Activity of Ajuga iva Extracts Against the African Cotton Leafworm Spodoptera littoralis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Extraction and Purification of Phytoecdysteroids
2.3. Identification of Phytoecdysteroids and Clerodanes
2.4. Biological Activity of A. iva Crude Leaf Extract against S. littoralis
2.5. Effect of A. iva Crude Leaf Extract and Purified Phytoecdysteroid Mixture on Larval Gut of S. littoralis
3. Results
3.1. Identification of Natural Phytoecdysteroids from A. iva by TLC Analysis
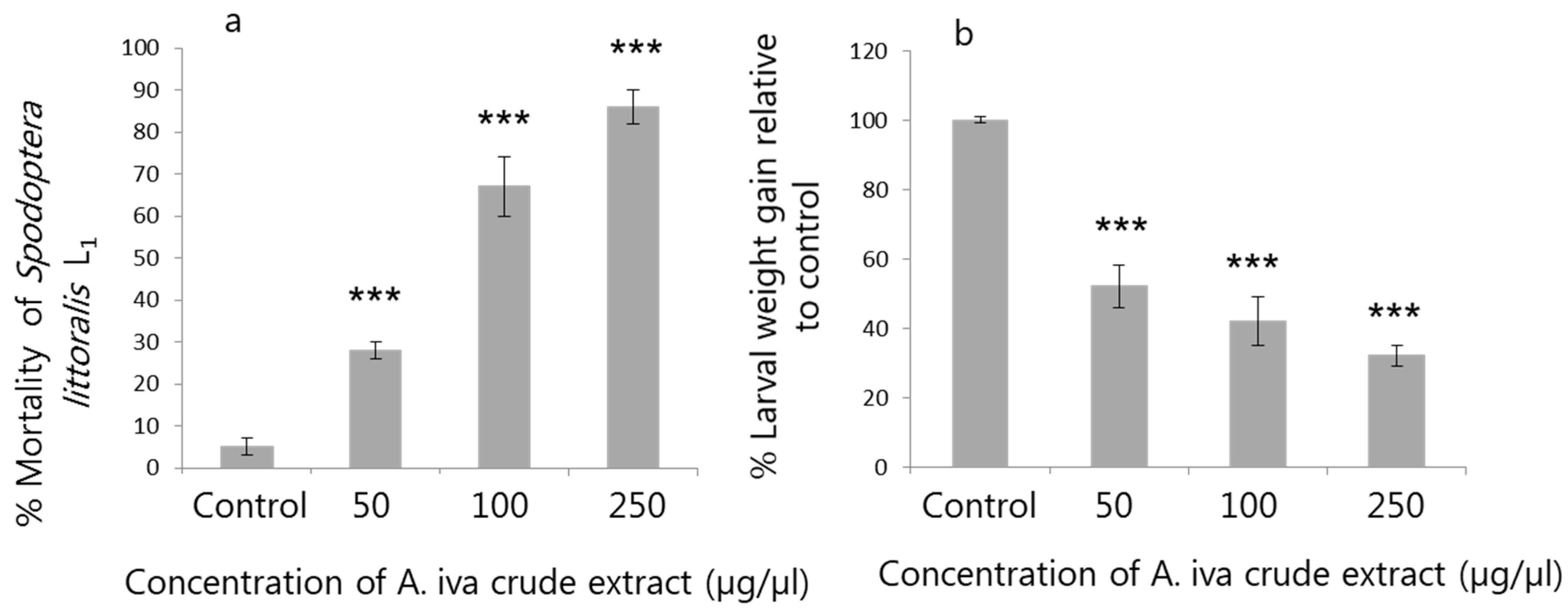
3.2. Effect of A. iva Crude Leaf Extract on First-Instar Larval Survival
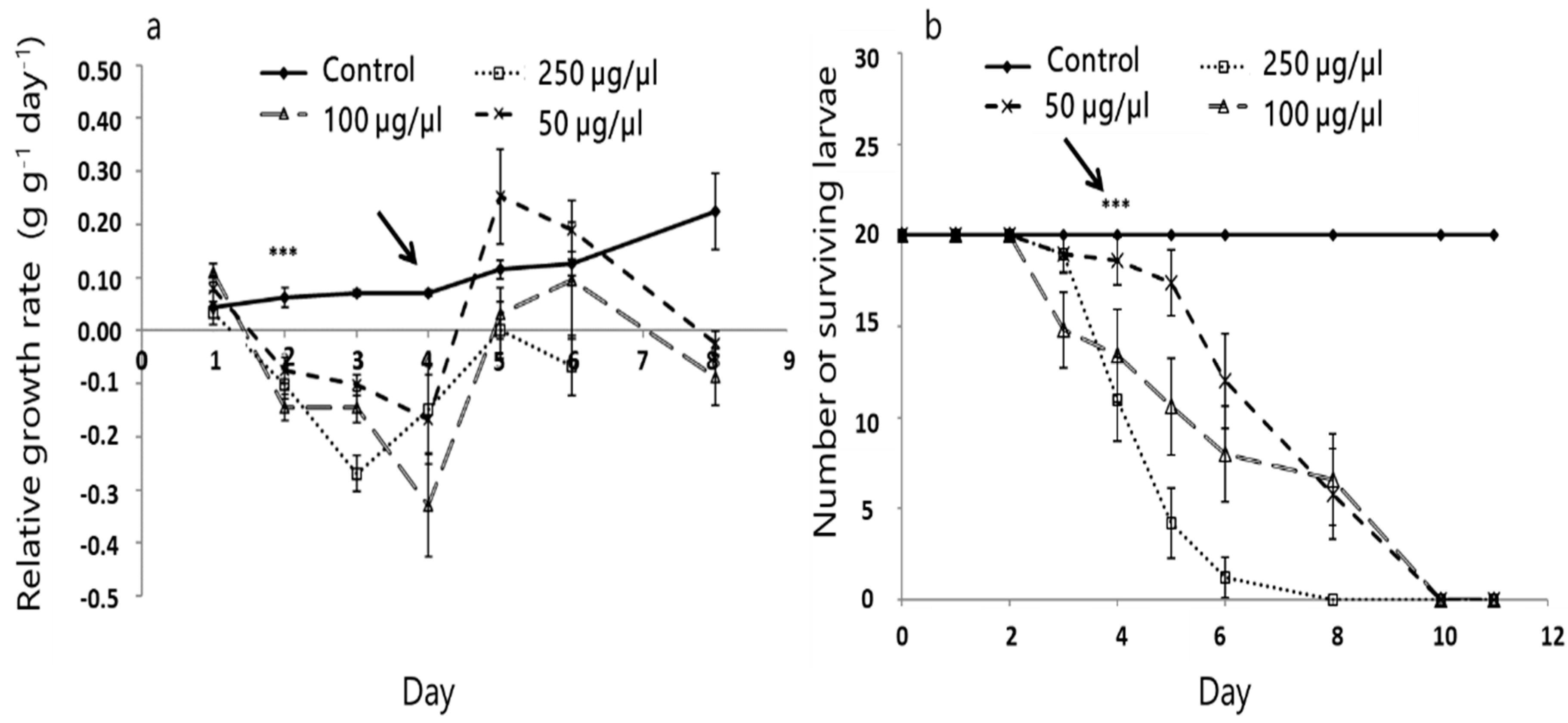
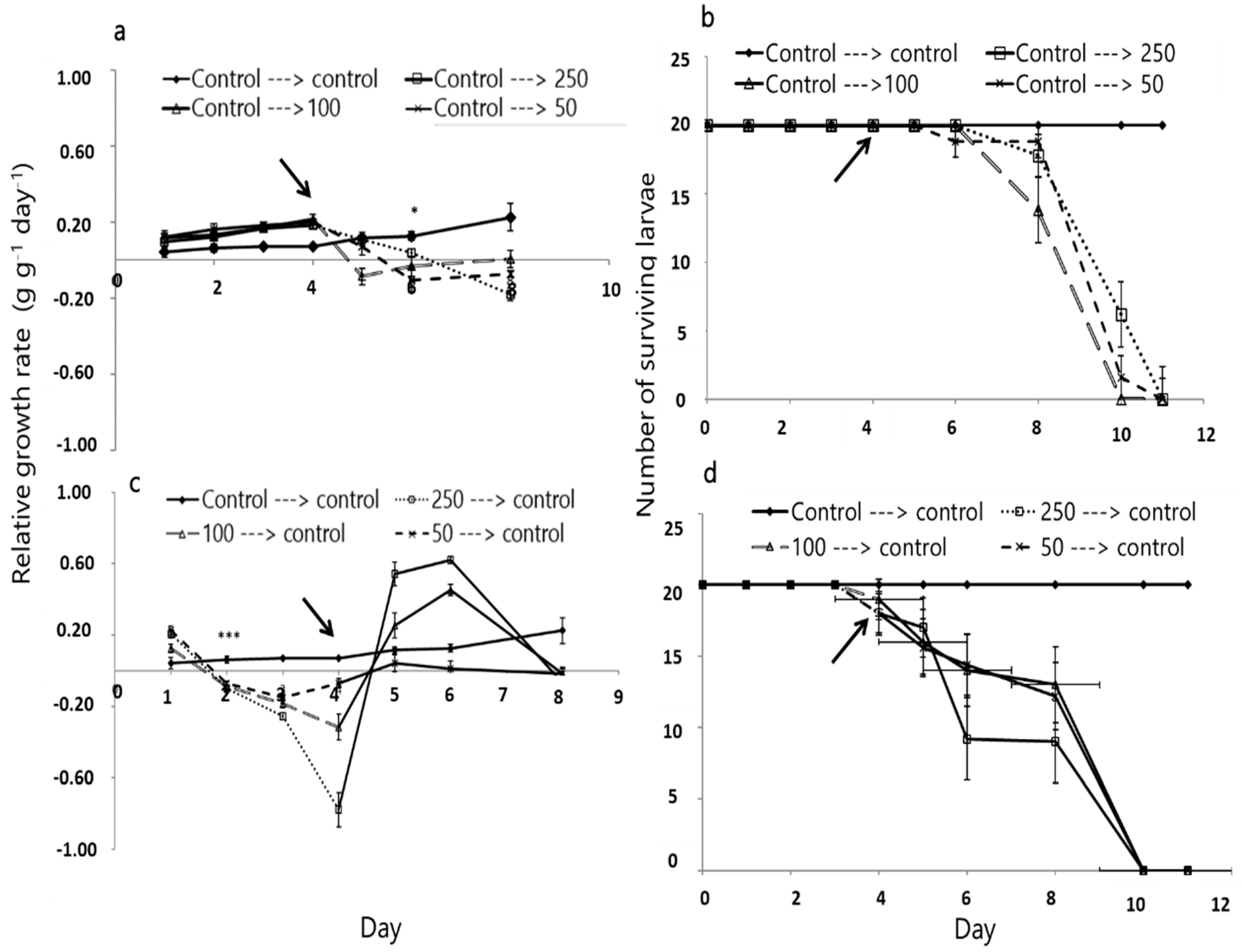
3.3. Effect of A. iva Crude Leaf Extract on Third-Instar Larval Survival and Development
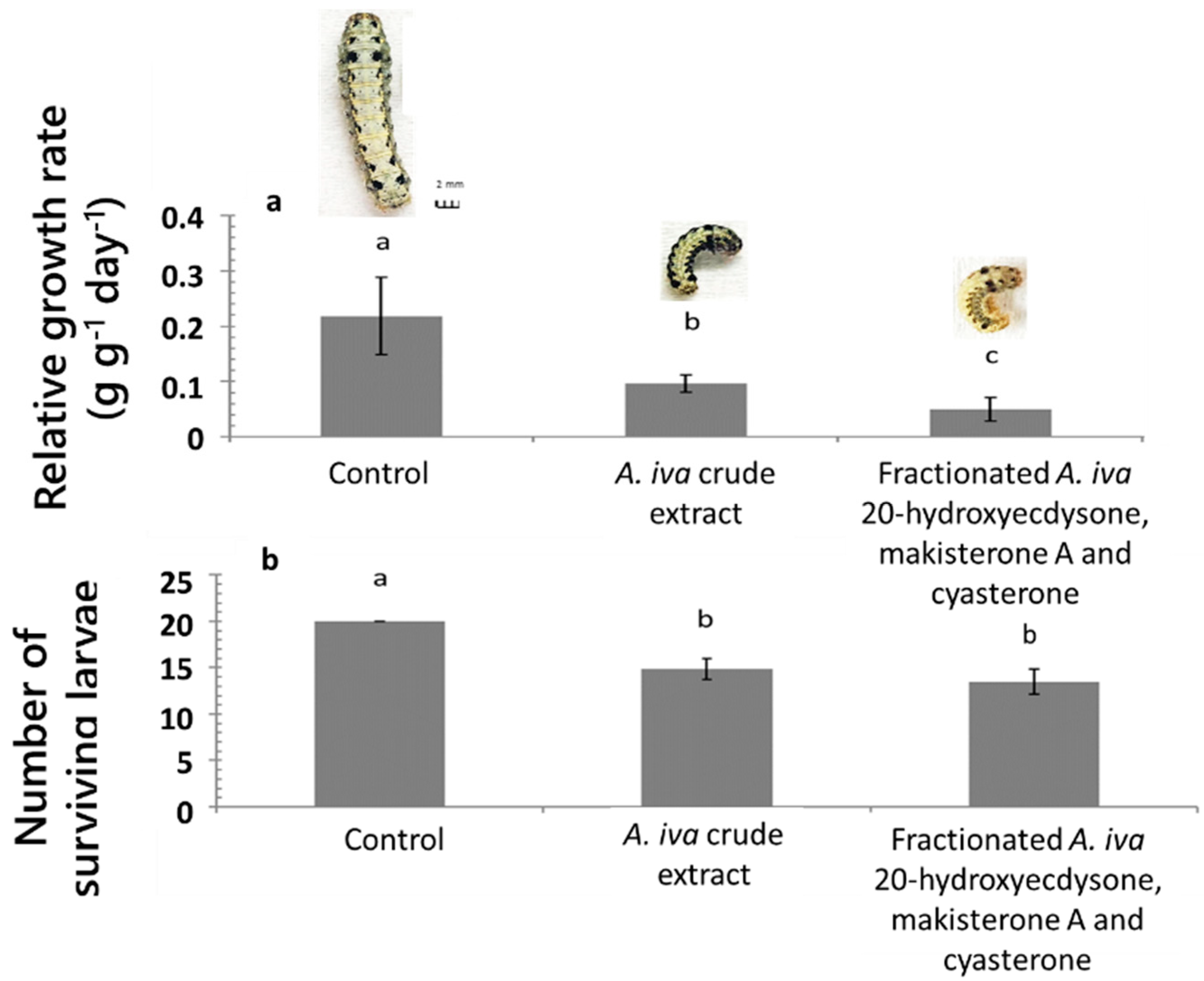
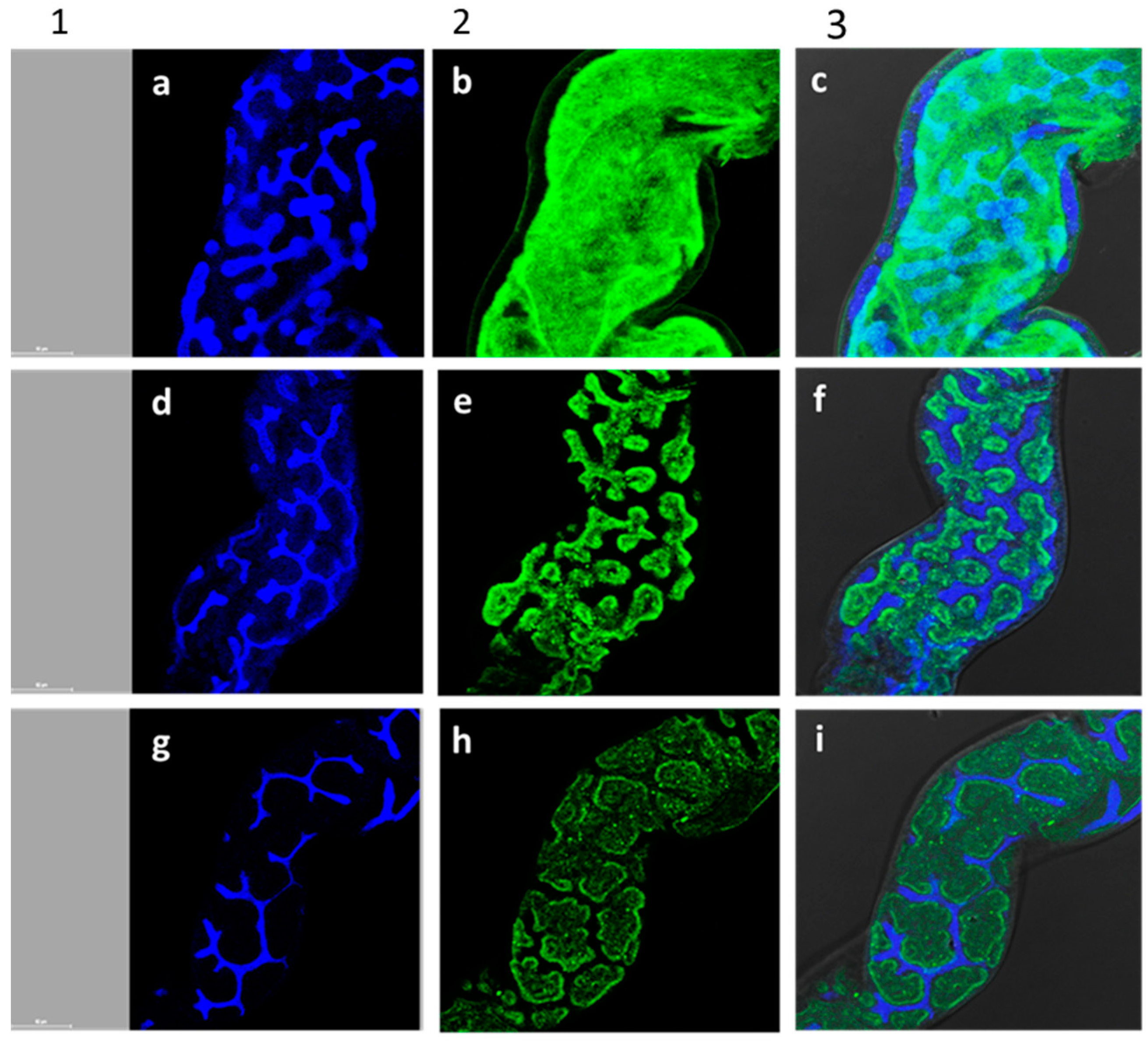
3.4. Effect of A. iva Crude Leaf Extract and Purified Phytoecdysteroid Mixture on Larval Gut of S. littoralis
3.5. Pupation of S. littoralis following Biological Activity Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martinez, S.S.; van Emden, H.F. Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) caused by Azadirachtin. Neotrop. Entomol. 2001, 30, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Hatem, A.E.S.; Aldebis, H.K.; Osuna, E.V. Effects of the Spodoptera littoralis granulovirus on the development and reproduction of cotton leafworm S. littoralis. Biol. Contr. 2011, 5, 192–199. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Khasdan, V.; Ishaaya, I. Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch. Insect Biochem. Physiol. 