Selectivity of Entomopathogenic Fungi to Chrysoperla externa (Neuroptera: Chrysopidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Fungi
2.2. Obtaining and Rearing Chrysoperla externa
2.3. Effects of the Entomopathogenic Fungi in the Chrysoperla externa Larvae
2.4. Statistical Analysis
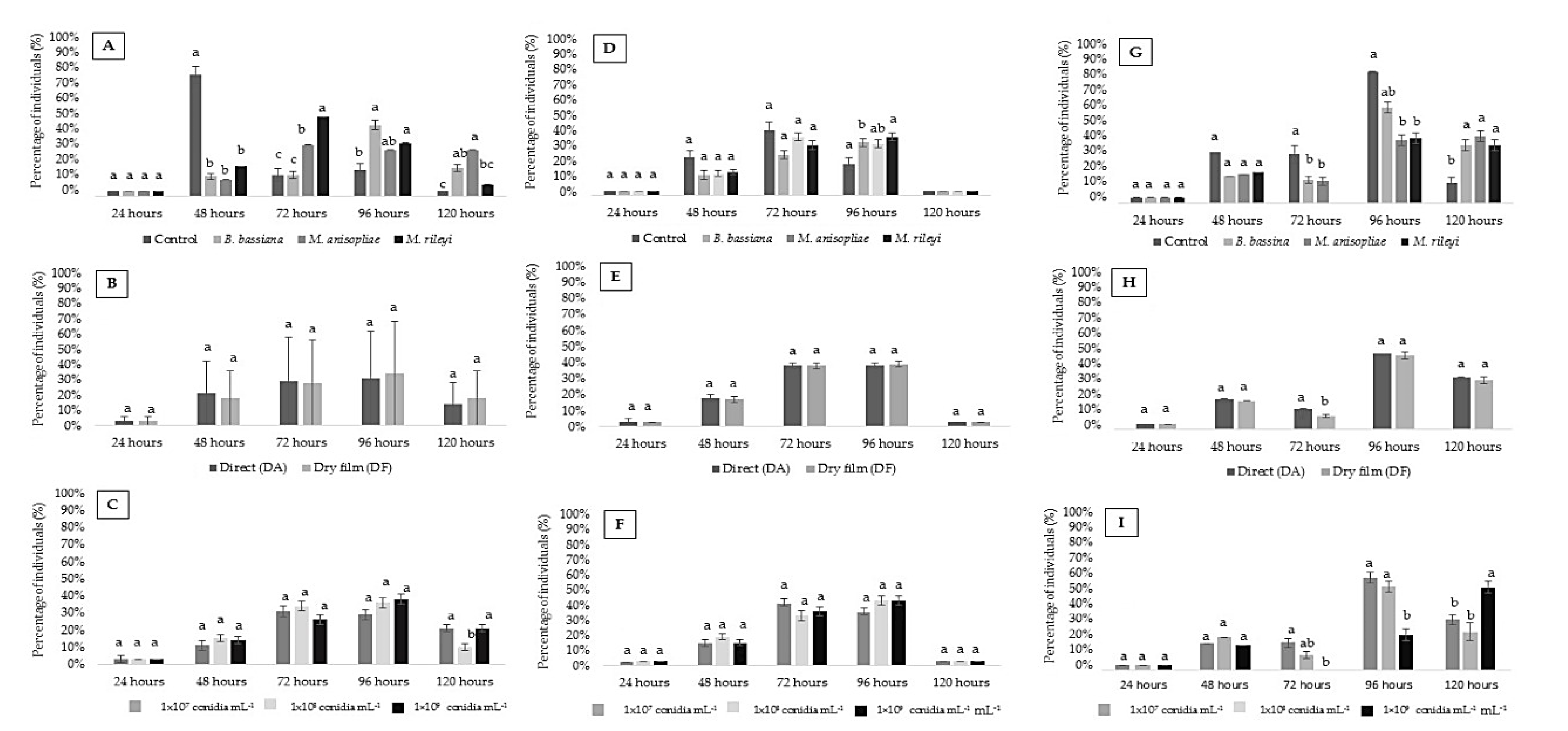
3. Results
3.1. Mortality of Chrysoperla externa Larvae
3.2. Change of Larval State of C. externa Larvae
3.3. Duration (Days) Pupal Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oswald, J.D. Neuropterida Species of the World. 2018. Available online: http://lacewing.tamu.edu/SpeciesCatalog/Main> (accessed on 3 January 2020).
