Lethal Doses of Saponins from Quillaja saponaria for Invasive Slug Arion vulgaris and Non-Target Organism Enchytraeus albidus (Olygochaeta: Enchytraeidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Maintenance of Invertebrates
2.2. Molluscicidal Activity of Saponins on A. vulgaris Slugs
2.3. Wormicidal Activity of Saponins on E. albidus Worms
2.4. Statistical Analysis
3. Results
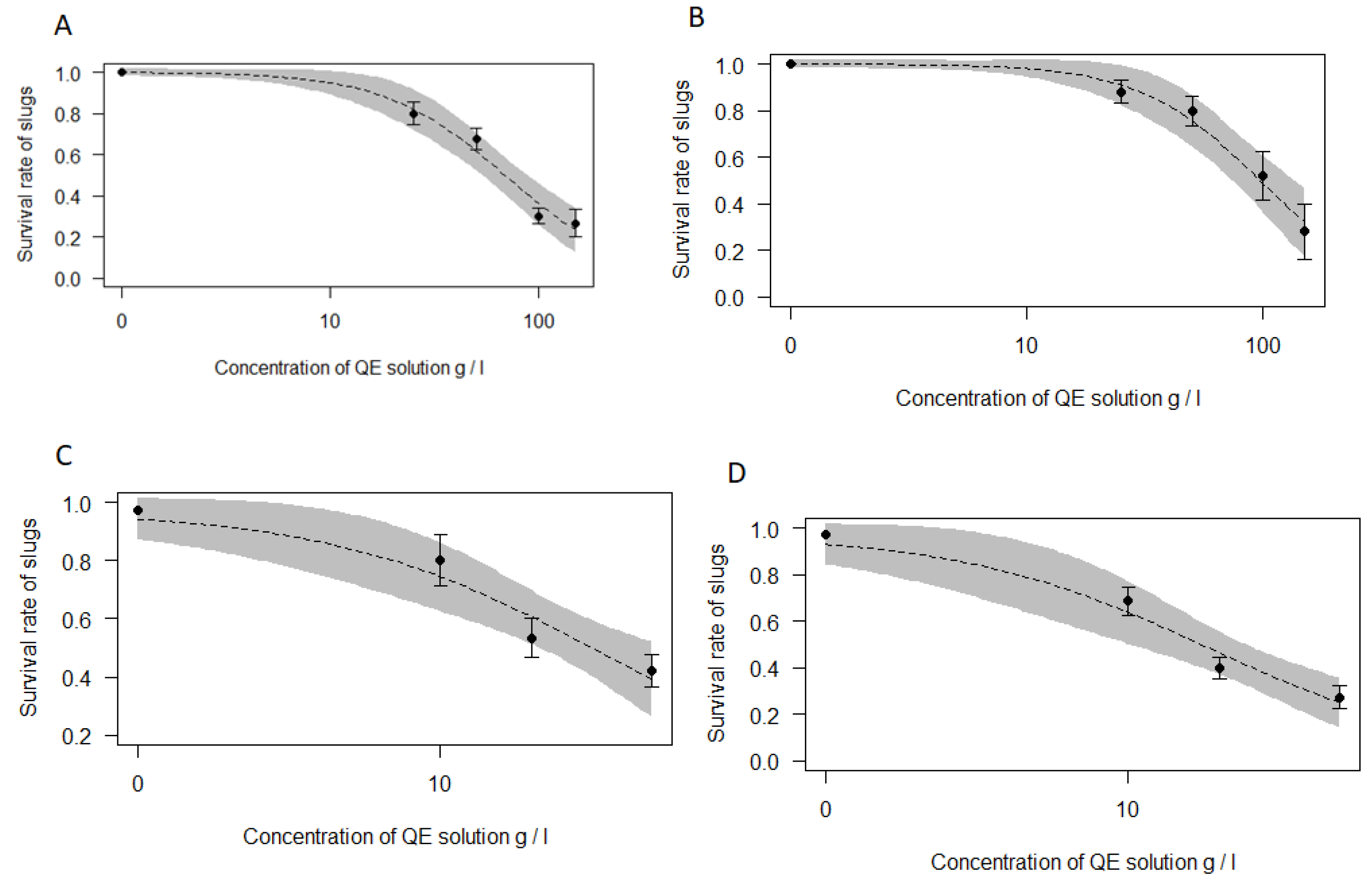
3.1. Molluscicidal Activity of Saponins on A. vulgaris Slugs
3.2. Wormicidal Activity of Saponins on E. albidus Worms
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- South, A. Terrestrial Slugs: Biology, Ecology and Control; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Karthiga, S.; Jegathambigai, V.; Karunaranthe, M.; Svinningen, A.; Mikunthan, G. Snails and slugs damaging the cut foliage, Cordyline fruticosa and use of biorationals towards their management. Commun. Agric. Appl. Biol. Sci. 2012, 77, 691–698. [Google Scholar] [PubMed]
- Norris, R.F.; Caswell-Chen, E.P.; Kogan, M. Concepts in Integrated Pest Management; Prentice Hall: Upper Saddle River, NJ, USA, 2003; ISBN 978-0-13-087016-2. [Google Scholar]
- Crowl, T.A.; Crist, T.O.; Parmenter, R.R.; Belovsky, G.; Lugo, A.E. The spread of invasive species and infectious disease as drivers of ecosystem change. Front. Ecol. Env. 2008, 6, 238–246. [Google Scholar] [CrossRef]
- Kerney, M.P. Atlas of the Land and Freshwater Molluscs of Britain and Ireland; Harley Books: Chichester, UK, 1999; ISBN 978-0-946589-48-7. [Google Scholar]
- Kozłowski, J. The distribution, biology, population dynamics and harmfulness of Arion lusitanicus Mabille, 1868 (Gastropoda: Pulmonata: Arionidae) in Poland. J. Plant Prot. Res. 2007, 47, 119–230. [Google Scholar]
- Nabeerasool, M.; Campen, A.; Polya, D.; Brown, N.; van Dongen, B. Removal of metaldehyde from water using a novel coupled adsorption and electrochemical destruction technique. Water 2015, 7, 3057–3071. [Google Scholar] [CrossRef] [Green Version]
- Capinera, J.L. Assessment of barrier materials to protect plants from Florida leatherleaf slug (Mollusca: Gastropoda: Veronicellidae). Fla. Entomol. 2018, 101, 373–381. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. Is a combination of different natural substances suitable for slug (Arion spp.) control? Span. J. Agric. Res. 2016, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Grubišić, D.; Gotlin Čuljak, T.; Mešić, A.; Juran, I.; Loparić, A.; Starčević, D.; Brmež, M.; Benković- Lačić, T. Slug control in leafy vegetable using nematode Phasmarhabditis hermaphrodita (Schneider). Appl. Ecol. Env. Res. 2018, 16, 1739–1747. [Google Scholar] [CrossRef]