2005, 58, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L. Phytoecdysteroids: Biological aspects. Phytochemistry 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Sandlund, L.; Kongshaug, H.; Horsberg, T.E.; Male, R.; Nilsen, F.; Dalvin, S. Identification and characterisation of the ecdysone biosynthetic genes neverland, disembodied and shade in the salmon louse Lepeophtheirus salmonis (Copepoda, Caligidae). PLoS ONE 2018, 13, e0191995. [Google Scholar] [CrossRef] [Green Version]
- Dinan, L. A strategy for the identification of ecdysteroid receptor agonists and antagonists from plants. Eur. J. Entomol. 1995, 92, 271–283. [Google Scholar]
- Sadek, M.M. Antifeedant and toxic activity of Adhatoda vasica leaf extract against Spodoptera littoralis (Lep., Noctuidae). J. Appl. Entomol. 2003, 127, 396–404. [Google Scholar] [CrossRef]
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: An update. J. Insect Sci. 2003, 3, 7. [Google Scholar] [CrossRef]
- Dinan, L. Ecdysteroid structure and hormonal activity. In Ecdysone: From Chemistry to Mode of Action; Koolman, J., Ed.; George Thieme Verlag: Stuttgart, Germany, 1989; pp. 345–354. [Google Scholar]
- Lafont, R. Ecdysteroids and related molecules in animals and plants. Arch. Insect Biochem. Physiol. 1997, 35, 3–20. [Google Scholar] [CrossRef]
- Grieneisen, M.L.; Warren, J.T.; Sakurai, S.; Gilbert, L.I. A putative route to ecdysteroids: Metabolism of cholesterol in vitro by mildly disrupted prothoracic glands of Manduca sexta. Insect Biochem. 1991, 21, 41–51. [Google Scholar] [CrossRef]
- Thummel, C.S.; Chory, J. Steroid signaling in plants and insects—common themes, different pathways. Genes Dev. 2002, 16, 3113–3129. [Google Scholar] [CrossRef] [Green Version]
- Riddiford, L.M.; Cherbas, P.; Truman, J.W. Ecdysone receptors and their biological actions. Vitam. Horm. 2000, 60, 1–73. [Google Scholar]