- Maia, W.J.M.S.C.; Souza, B.; Cruz, I.; Maia, T.J.A.F. Rhopalosiphum maidis (Fitch, 1856) (Hemiptera: Aphididae). Ciênc. Agrotecnol. 2004, 28, 1259–1268. [Google Scholar] [CrossRef]
- Pessoa, L.G.A.; Leite, M.V.; de Freitas, S.; Garbin, G.C. Efeito da variação da temperatura sobre o desenvolvimento embrionário e pós embrionário de Ceraeochrysa paraguaria (Navás) (Neuroptera: Chrysopidae). Arq. Inst. Biol. 2004, 71, 473–476. [Google Scholar]
- Pitwak, J.; Menezes, A.O., Jr.; Ventura, M.U. Development and reproductive performance of Chrysoperla externa (Neuroptera: Chrysopidae) using preys from wheat crop. Rev. Colomb. Entomol. 2016, 42, 118–123. [Google Scholar] [CrossRef]
- Pasini, R.A. Seletividade de Agrotóxicos Utilizados na Cultura do Trigo aos Predadores Chrysoperla Externa (Hagen, 1861) (Neuroptera: Chrysopidae) e Eriopis Connexa (Germar, 1824) (Coleoptera: Coccinellidae) em Condições de Laboratório e Semi-Campo. Tese (Doutorado) Programa de Pós-Graduação em Fitossanidade; Universidade Federal de Pelotas: Pelotas, Brazil, 2017; p. 154. (In Portuguese) [Google Scholar]
- De Silva, B.K.A.; de Godoy, M.S.; de Lima, A.G.; de Oliveira, A.K.S.; Pastori, P.L. Toxicity of insecticides used in muskmelon on first-instar larvae of Chrysoperla genanigra Freitas (Neuroptera: Chrysopidae). Rev. Caatinga 2017, 30, 662–669. [Google Scholar] [CrossRef]
- Hewlett, J.A.; Eubanks, M.D. The effects of sugarcane aphid density in sorghum on predation by lady beetles and lacewings. Biol. Control. 2018, 129, 171–177. [Google Scholar] [CrossRef]
- De Armas, F.S.; Grützmacher, A.D.; Nava, D.E.; Rakes, M.; Bueno, F.A.; Pasini, R.A. Selectivity of pesticides used in peach orchards to eggs and pupae of the predators Chrysoperla externa and Coleomegilla quadrifasciata. Semin. Ciênc. Agrár. 2019, 40, 1427–1440. [Google Scholar] [CrossRef]
- Santos, T.M.; Junior, A.L.B.B.; Soares, J.J. Influência de tricomas do algodoeiro sobre os aspectos biológicos e capacidade predatória de Chrysoperla externa (Hagen) alimentada com Aphis gossypii Glover. Bragantia 2003, 14, 243–254. [Google Scholar] [CrossRef]
- Bezerra, C.E.S.; Nogueira, C.A.F.; Shadow, M.C.D.S.; Demartelaere, A.C.C.; Araujo, E.L. Green lacewings (Neuroptera: Chrysopidae): Biological Aspects, potential use and future perspectives. Rev. Caatinga 2009, 22, 1–5. [Google Scholar]
- Alberquerque, S.A. Green lacewings (Neuroptera: Chrysopidae). In The Bioecology and Nutrition of Insects: Basis for the Integrated Management of Pests, 1st ed.; Panizzi, A.R., Parra, J.R.P., Eds.; Embrapa Technological Information: Brasilia, Brazil, 2009; Volume 23, pp. 969–1022. [Google Scholar]
- Bortoli, S.A.; Caetano, A.C.; Murata, A.T.; Oliveira, J.E.M. Development and predatory viability of Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae) in different prey. Bioterra 2006, 6, 152–154. [Google Scholar]
- Murata, T.; Caetano, C.; Bortoli, S.A.; Brito, C.H. Capacity of consumption of Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae) in different prey. Rev. Caatinga 2006, 19, 304–309. [Google Scholar]
- Albuquerque, D.F.A.; Cruz, I. Biological Aspects of Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae) having as a source of food eggs of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Seminar on Scientific Initiation Program PIBIC/BIC JÚNIOR, 2017, Sete Lagoas (submitted paper). In Proceedings of the Sete Lagoas: Embrapa Maize and Sorghum, Sete Lagoas, Brazil, 17 August 2017; Available online: http://www.alice.cnptia.embrapa.br/alice/handle/doc/1074573 (accessed on 10 August 2019).
