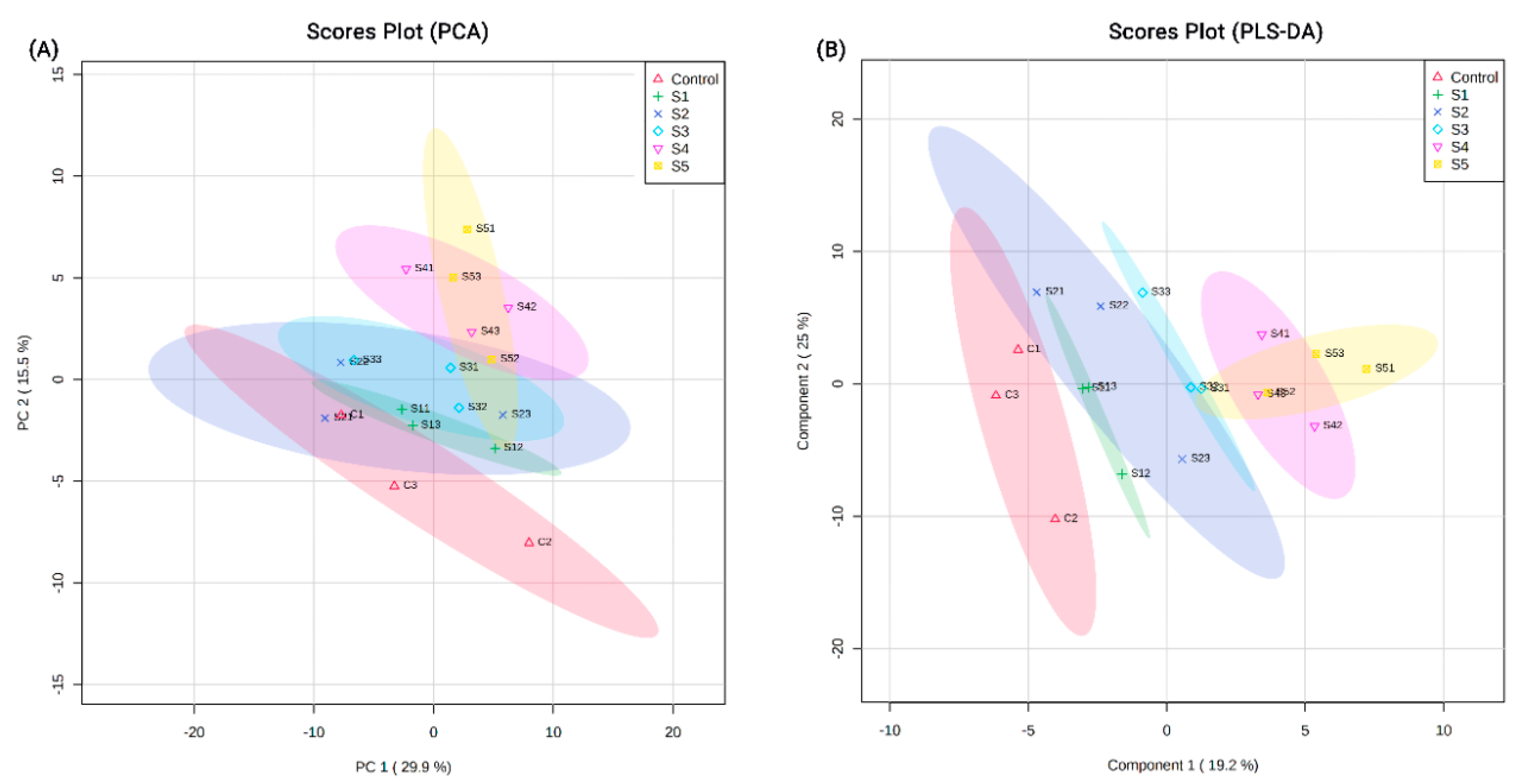
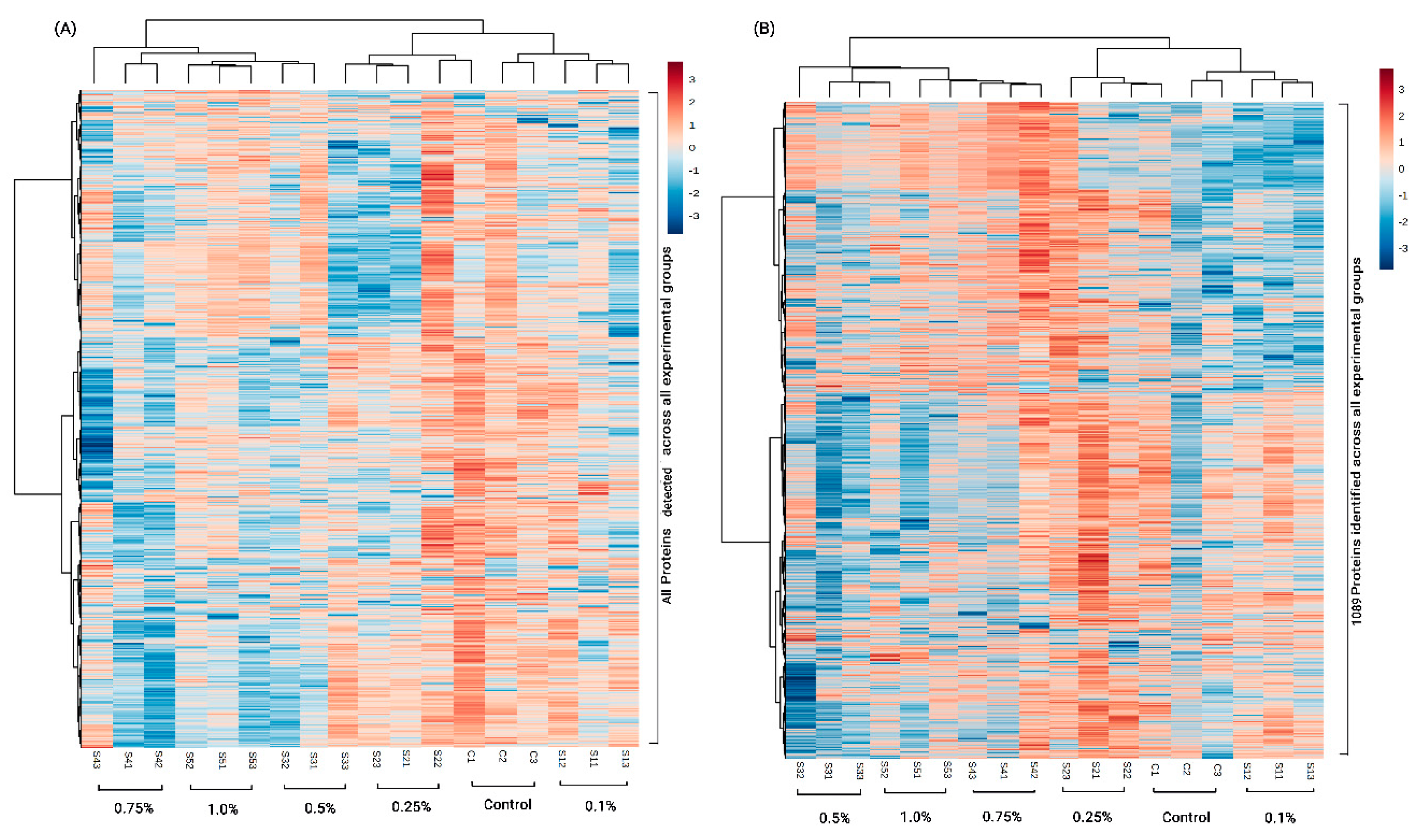
3. Results
3.1. Proteins Identified from Proteomic Analysis
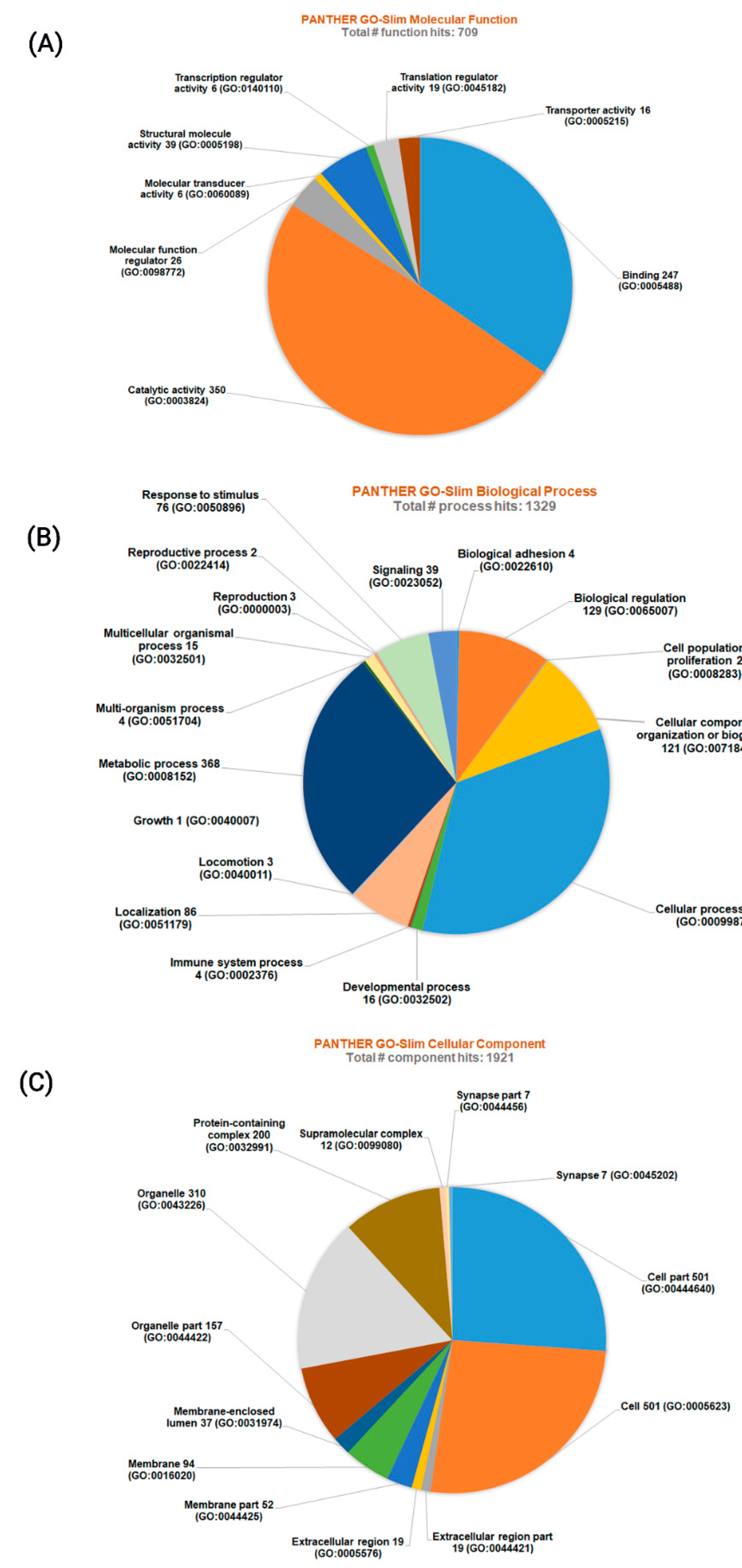
3.2. Protein-Protein Interactions and Functional Enrichments
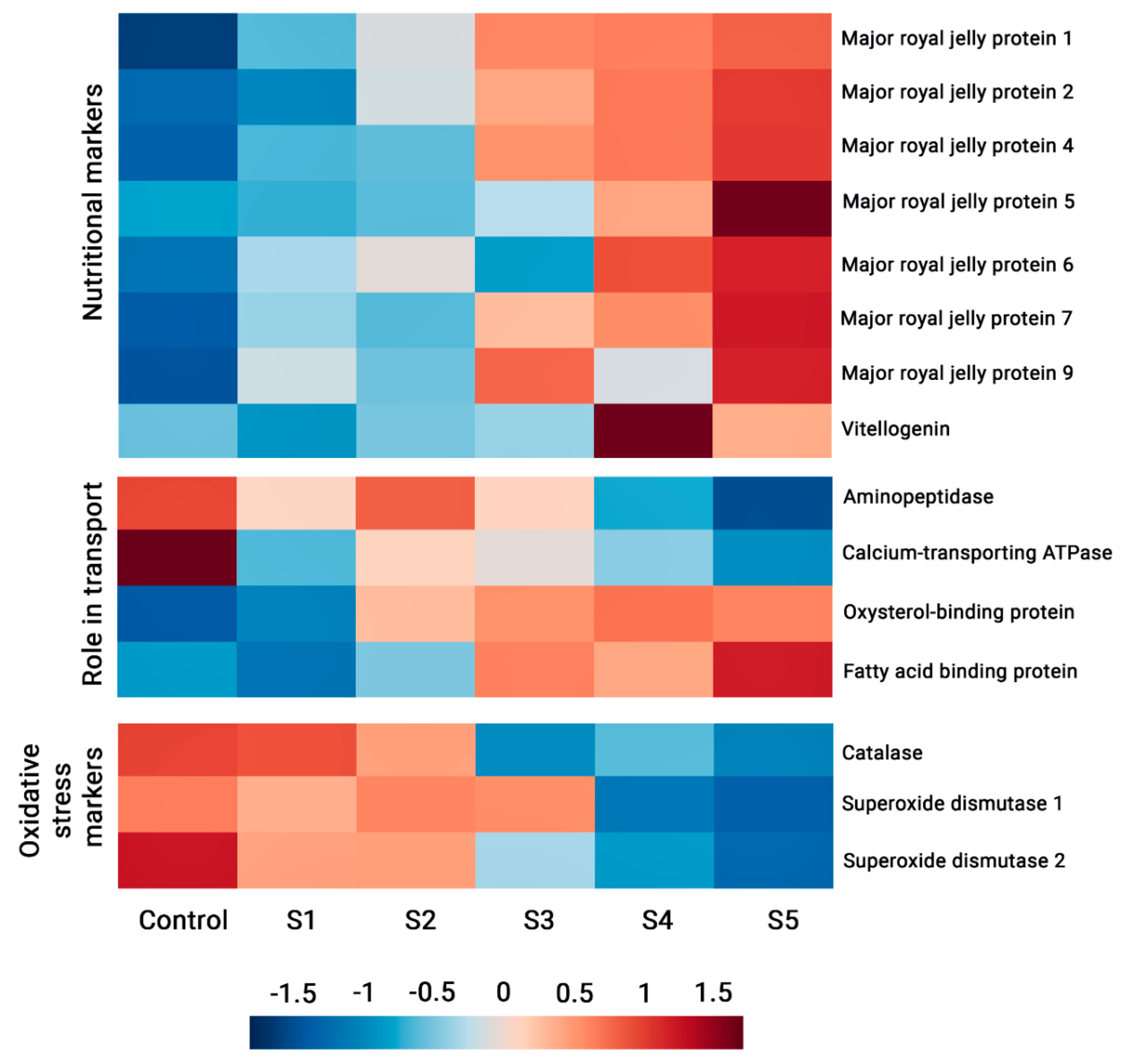
3.3. Classification of Proteins Identified
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alaux, C.; Ducloz, F.; Crauser, D.; Le Conte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010, 6, 562–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Alaux, C.; Dantec, C.; Parrinello, H.; Le Conte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and Varroa-parasitized bees. BMC Genom. 2011, 12, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmehl, D.R.; Teal, P.E.A.; Frazier, J.L.; Grozinger, C.M. Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J. Insect Physiol. 2014, 71, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; Morre, J.T.; Lucas, H.M.; Maier, C.S.; Sagili, R.R. The omics approach to bee nutritional landscape. Metabolomics 2019, 15, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, P.; Lucas, H.M.; Sagili, R.R. Evaluating effects of a critical micronutrient (24-methylenecholesterol) on honey bee physiology. Ann. Entomol. Soc. Am. 2019, 113, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behmer, S.T.; Nes, W.D. Insect sterol nutrition and physiology: A global overview. Adv. Insect Phys. 2003, 31, 1–72. [Google Scholar]
- Carvalho, M.; Schwudke, D.; Sampaio, J.L.; Palm, W.; Riezman, I.; Dey, G.; Gupta, G.D.; Mayor, S.; Riezman, H.; Shevchenko, A.; et al. Survival strategies of a sterol auxotroph. Development 2010, 137, 3675–3685. [Google Scholar] [CrossRef] [Green Version]