- Grimm, B. Effect of nematode Phasmarhabditis hermaphrodita on young stages of the pest slug Arion lusitanicus. J. Moll. Stud. 2002, 68, 25–28. [Google Scholar] [CrossRef]
- Laznik, Z.; Bohinc, T.; Franin, K.; Majić, I.; Trdan, S. Efficacy of invasive alien plants in controlling Arionidae slugs. Span. J. Agric. Res. 2020, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- González-Cruz, D.; San Martín, R. Molluscicidal effects of saponin-rich plant extracts on the grey field slug. Cienc. Inv. Agr. 2013, 40, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Güçlü-Üstündağ, Ö.; Mazza, G. Saponins: Properties, applications and processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Melzig, M.F.; Fuchs, H.; Weng, A. Chemistry and pharmacology of saponins: Special focus on cytotoxic properties. Botanics 2011, 1, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Wang, Y.-M.; Wang, J.-F.; Xue, Y.; Li, Z.-J.; Nagao, K.; Yanagita, T.; Xue, C.-H. Dietary saponins of sea cucumber alleviate orotic acid-induced fatty liver in rats via PPARa and SREBP-1c signaling. Lipids Health Dis. 2010, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Walkowicz, W.E.; Fernández-Tejada, A.; George, C.; Corzana, F.; Jiménez-Barbero, J.; Ragupathi, G.; Tan, D.S.; Gin, D.Y. Quillaja saponin variants with central glycosidic linkage modifications exhibit distinct conformations and adjuvant activities. Chem. Sci. 2016, 7, 2371–2380. [Google Scholar] [CrossRef] [Green Version]
- Slotsbo, S.; Hansen, L.M.; Holmstrup, M. Low temperature survival in different life stages of the Iberian slug, Arion lusitanicus. Cryobiol. 2011, 62, 68–73. [Google Scholar] [CrossRef]
- Pulleman, M.; Creamer, R.; Hamer, U.; Helder, J.; Pelosi, C.; Pérès, G.; Rutgers, M. Soil biodiversity, biological indicators and soil ecosystem services-an overview of European approaches. Curr. Opin. Env. Sust. 2012, 4, 529–538. [Google Scholar] [CrossRef]
- Erséus, C.; Klinth, M.J.; Rota, E.; De Wit, P.; Gustafsson, D.R.; Martinsson, S. The popular model annelid Enchytraeus albidus is only one species in a complex of seashore white worms (Clitellata, Enchytraeidae). Org. Divers. Evol. 2019, 19, 105–133. [Google Scholar] [CrossRef] [Green Version]
- Rowson, B.; Turner, J.A.; Anderson, R.; Symondson, B. The Slugs of Britain and Ireland: Identification, Understanding and Control; Field Studies Council: Shropshire, UK, 2014. [Google Scholar]
- Bindesbøl, A.-M.; Bayley, M.; Damgaard, C.; Holmstrup, M. Life-history traits and population growth rate in the laboratory of the earthworm Dendrobaena octaedra cultured in copper-contaminated soil. Appl. Soil Ecol. 2007, 35, 46–56. [Google Scholar] [CrossRef]