- Otey, C.A.; Pavalko, F.M.; Burridge, K. An interaction between α-actinin and the β1 integrin subunit in vitro. J. Cell Biol. 1990, 111, 721–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belles, X.; Piulachs, M.D. Ecdysone signaling and ovarian development in insects: From stem cells to ovarian follicle formation. Biochim. Biophys. Acta 2014, 1849, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I. Tyrosinase inhibitors from plants. In Phytochemicals for Pest Control; Hedin, P., Hollingsworth, R., Masler, E., Miyamoto, J., Thompson, D., Eds.; American Chemical Society: Washington DC, USA, 1997; Volume 685, pp. 311–326. [Google Scholar]
- Tomás, J.; Camps, F.; Claveria, E.; Coll, J.; Melé, E.; Messeguer, J. Composition and location of phytoecdysteroids in Ajuga reptans in vivo and in vitro cultures. Phytochemistry 1992, 3, 1585–1591. [Google Scholar] [CrossRef]
- Wessner, M.; Champion, B.; Girault, J.P.; Kaouadji, N.; Saidi, B.; Lafont, R. Ecdysteroids from Ajuga iva. Phytochemistry 1992, 31, 3785–3788. [Google Scholar] [CrossRef]
- Coll, J.; Tandrón, Y. Isolation and identification of neo-clerodane diterpenes from Ajuga remota by high-performance liquid chromatography. Phytochem. Anal. 2005, 16, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Coll, J.; Tandrón, Y.A.; Pant, A.K.; Mathela, C.S. Phytoecdysteroids from Ajuga macrosperma var. breviflora roots. J. Nat. Prod. 2008, 71, 1294–1296. [Google Scholar] [CrossRef]
- Castro, A.; Coll, J.; Arfan, M. Neo-clerodane diterpenoids from Ajuga bracteosa. J. Nat. Prod. 2011, 74, 1036–1041. [Google Scholar] [CrossRef]