- Hobson, R.P. On a fat-soluble growth factor required by blow-fly larvae: Distribution and properties. Biochem. J. 1935, 29, 1292–1296. [Google Scholar] [CrossRef]
- Vanderplanck, M.; Moerman, R.; Rasmont, P.; Lognay, G.; Wathelet, B.; Wattiez, R.; Michez, D. How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS ONE 2014, 9, e86209. [Google Scholar] [CrossRef]
- Ferreira-Caliman, M.J.; da Silva, C.I.; Mateus, S.; Zucchi, R.; do Nascimento, F.S. Neutral sterols of cephalic glands of stingless bees and their correlation with sterols from pollen. Psyche 2012, 982802, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Svoboda, J.A.; Thompson, M.A.; Herbert, E.W.; Shortino, T.J.; Szczepanik-Vanleeuwen, P.A. Utilization and metabolism of dietary sterols in the honey bee and the yellow fever mosquito. Lipids 1982, 17, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, J.; Herbert, E.W., Jr.; Thompson, M.J.; Feldlaufer, M.F. Selective sterol transfer in the honey bee: Its significance and relationship to other Hymenoptera. Lipids 1986, 21, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.W.; Svoboda, J.A., Jr.; Thompson, M.J.; Shimanuki, H. Sterol utilization in honey bees fed a synthetic diet: Effects on brood rearing. J. Insect Physiol. 1980, 26, 287–289. [Google Scholar] [CrossRef]
- Feldlaufer, M.F. Biosynthesis of makisterone A and 20-hydroxyecdysone from labeled sterols by the honey bee. Arch. Insect Biochem. Physiol. 1986, 3, 415–421. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Lucas, H.M.; Sagili, R.R. Novel insights into dietary phytosterol utilization and its fate in honey bees (Apis mellifera L.). Molecules 2020, 25, 571. [Google Scholar] [CrossRef] [Green Version]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Knecht, D.; Kaatz, H.H. Patterns of larval food production by hypopharyngeal glands in adult worker honey bees. Apidologie 1990, 21, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Crailsheim, K.; Schneider, L.H.W.; Hrassnigg, N.; Bühlmann, G.; Brosch, U.; Gmeinbauer, R.; Schöffmann, B. Pollen consumption and utilization in worker honeybees (Apis mellifera carnica), dependence on individual age and function. J. Insect Physiol. 1992, 38, 409–419. [Google Scholar] [CrossRef]
- Zheng, A.; Li, J.; Begna, D.; Fang, Y.; Feng, M.; Song, F. Proteomic analysis of honeybee (Apis mellifera L.) pupae head development. PLoS ONE 2011, 6, e20428. [Google Scholar] [CrossRef] [Green Version]
- Nie, H.; Liu, X.; Pan, J.; Li, W.; Li, Z.; Zhang, S.; Chen, S.; Miao, X.; Zheng, N.; Su, S. Identification of genes related to high royal jelly production in the honey bee (Apis mellifera) using microarray analysis. Genet. Mol. Biol. 2017, 40, 781–789. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.Q.; Zheng, H.Q.; Corona, M.; Pirk, C.; Meng, F.; Zheng, Y.F.; Hu, F.L. Comparative transcriptome analysis on the synthesis pathway of honey bee (Apis mellifera) mandibular gland secretions. Sci. Rep. 2017, 7, 4530. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fan, R.-L.; Ji, W.-N.; Zhang, W.-W.; Chen, X.-M.; Wang, S.; Yin, L.; Gao, F.-C.; Chen, G.-H.; Ji, T. Transcriptome analysis of newly emerged honeybees exposure to sublethal carbendazim during larval stage. Front. Genet. 2018, 9, 426. [Google Scholar] [CrossRef] [Green Version]