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invert. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G. The entomopathogenic fungi Isaria farinose (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): Biology, ecology and use in biological control. Biocont. Sci. Technol. 2008, 18, 865–901. [Google Scholar] [CrossRef]
- Mora, M.A.E.; Castilho, A.M.C.; Fraga, M.E. Fungos entomopatogenicos: Enzimas, toxinas e fatores que afetam a diversidade. Rev. Bras. Prod. Agroind. 2016, 18, 335–349. [Google Scholar] [CrossRef]
- Fathipour, Y.; Sedaratian, A. integrated management systems of cultivation of soy Helicoverpa armigera. In Elshemy Ha Resistance a Pests of Soybeans; InTeOpP: Cairo, Egypt, 2013; pp. 231–280. [Google Scholar]
- De Bueno, A.F.; Carvalho, G.A.; dos Santos, A.C.; Sosa-Gómez, D.R.; da Silva, D.M. Pesticide selectivity to natural enemies: Challenges and constraints for research and field recommendation. Ciênc. Rural 2017, 47, 1–10. [Google Scholar] [CrossRef]
- Fernandes, F.L.; Bacci, L.; Fernandes, M.S. Impact and selectivity of insecticides to predators and parasitoids. EntomoBrasilis 2010, 3, 1–10. [Google Scholar] [CrossRef]
- De Bueno, A.F.; Batistela, M.J.; de Bueno, R.C.O.F.; de França-Neto, J.B.; Nishikawa, M.A.N.; Filho, A.L. Effects of integrated pest management, biological control and prophylactic use of insecticides on the management and sustainability of soybean. Crop. Prot. 2011, 30, 937–945. [Google Scholar] [CrossRef]
- Ndakidemi, B.; Mtei, K.; Ndakidemi, P.A. Impacts of synthetic and botanical pesticides on beneficial insects. Agric. Sci. 2016, 07, 364–372. [Google Scholar] [CrossRef]
- Sosa-Gómez, D.R. Seletividade de agroquímicos para fungos entomopatogênicos. Embrapa Soja Outras Publicações Científicas. 2005. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPSO-2009-09/28931/1/seletiv_fung.pdf (accessed on 24 August 2020).
- Rimoldi, F.; Schneider, M.I.; Ronco, A. Short and long-term effects of endosulfan, cypermethrin, spinosad, and methoxyfenozide on adults of Chrysoperla externa (Neuroptera: Chrysopidae). J. Econ. Entomol. 2012, 105, 1982–1987. [Google Scholar] [CrossRef] [PubMed]
- Rugno, G.R.; Zanardi, O.Z.; Yamamoto, P.T. Are the Pupae and Eggs of the Lacewing Ceraeochrysa cubana (Neuroptera: Chrysopidae) Tolerant to Insecticides? J. Econ. Entomol. 2015, 108, 2630–2639. [Google Scholar] [CrossRef]
- Pasini, R.A.; Grützmacher, A.D.; de Pazini, J.B.; de Armas, F.S.; Bueno, F.A.; Pires, S.N. Side effects of insecticides used in wheat crop on eggs and pupae of Chrysoperla externa and Eriopis connexa. Phytoparasitica 2018, 46, 115–125. [Google Scholar] [CrossRef]
- De Soares, A.F.; Carvalho, G.A. Physiological selectivity of insecticides to eggs and larvae of predator Chrysoperla externa (HAGEN) (Neuroptera: Chrysopidae). Coffee Sci. 2018, 13, 292–303. [Google Scholar] [CrossRef]
- Rugno, G.R.; Zanardi, O.Z.; Parra, J.R.P.; Yamamoto, P.T. Lethal and Sublethal Toxicity of Insecticides to the Lacewing Ceraeochrysa cubana. Neotrop. Entomol. 2019, 48, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Generoso, A.R. Compatibility of Beauveria bassiana and Paecilomyces fumosoroseus with Chrysoperla externa (Neuroptera: Chrysopidae) and Methodology for Evaluating Selectivity. Master’s Thesis, Paulista State University, Jaboticabal-SP, Brazil, 2002; p. 63. [Google Scholar]