- Grace, M.H.; Cheng, D.M.; Raskin, L.; Lilaa, M.A. Neo-clerodane diterpenes from Ajuga turkestanica. Phytochem. Lett. 2008, 1, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Li, Y.; Jin, D.Q.; Guo, P.; Xu, J.; Guo, Y.; Zhang, L. Structure elucidation and inhibitory effects on NO production of clerodane diterpenes from Ajuga decumbens. Planta Med. 2012, 78, 1579–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lva, H.; Luoa, J.; Konga, L. A new neo-clerodane diterpene from Ajuga decumbens. Nat. Prod. Res. 2014, 28, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Guibout, l.; Mamadalieva, N.; Balducci, C.; Giraultd, J.P.; Lafonta, R. The minor ecdysteroids from Ajuga turkestanica. Phytochem. Anal. 2015, 26, 293–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taha-Salaime, L.; Davidovich-Rikanati, R.; Sadeh, A.; Abu-Nassar, J.; Marzouk-Kheredin, S.; Yahyaa, Y.; Ibdah, M.; Ghanim, M.; Lewinsohn, E.; Inbar, M.; et al. Phytoecdysteroid and clerodane content in three wild Ajuga species in Israel. ACS Omega 2019, 4, 2369–2376. [Google Scholar] [CrossRef] [Green Version]
- Dinan, L. Distribution and levels of phytoecdysteroids within individual plants of species of the Chenopodiaceae. Eur. J. Entomol. 1995, 92, 295–300. [Google Scholar]
- Kubo, I.; Klocke, J.A. Isolation of phytoecdysone as insect ecdysis inhibitors and feeding deterrent. In Plant Resistance to Insects; Hedin, P.A., Ed.; American Chemical Society: Washington, DC, USA, 1983; Chapter 19; pp. 329–346. [Google Scholar]
- Aly, R.; Ravid, U.; Abu-Nassar, J.; Botnick, I.; Lebedev, J.; Gal, S.; Ziadna, H.; Achdari, G.; Smirov, E.; Meir, A.; et al. Biological activity of natural phytoecdysteroids from Ajuga iva against the sweet potato whitefly Bemisia tabaci and the persea mite Oligonychus perseae. Pest Manage. Sci. 2011, 67, 1493–1498. [Google Scholar] [CrossRef]