- Zaluski, R.; Bittarello, A.C.; Vieira, J.C.S.; Braga, C.P.; Padilha, P.D.M.; da Silva Fernandes, M.; de Souza Bovi, T.; de Oliveira Orsi, R. Modification of the head proteome of nurse honeybees (Apis mellifera) exposed to field-relevant doses of pesticides. Sci. Rep. 2020, 10, 2190. [Google Scholar] [CrossRef] [Green Version]
- Milone, J.P.; Chakrabarti, P.; Sagili, R.R.; Tarpy, D.R. Colony-level pesticide exposure affects honey bee (Apis mellifera L.) royal jelly production and nutritional composition. Chemosphere 2021, 263, 128183. [Google Scholar] [CrossRef]
- Troyer, R.M.; Ruby, C.E.; Goodall, C.P.; Yang, L.; Maier, C.S.; Albarqi, H.A.; Brady, J.V.; Bathke, K.; Taratula, O.; Mourich, D.; et al. Exosomes from Osteosarcoma and normal osteoblast differ in proteomic cargo and immunomodulatory effects on T cells. Exp. Cell Res. 2017, 358, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; von Mering, C.; et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.; Poudel, S.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. PANTHER version 10: Expanded protein families and functions, and analysis tools. Nucleic Acids Res. 2016, 44, D336–D342. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Diz, A.P.; Carvajal-Rodríguez, A.; Skibinski, D.O.F. Multiple hypothesis testing in proteomics: A strategy for experimental work. Mol. Cell. Proteomics 2011, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kucharski, R.; Maleszka, R.A. Royal jelly protein is expressed in a subset of kenyon cells in the mushroom bodies of the honey bee brain. Naturwissenschaften 1998, 85, 343–346. [Google Scholar] [CrossRef]