- Pessoa, L.G.A.; Cavalcanti, R.S.; Junior, A.M.; Souza, B. Compatibility between Beauveria bassiana and predator Chrysoperla externa in the laboratory. Pesq. Agropecu. Bras. 2005, 40, 617–619. [Google Scholar] [CrossRef]
- Cardoso, E.R.; Freitas, S.; Nunes, H.T.; Pessoa, L.G.A. Selectivity of Lecanicillium lecanii and Metarhizium anisopliae for first instar larvae of Ceraeochrysa cincta (Neuroptera: Chrysopidae) in laboratory. Acta Sci. Agron. 2007, 29, 563–568. [Google Scholar] [CrossRef]
- Schlick, E.C.; Toscano, L.C.; Souza, G.D.; Peres, A.J.A.; Dias, P.M.; Maruyama, W.I. Compatibilidade de Metarhizium anisopliae (Metschnikoff) Sorokin (Hypocreales: Clavicipitaceae) com Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae). EntomoBrasilis 2015, 8, 189–195. [Google Scholar]
- Consechi, M.R. Parâmetros a Serem Considerados nas Pulverizações do Fungo Isaria Fumosorosea Para o Manejo de Diaphorina Citri. Doctor’s Thesis, University of São Paulo—School of Agriculture “Luiz de Queiroz”, Piracicaba-SP, Brazil, 2017; p. 134. (In Porutguese). [Google Scholar]
- Dias, P.M.; Filho, L.M.A.; Pessoa, L.G.A.; Loureiro, E.S.; Ramalho, K.F. Production of Metarhizium rileyi in different mixtures of rice and sorghum. In Proceedings of the XII Agronomic Week Cassilândia & V Week of Research of the Post-Graduation, Cassilândia-MS, Brazil, 12 September 2017; p. 99. [Google Scholar]
- Ribeiro, M.J. Biology of Chrysoperla externa (Hagen, 1861) (Neuroptera, Chrysopidae) Fed with Different Diets. Master’s Thesis, School of Agriculture of Lavras, Lavras-MG, Brazil, 1988; p. 131. [Google Scholar]
- Dias, P.M.; Loureiro, E.S.L.; Pessoa, L.G.A.; Neto, F.M.O.; Tosta, R.A.S.; Teodoro, P.E. Interactions between Fungal-Infected Helicoverpa armigera and the Predator Chrysoperla externa. Insects 2019, 10, 309. [Google Scholar] [CrossRef]
- Thungrabeab, M.; Angma, S. Effect of entomopathogenic fungus, Beauveria bassiana (BALSAM) and Metarhizium anisopliae (Metsch) on non-target insects. KMITL Sci. Technol. J. 2007, 7, 1–10. [Google Scholar]
- Hassan, S.A. Standardized techniques for testing side-effects of pesticides on beneficial arthropods in the laboraary. ZeitscbriflfUr Pflanz. FIanzenscbutz 1977, 84, 158–163. [Google Scholar]
- Hassan, S.A. Standard methods a test the Sideeffects of pesticides on natural enemies of insects and mites developed by the IOBC/WPRS Wprk Group ‘Pesticides and Benefitial Organisms’. EPPO Bull. 1985, 15, 214–255. [Google Scholar] [CrossRef]
- Freitas, S.; Penny, N.D. The green lacewings (Neuroptera: Chrysopidae) of Brazilian agro-ecosystems. Acad. Sci. 2001, 50, 245–395. [Google Scholar]
- Bhering, L.L. Rbio: A Tool for Biometric And Statistical Analysis Using The R Platform. Crop. Breed. Appl. Biotech. 2017, 17, 187–190. [Google Scholar] [CrossRef]
- Alves, S.B.; Lopes, R.B. Microbial Control of Pests in Latin America; FEALQ: Piracicaba, Brazil, 2008; p. 414. [Google Scholar]
- Alves, S.B.; Milk, L.H.; Filho, A.B.; Almeida, J.E.M.; Marques, E.J. Massal production of entomopathogenic fungi in Latin America. In Microbial Control of Pests in Latin America, 2nd ed.; Alves, S.B., Lopes, R.B., Eds.; FEALQ: Piracicaba, Brazil, 2008; pp. 215–237. [Google Scholar]
- Leger, R.J.; Wang, C. Genetic engineering of fungal biocontrol agents to achieve efficacy against insect pests. Appl. Microb. Biotechnol. 2010, 85, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, M.G. Advances in fundamental and applied studies in china of fungal biocontrol agents for use against arthropod pests. Biol. Control. 2014, 68, 129–135. [Google Scholar] [CrossRef]