- Camps, F.; Coll, J. Insect allelochemicals from Ajuga plants. Phytochemistry 1993, 32, 1361–1370. [Google Scholar] [CrossRef]
- Hussain, J.; Begum, N.; Hussain, H.; Khan, F.U.; Rehman, N.U.; Al-Harrasi, A.; Ali, L. Ajuganane: A new phenolic compound from Ajuga bracteosa. Nat. Prod. Commun. 2012, 7, 615–616. [Google Scholar] [CrossRef] [Green Version]
- Kubo, I.; Asaka, Y.; Shibata, K. Insect growth inhibitory nor-diterpenes, cis-dehydrocrotonin and trans-dehydrocrotonin, from Croton cajucara. Phytochemistry 1991, 30, 2545–2546. [Google Scholar] [CrossRef]
- Koul, O. Insect feeding deterrents in plants. Indian Rev. Life Sci. 2016, 2, 97–125. [Google Scholar]
- Li, R.; Morris-Natschke, S.L.; Lee, K.H. Clerodane diterpenes: Sources, structures, and biological activities. Nat. Prod. Rep. 2016, 33, 1166–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darby, I.A.; Bisucci, T.; Desmoulière, A.; Hewitson, T.D. Methods in Molecular Biology: In Situ Hybridization Protocols. In In Situ Hybridization Using cRNA Probes; Humana Press Inc.: Totowa, NJ, USA, 2006; Chapter 3; Volume 326, pp. 17–31. [Google Scholar]
- Elmenofy, W.; Salem, R.; Osman, E.; Yasser, N.; Abdelmawgod, A.; Saleh, M.; Zaki, A.; Hanafy, E.; Tamim, S.; Amin, S.; et al. Evaluation of two viral isolates as a potential biocontrol agent against the Egyptian cotton leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2020, 30, 4–11. [Google Scholar] [CrossRef]
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Davies, T.G.E.; Nauen, R. Diamide resistance: 10 years of lessons from lepidopteran pests. J. Pest Sci. 2020, 93, 911–928. [Google Scholar] [CrossRef] [Green Version]