- Drapeau, M.D.; Albert, S.; Kucharski, R.; Prusko, C.; Maleszka, R. Evolution of the yellow/major royal jelly protein family and the emergence of social behavior in honey bees. Genome Res. 2006, 16, 1385–1394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef]
- Tian, W.; Li, M.; Guo, H.; Peng, W.; Xue, X.; Hu, Y.; Liu, Y.; Zhao, Y.; Fang, X.; Wang, K.; et al. Architecture of the native major royal jelly protein 1 oligomer. Nat. Commun. 2018, 9, 3373. [Google Scholar] [CrossRef]
- Wyatt, G.R.; Davey, K.G. Cellular and molecular actions of juvenile hormone. II. Roles of juvenile hormone in adult insects. Adv. Insect Physiol. 1996, 26, 100–155. [Google Scholar]
- Bellés, X. Vitellogenesis directed by juvenile hormone. In Reproductive Biology of Invertebrates, Recent Progress in Vitellogenesis; Raikhel, A., Ed.; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Nelson, C.M.; Ihle, K.E.; Fondrk, M.K.; Page, R.E., Jr.; Amdam, G.V. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007, 5, e62. [Google Scholar] [CrossRef]
- Amdam, G.V.; Fennern, E.; Havukainen, H. Vitellogenin in honey bee behavior and lifespan. In Honeybee Neurobiology and Behavior, 2nd ed.; Galizia, C., Eisenhardt, D., Giurfa, M., Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Seehuus, S.-C.; Norberg, K.; Gimsa, U.; Krekling, T.; Amdam, G.V. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc. Natl. Acad. Sci. USA 2006, 103, 962–967. [Google Scholar] [CrossRef] [Green Version]
- Weisiger, R.A. Cytosolic fatty acid binding proteins catalyze two distinct steps in intracellular transport of their ligands. Mol. Cell. Biochem. 2002, 239, 35–42. [Google Scholar] [CrossRef]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489. [Google Scholar] [CrossRef] [Green Version]