- Iglesias, J.; Castillejo, J.; Ester, A. Laboratory evaluation of potential molluscicides for the control of eggs of the pest slug Deroceras reticulatum (Müller) (Pulmonata: Limacidae). Int. J. Pest. Manag. 2002, 48, 19–23. [Google Scholar] [CrossRef]
- Souza, B.A.; Silva, L.C.D.; Chicarino, E.D.; Bessa, E.C.A. Preliminary phytochemical screening and molluscicidal activity of the aqueous extract of Bidens pilosa Linné (Asteraceae) in Subulina octona (Mollusca, Subulinidade). Acad. Bras. Ciênc. 2013, 85, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD Guidelines for the Testing of Chemicals (No. 220). Enchytraeid Reproduction Test; Organization for Economic Co-operation and Development: Paris, France, 2016. [Google Scholar] [CrossRef]
- Ritz, C.; Streibig, J. Bioassay analysis using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef] [Green Version]
- De Mendes, R.J.A.; Pereira Filho, A.A.; Nogueira, A.D.J.L.; Araújo, K.R.F.; França, C.R.C.; Carvalho, I.B.D.; Silva, N.M.L.D.; Azevedo, A.S.; Rosa, I.G. Evaluation of molluscicidal activity of three mangrove species (Avicennia schaueriana, Laguncularia racemosa and Rhizophora mangle) and their effects on the bioactivity of Biomphalaria glabrata Say, 1818. Rev. Inst. Med. Trop. S. Paulo 2018, 60, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Barone, M.; Frank, T. Effects of plant extracts on the feeding behaviour of the slug Arion lusitanicus. Ann. Appl. Biol. 1999, 134, 341–345. [Google Scholar] [CrossRef]
- Huang, H.-C.; Liao, S.-C.; Chang, F.-R.; Kuo, Y.-H.; Wu, Y.-C. Molluscicidal saponins from Sapindus mukorossi, inhibitory agents of golden apple snails, Pomacea canaliculata. J. Agric. Food Chem. 2003, 51, 4916–4919. [Google Scholar] [CrossRef]
- Chaieb, I.; Tayeb, W. Comparison of the molluscicidal activity of Cestrum parqui (Solanaceae) and Quillaja saponaria (Quillajaceae) saponins. Tunis. J. Med. Plants Nat. Prod. 2009, 2, 31–35. [Google Scholar]
- Chen, Y.Q.; Xu, Q.M.; Liu, Y.L.; Li, X.R.; Yang, S.L.; Zhuge, H.X. Laboratory evaluation of the molluscicidal activity of Pulsatilla Chinensis (Bunge) regel saponins against the snail Oncomelania Hupensis. Biomed. Env. Sci. 2012, 25, 224–229. [Google Scholar] [CrossRef]
- Adewumi, A.A.J.; Aina, V.O.; Zhang, C.S.; Che, Z. Assessment of the molluscicidal activities of Sasanqua saponin. Curr. Res. J. Biol. Sci. 2013, 5, 1–4. [Google Scholar] [CrossRef]
- Frank, T.; Biert, K.; Speiser, B. Feeding deterrent effect of carvone, a compound from caraway seeds, on the slug Arion lusitanicus. Ann. Appl. Biol. 2002, 141, 93–100. [Google Scholar] [CrossRef]