- Hatem, A.E.; Amer, R.A.M.; Vargas-Osuna, E. Combination effects of Bacillus thuringiensis cry1Ac toxin and nucleopolyhedrovirus or granulovirus of Spodoptera littoralis on the cotton leafworm. Egypt. J. Biol. Pest Control 2012, 22, 115–120. [Google Scholar]
- MagholiFard, Z.; Hesami, S.; Marzban, R.; Salehi Jouzani, G. Individual and combined biological effects of Bacillus thuringiensis and Multicapsid nucleopolyhedrovirus on the biological stages of Egyptian cotton leafworm, Spodoptera littoralis (B.) (Lep.: Noctuidae). J. Agric. Sci. Technol. 2020, 22, 465–476. [Google Scholar]
- Ma, W.C. Dynamics of Feeding Responses in Pieris brassicae L. as a Function of Chemosensory Input: A Behavioural, Ultrastructural and Electrophysiological Study. Ph.D. Thesis, Landbouwhogeschool, Wageningen, The Netherlands, 1972. [Google Scholar]
- Tanaka, Y.; Yukuhiro, F. Ecdysone has an effect on the regeneration of midgut epithelial cells that is distinct from 20-hydroxyecdysone in the silk worm Bombyx mori. Gen. Comp. Endocrinol. 1999, 116, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Rharrabe, K.; Bouayad, N.; Sayah, F. Effects of ingested 20-hydroxyecdysone on development and midgut epithelial cells of Plodia interpunctella (Lepidoptera, Pyralidae). Pestic. Biochem. Physiol. 2009, 93, 112–119. [Google Scholar] [CrossRef]
- Wadsworth, T.; Carriman, A.; Gutierrez, A.A.; Moffatt, C.; Fuse, M. Ecdysis behaviors and circadian rhythm of ecdysis in the stick insect, Carausius morosus. J. Insect Physiol. 2014, 71, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Takeda, S. Ecdysone and 20-hydroxyecdysone supplements to the diet affect larval development in the silkworm, Bombyx mori, differently. J. Insect Physiol. 1993, 39, 805–809. [Google Scholar] [CrossRef]