- Alves, S.B.; Moino, J.R.A.; Almeida, J.E.M. Produtos fitossanitários e entomopatógenos. In Controle Microbiano de Insetos; Alves, S.B., Ed.; Fealq: Piracicaba, Brazil, 1998; pp. 217–238. [Google Scholar]
- Ausique, J.J.S.; D’Alessandro, C.P.; Conceschi, R.M.; Mascarin, G.M.; Delalibera, I.J. Efficacy of entomopathogenic fungi against adults of Diaphorina citri for field laboratory applications. J. Pest. Sci. 2017, 90, 947–960. [Google Scholar] [CrossRef]
- Arnosti, A.; Junior, I.D.; Conceschi, M.R.; D’Alessandro, C.P.; Travaglini, R.V.; Camargo-Mathias, M.I. Interactions of adjuvants on adhesion and germination of Isaria fumosorosea on adults of Diaphorina citri. Sci. Agric. 2019, 76, 487–493. [Google Scholar] [CrossRef]
- Alves, S.B.; Padua, L.E.M.; Azevedo, E.M.V.M.; Almeida, L.C. Controle da broca da cana-de-acucar pelo uso de Beauveria bassiana. Pesq. Agrop. Bras. 1985, 20, 403–406. [Google Scholar]
- Faria, L.L.F.; Oliveira, J.V.; Barros, R. Patogenicidade do fungo Beauveria bassiana (Bals.) Vuill., em lagartas de Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera, Noctuidae) sob condições de Laboratorio. Cad. Omega Sér. Agron. 1992, 4, 207–217. [Google Scholar]
- Lecuona, R.E.; Tigano, M.S.; Diaz, B.M. Characterization and pathogenicity of Beauveria bassiana against Diatraea saccharalis (F.) (Lepidoptera; Pyralidae) in Argentina. An. Soc. Entomol. Bras. 1996, 25, 299–307. [Google Scholar]
- Silva, R.B.Q.; Veiga, A.F.S.L. Patogenicidade de Beauveria bassiana (Bals.) e Metarhizium anisopliae (Metsch.) Sorok. sobre Castnia icarus (Cramer, 1775). Rev. Agric. 1998, 73, 119–127. [Google Scholar]
- Leyva, O.E.; Villalon, E.M.; Ávila, R.A.; Bulet, D.B.B. Susceptibilidad de Chrysopa exterior Navas a Beauveria bassiana (Blasamo) Vuillemin cepa LBB-1 en condiciones de laboratorio. Fitosanidad 2011, 15, 51–57. [Google Scholar]
- López, M.A.C.; Osorio, J.W.M. Compatibilidad de Beauveria bassiana Y Metarhizium anisopliae com Chrysoperla externa depredador de Trialeurodes vaporariorum. Chliean J. Agric. Anim. Sci. 2019, 35, 38–48. [Google Scholar]
- Ventura, M.A.; Ribeiro, C.; Garcia, V. Susceptibility of third instar larvae of the green lacewing Chrysoperla kolthoffi (Navas) (Insecta: Neuroptera: Chrysopidae) to the entomopathogenic fungus Metarhizium anisopliae (Metschnikoff) Sorokin var. anisopliae Tulloch in the laboratory. In Proceedings of the Fifth International Symposium on Neuropterology, Cairo, Egypt, 2–6 May 1994; pp. 241–249. [Google Scholar]
- Ventura, M.A.; Garcia, V.; Canard, M. Efeito da antibiose causado pelo fungo entomopatogênico Metarhizium anisopliae (Metschnikoff) Sorokin variedade anisopliae Tulloch, a uma “crisopideo verde comum” Chrysoperla kolthoffi (Navás) (Neuroptera: Chrysopidae). J. Neuropterol. 2000, 3, 33–41. [Google Scholar]
- Maia, W.J.S.; Carvalho, C.F.; Souza, B. Exigências térmicas de Chrysoperla externa (Hagen, 1861) (Neuroptera: Chrysopidae) alimentada com Schizaphis graminum (Rondani, 1852) (Hemiptera: Aphididae) em condições laboratório. Ciênc. Agrotecnol. 2000, 24, 81–86. [Google Scholar]
- Silva, C.G. Desenvolvimento das fases imaturas de Chrysoperla externa alimentadas com ninfas de Bemisia tabaci criadas em três hospedeiros. Pesq. Agrop. Bras. 2004, 39, 1065–1070. [Google Scholar] [CrossRef]