- Potter, D.A.; Redmond, C.T.; Meepagala, K.M.; Williams, D.W. Managing earthworm casts (Oligochaeta: Lumbricidae) in turfgrass using a natural byproduct of tea oil (Camellia sp.) manufacture. Pest. Manag. Sci. 2010, 66, 439–446. [Google Scholar] [CrossRef]
- Fischer, M.J.C.; Pensec, F.; Demangeat, G.; Farine, S.; Chong, J.; Ramírez-Suero, M.; Mazet, F.; Bertsch, C. Impact of Quillaja saponaria saponins on grapevine ecosystem organisms. Antonie Van Leeuwenhoek 2011, 100, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Anto, F.; Aryeetey, M.E.; Anyorigiya, T.; Asoala, V.; Kpikpi, J. The relative susceptibilities of juvenile and adult Bulinus globosus and Bulinus truncatus to the molluscicidal activities in the fruit of Ghanaian Blighia sapida, Blighia unijugata and Balanites aegyptiaca. Ann. Trop. Med. Parasitol. 2005, 99, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, D.; Parashar, B.; Gupta, A.; Jeevaratnam, K.; Prakash, S. Molluscicidal effect of nicotinanilide and its intermediate compounds against a freshwater snail Lymnaea luteola, the vector of animal schistosomiasis. Mem. Inst. Oswaldo Cruz 2004, 99, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; W. H. Freeman: New York, NY, USA, 2012. [Google Scholar]
- Lee, R.E.; Damodaran, K.; Yi, S.X.; Lorigan, G.A. Rapid cold-hardening increases membrane fluidity and cold tolerance of insect cells. Cryobiology 2006, 52, 459–463. [Google Scholar] [CrossRef]
- Chaieb, I. Saponins as insecticides: A review. Tunis. J. Plant Prot. 2010, 5, 39–50. [Google Scholar]
- Dowd, P.F.; Berhow, M.A.; Johnson, E.T. Differential activity of multiple saponins against omnivorous insects with varying feeding preferences. J. Chem. Ecol. 2011, 37, 443–449. [Google Scholar] [CrossRef]
- Holmstrup, M.; Krogh, P.H. Effects and risk assessment of linear alkylbenzene sulfonates in agricultural soil. 3. Sublethal effects on soil invertebrates. Env. Toxicol. Chem. 2001, 20, 1673–1679. [Google Scholar] [CrossRef]
- Römbke, J. Ecotoxicological laboratory tests with enchytraeids: A review. Pedobiologia 2003, 47, 607–616. [Google Scholar] [CrossRef]

| Species of Groups | Number of Individual | Temperature (°C) | Weight of Animal, Av ± SE (g) | Duration of Exposure (Days) |
|---|---|---|---|---|
| A. vulgaris ad. | 125 | +15 | 8.3 ± 2.5 | 2 |
| A. vulgaris ad. | 255 | +2 | 8.4 ± 0.3 | 2 |
| A. vulgaris ad. | 170 | −1 | 8.7 ± 0.3 | 2 |
| A. vulgaris juv. | 125 | +15 | 0.05 ± 0.01 | 2 |
| E. albidus | 350 | +6 | 0.04 ± 0.001 | 14 |
| E. albidus | 230 | −1 | 0.04 ± 0.001 | 14 |