- Kubo, I.; Klocke, J.A.; Asano, S. Insect ecdysis inhibitors from the East African medicinal plant Ajuga remota (Labiatae). Agric. Biol. Chem. 1981, 45, 1925–1927. [Google Scholar] [CrossRef]
- Slama, K.; Abubakirov, N.K.; Gorovits, M.B.; Baltaev, U.A.; Saatov, Z. Hormonal activity of ecdysteroids from certain Asiatic plants. Insect Biochem. Mol. Biol. 1993, 23, 181–185. [Google Scholar] [CrossRef]
- Kubo, B. Insect control agents from tropical plants. In Phytochemical Potential of Tropical Plants; Downum, K.R., Romeo, J.T., Stafford, H.A., Eds.; Plenum Press: New York, NY, USA, 1993; pp. 133–151. [Google Scholar]
- Tanaka, Y. The different effects of ecdysone and 20-hydroxyecdysone on the induction of larval ecdysis in the silkworm, Bombyx mori (Lepidoptera: Bombycidae). Eur. J. Entomol. 1995, 92, 155–160. [Google Scholar]
- Swevers, L.; Iatrou, K. The ecdysone regulatory cascade and ovarian development in lepidopteran insects: Insights from the silk moth paradigm. Insect Biochem. Mol. Biol. 2003, 33, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Arnault, C.; Slama, K. Dietary effects of phytoecdysones in the leek-moth, Acrolepiopsis assectella Zell. (Lepidoptera: Acrolepiidae). J. Chem. Ecol. 1986, 12, 1979–1986. [Google Scholar] [CrossRef]
- Carley, W.W.; Barak, L.S.; Webb, W.W. F-actin aggregates in transformed cells. J. Cell Biol. 1981, 90, 797–802. [Google Scholar] [CrossRef]
- Meyer, R.K.; Burger, M.M.; Tschannen, R.; Schafer, R. Actin filament bundles in vaccinia virus infected fibroblasts. Arch. Virol. 1981, 67, 11–18. [Google Scholar] [CrossRef]
- Bohn, W.; Rutter, G.; Hohenberg, H.; Mannweiler, K.; Nobis, P. Involvement of actin microfilaments in budding of measles virus: Studies on cytoskeletons of infected cells. Virology 1986, 149, 91–106. [Google Scholar] [CrossRef]