- Gupta, S.; Krasnoff, S.B.; Roberts, D.W.; Renwick, J.A.A.; Brinen, L.S.; Clardy, J. Structure of efrapeptins from the fungus Tolypocladium niveum: Peptide inhibitors of mitochondrial ATPase. J. Org. Chem. 1992, 57, 2306–2313. [Google Scholar] [CrossRef]
- Vestergaard, S.; Gillespie, A.T.; Butt, T.M.; Schreiter, G.; Eilenberg, J. Pathogenicity of the Hyphomycete fungi Verticillium lecanii and Metarhizium anisopliae to the western thrips, Frankliniella occidentalis. Biocont. Sci. Technol. 1995, 5, 185–192. [Google Scholar] [CrossRef]
- Leal, S.C.M.; Bertioli, D.J.; Butt, T.M.; Carter, J.H.; Burrows, P.R.; Peberdy, J.F. Amplification and restriction endonuclease digestion of the Pr1 gene for the detection and characterization of Metarhizium anisopliae. Mycol. Res. 1997, 101, 257–265. [Google Scholar] [CrossRef]
- Lacey, L.A.; Goettel, L.A. Current developments in microbial control of insect pests and prospects for the early 21st century. Entomophaga 1995, 40, 3–27. [Google Scholar] [CrossRef]
- Hajek, A.E.; Eastburn, C.C. Attachment and germination of Entomophaga maimaiga conidia on host and non-host larval cuticle. J. Invertebr. Pathol. 2003, 82, 12–22. [Google Scholar] [CrossRef]
- Bianco, L.; Perrota, G. Methodologies and perspectives of proteomics applied to filamentous fungi: From sample preparation to secretoma analisys. Int. J. Mol. Sci. 2015, 16, 5803–5820. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.C. Virulência de Nomuraea rileyi à Spodoptera frugiperda e Perfil Protéico do Secretoma em Presença da Cutícula do Inseto. Master’s Thesis, University of Caxias do Sul, Rio Grande do Sul-RS, Brazil, 2016; p. 38. (In Portuguese). [Google Scholar]
- Portilla, M.; Snodgrass, G.; Luttrell, R. Lethal and Sub-Lethal Effects of Beauveria bassiana (Cordycipitaceae) Strain NI8 on Chrysoperla rufilabris (Neuroptera: Chrysopidae). Flor. Entomol. 2017, 100, 627–633. [Google Scholar] [CrossRef]
- Portilla, M.; Luttrell, R.; Snodgrass, G.; Zhu, Y.C.; Riddick, E. Lethality of the entomopatoghenic fungus Beauveria bassiana NI8 strain in Lygus lineolaris (Hemiptera: Miridae) and its possible impact on beneficial arthropods. J. Entomol. Sci. 2017, 52, 352–369. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mingotti Dias, P.; de Souza Loureiro, E.; Amorim Pessoa, L.G.; Reis Devoz, G.L.; Bárbaro Barbosa Junior, G.; Macali Werner, A.; Navarrete, A.A.; Teodoro, P.E. Selectivity of Entomopathogenic Fungi to Chrysoperla externa (Neuroptera: Chrysopidae). Insects 2020, 11, 716. https://doi.org/10.3390/insects11100716
Mingotti Dias P, de Souza Loureiro E, Amorim Pessoa LG, Reis Devoz GL, Bárbaro Barbosa Junior G, Macali Werner A, Navarrete AA, Teodoro PE. Selectivity of Entomopathogenic Fungi to Chrysoperla externa (Neuroptera: Chrysopidae). Insects. 2020; 11(10):716. https://doi.org/10.3390/insects11100716
Chicago/Turabian StyleMingotti Dias, Pamella, Elisângela de Souza Loureiro, Luis Gustavo Amorim Pessoa, Gabriel Luiz Reis Devoz, Gilson Bárbaro Barbosa Junior, Allan Macali Werner, Acacio Aparecido Navarrete, and Paulo Eduardo Teodoro. 2020. "Selectivity of Entomopathogenic Fungi to Chrysoperla externa (Neuroptera: Chrysopidae)" Insects 11, no. 10: 716. https://doi.org/10.3390/insects11100716
APA StyleMingotti Dias, P., de Souza Loureiro, E., Amorim Pessoa, L. G., Reis Devoz, G. L., Bárbaro Barbosa Junior, G., Macali Werner, A., Navarrete, A. A., & Teodoro, P. E. (2020). Selectivity of Entomopathogenic Fungi to Chrysoperla externa (Neuroptera: Chrysopidae). Insects, 11(10), 716. https://doi.org/10.3390/insects11100716