- Raychaudhuri, S.; Prinz, W.A. The diverse functions of oxysterol-binding proteins. Annu. Rev. Cell Dev. Biol. 2010, 26, 157–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A. Aminopeptidases: Structure and function. FASEB J. 1993, 7, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.P.; Buckby, L.E.; Empson, R.M. Expression of plasma membrane Ca2+ ATPase family members and associated synaptic proteins in acute and cultured organotypic hippocampal slices from rat. Develop. Brain Res. 2004, 152, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; Rana, S.; Sarkar, S.; Smith, B.; Basu, P. Pesticide-induced oxidative stress in laboratory and field populations of native honey bees along intensive agricultural landscapes in two Eastern Indian states. Apidologie 2015, 46, 107–129. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, J. Universal behavior of membranes with sterols. Biophys. J. 2006, 90, 1639–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakrabarti, P.; Sagili, R.R. Changes in Honey Bee Head Proteome in Response to Dietary 24-Methylenecholesterol. Insects 2020, 11, 743. https://doi.org/10.3390/insects11110743
Chakrabarti P, Sagili RR. Changes in Honey Bee Head Proteome in Response to Dietary 24-Methylenecholesterol. Insects. 2020; 11(11):743. https://doi.org/10.3390/insects11110743
Chicago/Turabian StyleChakrabarti, Priyadarshini, and Ramesh R. Sagili. 2020. "Changes in Honey Bee Head Proteome in Response to Dietary 24-Methylenecholesterol" Insects 11, no. 11: 743. https://doi.org/10.3390/insects11110743
APA StyleChakrabarti, P., & Sagili, R. R. (2020). Changes in Honey Bee Head Proteome in Response to Dietary 24-Methylenecholesterol. Insects, 11(11), 743. https://doi.org/10.3390/insects11110743