- Mortara, R.A.; Koch, G.L.E. An association between actin and nucleocapsid polypeptides in isolated murine retroviral particles. J. Submicrosc. Cytol. Pathol. 1989, 21, 295–306. [Google Scholar]
- Jackson, P.; Bellett, A.J.D. Relationship between organization of the actin cytoskeleton and the cell cycle in normal and adenovirus-infected rat cells. J. Virol. 1989, 63, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.; Goldberg, A.R. Changes in microfilament organization and surface topography upon transformation of chick embryo fibroblasts with Rous sarcoma virus. Proc. Natl. Acad. Sci. USA 1976, 73, 4065–4069. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha-Salaime, L.; Lebedev, G.; Abo-Nassar, J.; Marzouk, S.; Inbar, M.; Ghanim, M.; Aly, R. Activity of Ajuga iva Extracts Against the African Cotton Leafworm Spodoptera littoralis. Insects 2020, 11, 726. https://doi.org/10.3390/insects11110726
Taha-Salaime L, Lebedev G, Abo-Nassar J, Marzouk S, Inbar M, Ghanim M, Aly R. Activity of Ajuga iva Extracts Against the African Cotton Leafworm Spodoptera littoralis. Insects. 2020; 11(11):726. https://doi.org/10.3390/insects11110726
Chicago/Turabian StyleTaha-Salaime, Leena, Galina Lebedev, Jackline Abo-Nassar, Sally Marzouk, Moshe Inbar, Murad Ghanim, and Radi Aly. 2020. "Activity of Ajuga iva Extracts Against the African Cotton Leafworm Spodoptera littoralis" Insects 11, no. 11: 726. https://doi.org/10.3390/insects11110726
APA StyleTaha-Salaime, L., Lebedev, G., Abo-Nassar, J., Marzouk, S., Inbar, M., Ghanim, M., & Aly, R. (2020). Activity of Ajuga iva Extracts Against the African Cotton Leafworm Spodoptera littoralis. Insects, 11(11), 726. https://doi.org/10.3390/insects11110726