| Species | Life Stage | Temperature (°C) | LC50 | Slope | χ2 (df, p) |
|---|---|---|---|---|---|
| A. vulgaris | Adult | +15 | 68.52 ± 8.38 | 1.51 ± 0.29 | 15.68 (27, 0.9588) |
| A. vulgaris | Juvenile | +15 | 96.94 ± 13.84 | 1.71 ± 0.4 | 16.731 (17, 0.4727) |
| A. vulgaris | Adult | +2 | 33.52 ± 7.49 | 0.98 ± 0.31 | 29.077 (30, 0.5135) |
| A. vulgaris | Adult | −1 | 18.38 ± 3.64 | 1.06 ± 0.3 | 20.986 (31, 0.9123) |
| E. albidus | Adult | +6 | 2.72 ± 0.09 | 11.85 ± 2.45 | 22.792 (29, 0.7857) |
| E. albidus | Adult | −1 | 2.07 ± 0.11 | 9.09 ± 2.61 | 51.252 (46, 0.2753) |
| Species and Age Group | Temperature (°C) | Duration of Exposure (Days) | Plant Aqueous (or Methanol) Extract | LC50 (g/L) | Reference |
|---|---|---|---|---|---|
| Molluscs | |||||
| Arion vulgaris, ad. | +15 | 2 | Quillaja saponaria | 68.52 | Present study |
| +2 | 2 | 33.52 | |||
| −1 | 2 | 18.38 | |||
| A. vulgaris juv. | +15 | 2 | Q. saponaria | 96.94 | Present study |
| Deroceras | +15 | 5 | Q. saponaria | 40 | [13] |
| reticulatum, ad. | +15 | 5 | Gleditchia amorphoides | ≈20 | |
| +15 | 5 | Camelia oleifera | ≈20 | ||
| Subulina octona, ad. | +21–24 | 2 | Bidens pilosa | ≈51.4 | [25] |
| Theba pisana, ad. | +21–24 | 1 | Q. saponaria | ≈0.54 | [31] |
| +21–24 | 1 | Cestrum parqui * | ≈0.04 | ||
| T. pisana, juv. | +21–24 | 1 | Q. saponaria | ≈0.29 | |
| +21–24 | 1 | C. parqui * | ≈0.01 | ||
| Pomacea canaliculata, ad. | +21–24 | 2 | Sapindus mucorossi * | ≈0.02 | [30] |
| Oncomelania hupensis, ad. | +25 | 1 | Pulsatilla chinensis | ≈0.001 | [32] |
| +21–24 | 2 | Camelia sasanqua | ≈0.002 | [33] | |
| Worms | |||||
| Enchytraeus albidus | +6 | 14 | Q. saponaria | 2.84 | Present study |
| (Olygochaeta), ad. | −1 | 14 | 2.16 | ||
| Xyphinema index | +22 | 3 | Q. saponaria | ≈0.15 | [36] |
| (Nematode), ad. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adomaitis, M.; Skujienė, G. Lethal Doses of Saponins from Quillaja saponaria for Invasive Slug Arion vulgaris and Non-Target Organism Enchytraeus albidus (Olygochaeta: Enchytraeidae). Insects 2020, 11, 738. https://doi.org/10.3390/insects11110738
Adomaitis M, Skujienė G. Lethal Doses of Saponins from Quillaja saponaria for Invasive Slug Arion vulgaris and Non-Target Organism Enchytraeus albidus (Olygochaeta: Enchytraeidae). Insects. 2020; 11(11):738. https://doi.org/10.3390/insects11110738
Chicago/Turabian StyleAdomaitis, Mantas, and Grita Skujienė. 2020. "Lethal Doses of Saponins from Quillaja saponaria for Invasive Slug Arion vulgaris and Non-Target Organism Enchytraeus albidus (Olygochaeta: Enchytraeidae)" Insects 11, no. 11: 738. https://doi.org/10.3390/insects11110738





