Factors Affecting the Reproduction and Mass-Rearing of Sclerodermus brevicornis (Hymenoptera: Bethylidae), a Natural Enemy of Exotic Flat-Faced Longhorn Beetles (Coleoptera: Cerambycidae: Lamiinae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing Psacothea hilaris hilaris Host Stock
2.2. Rearing Corcyra cephalonica Host Stock
2.3. Rearing Sclerodermus brevicornis Parasitoid Stock
2.4. Capacity of Multi-Foundress Groups to Utilize Successive Hosts
2.5. Reproductive Capacity of Individual Females
2.6. Longevity of Sclerodermus brevicornis Females Stored at Different Temperatures
2.7. Post-Storage Reproductive Performance of Sclerodermus brevicornis Females
2.8. Statistical Analysis
3. Results
3.1. Capacity of Multi-Foundress Groups to Utilize Successive Hosts
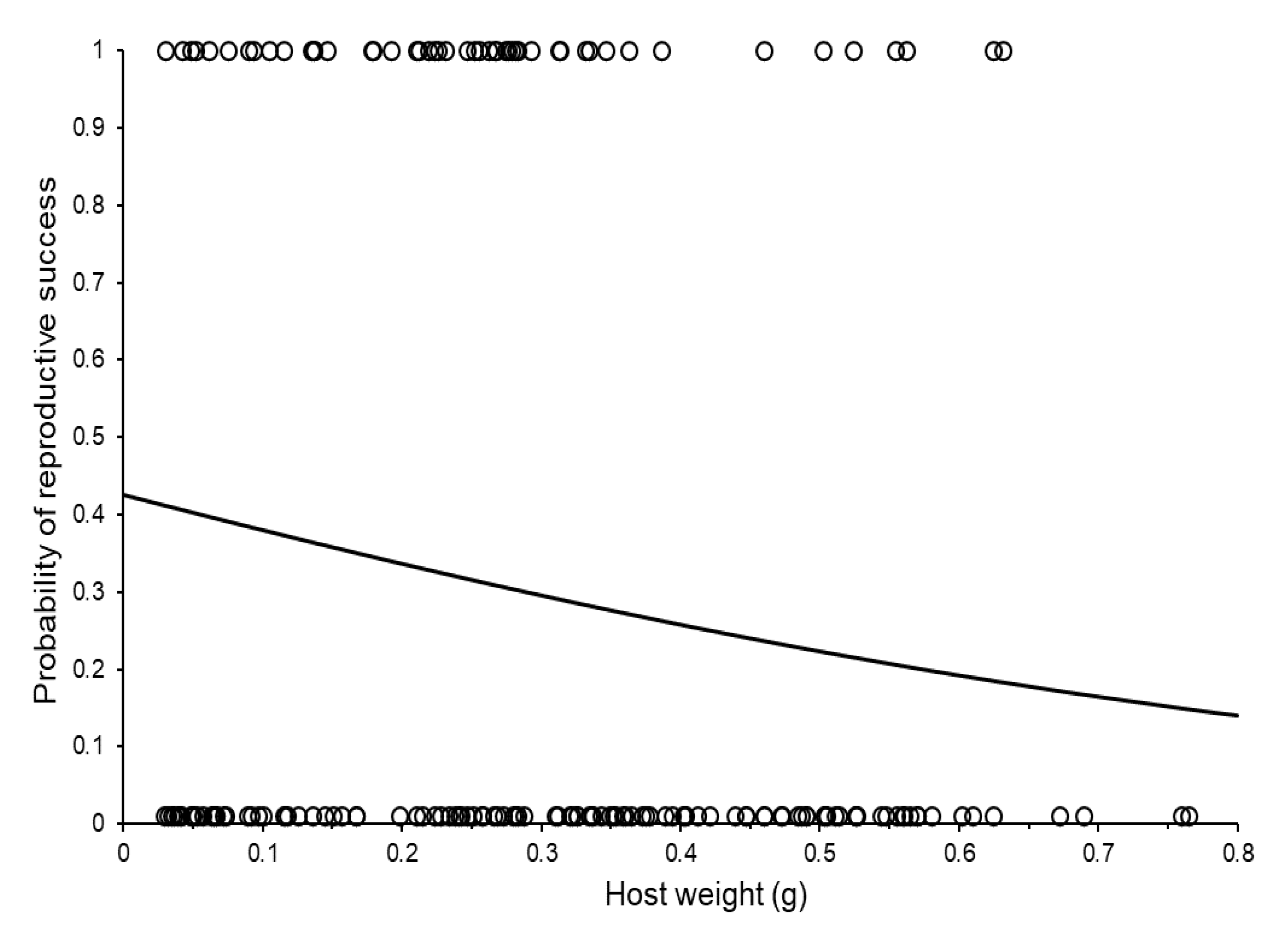
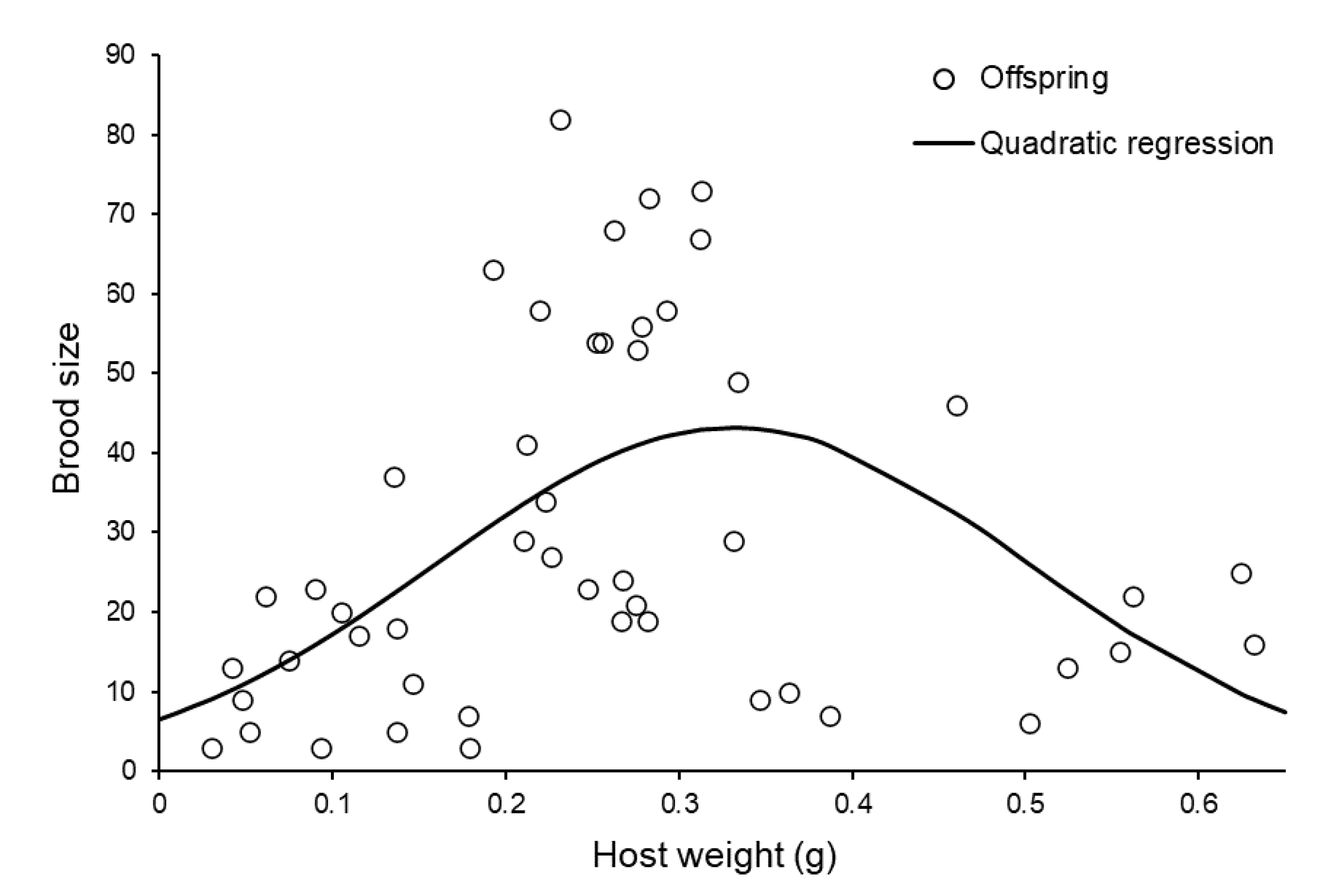
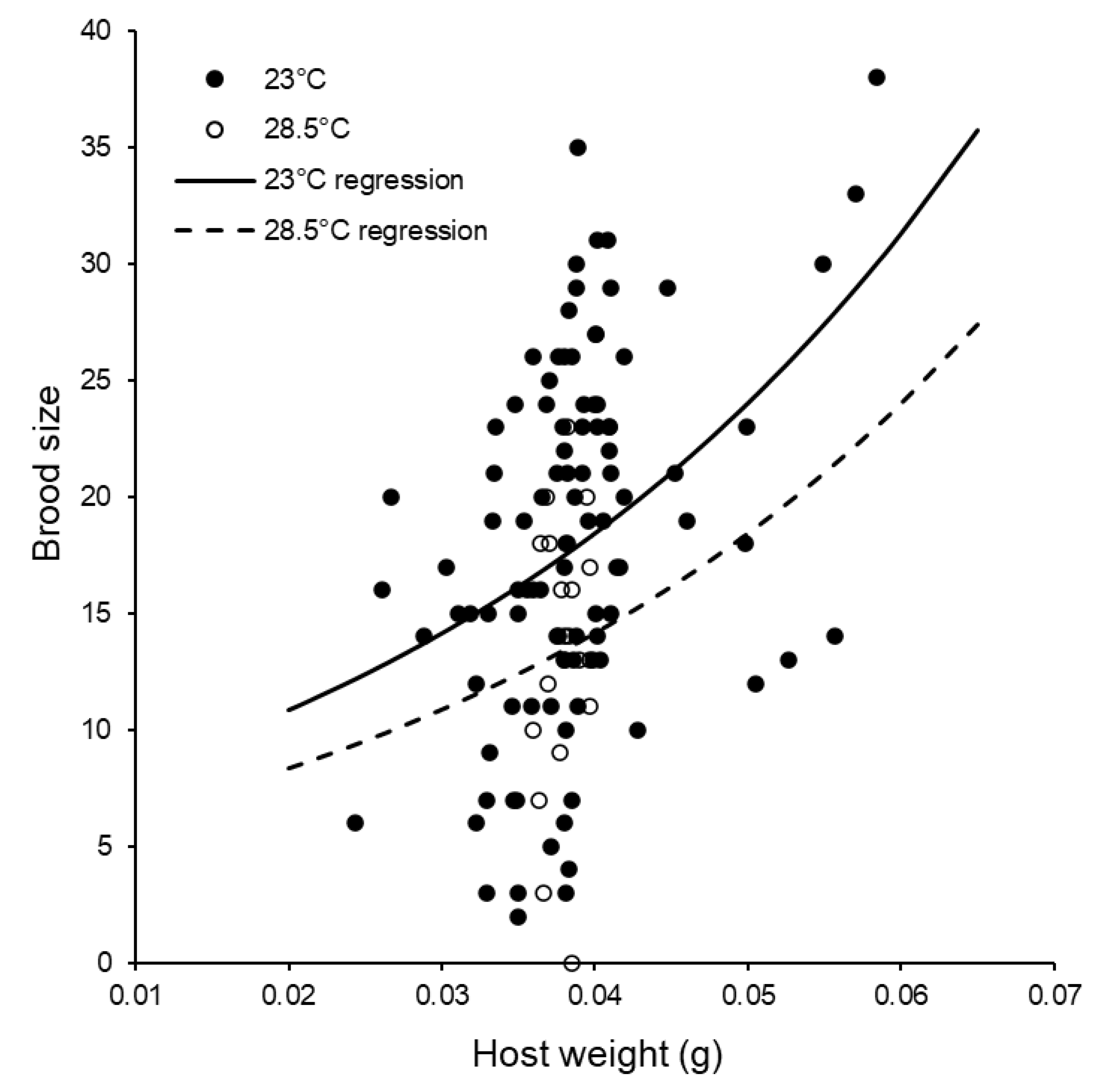
3.2. Reproductive Capacity of Individual Females
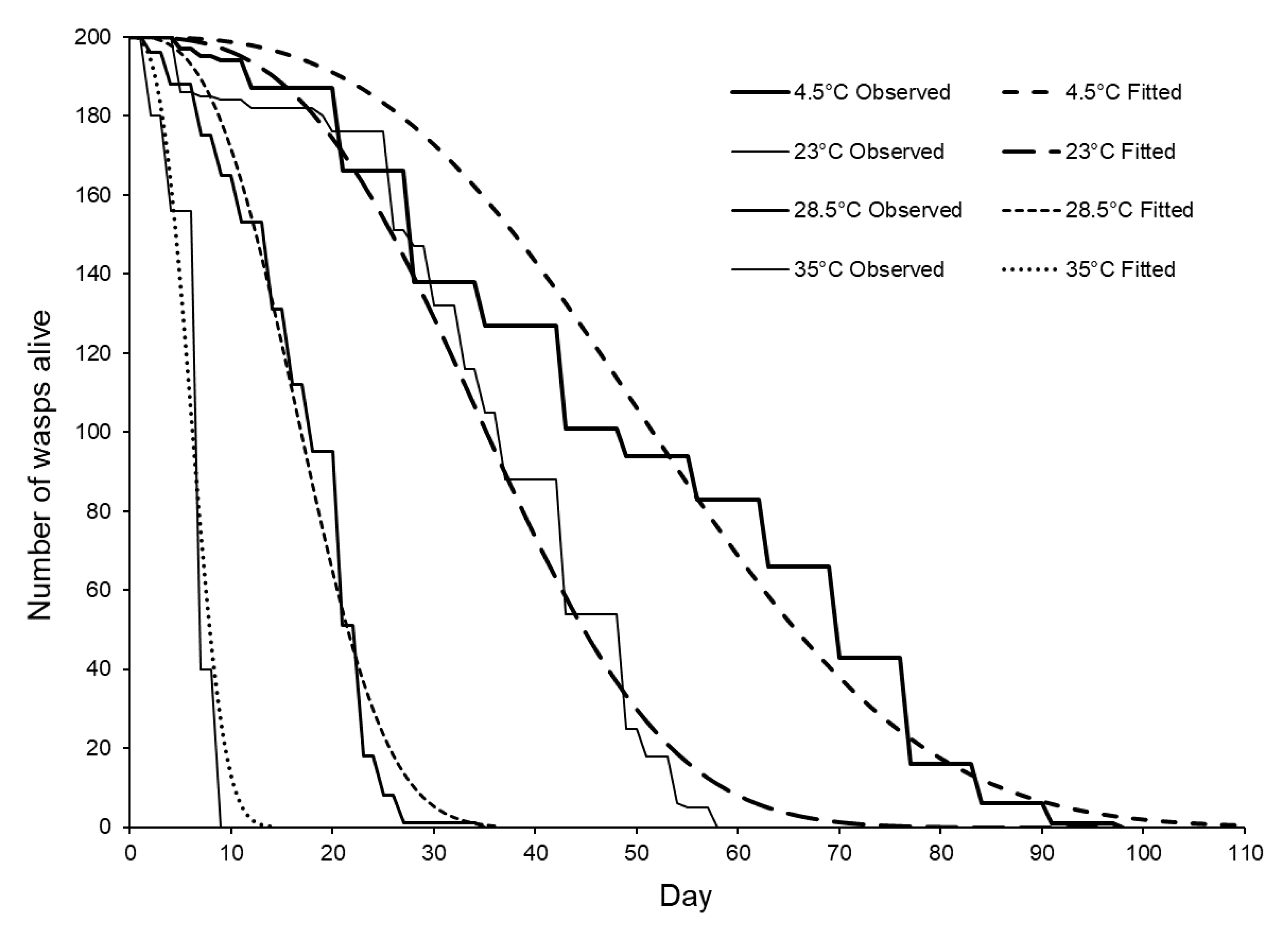
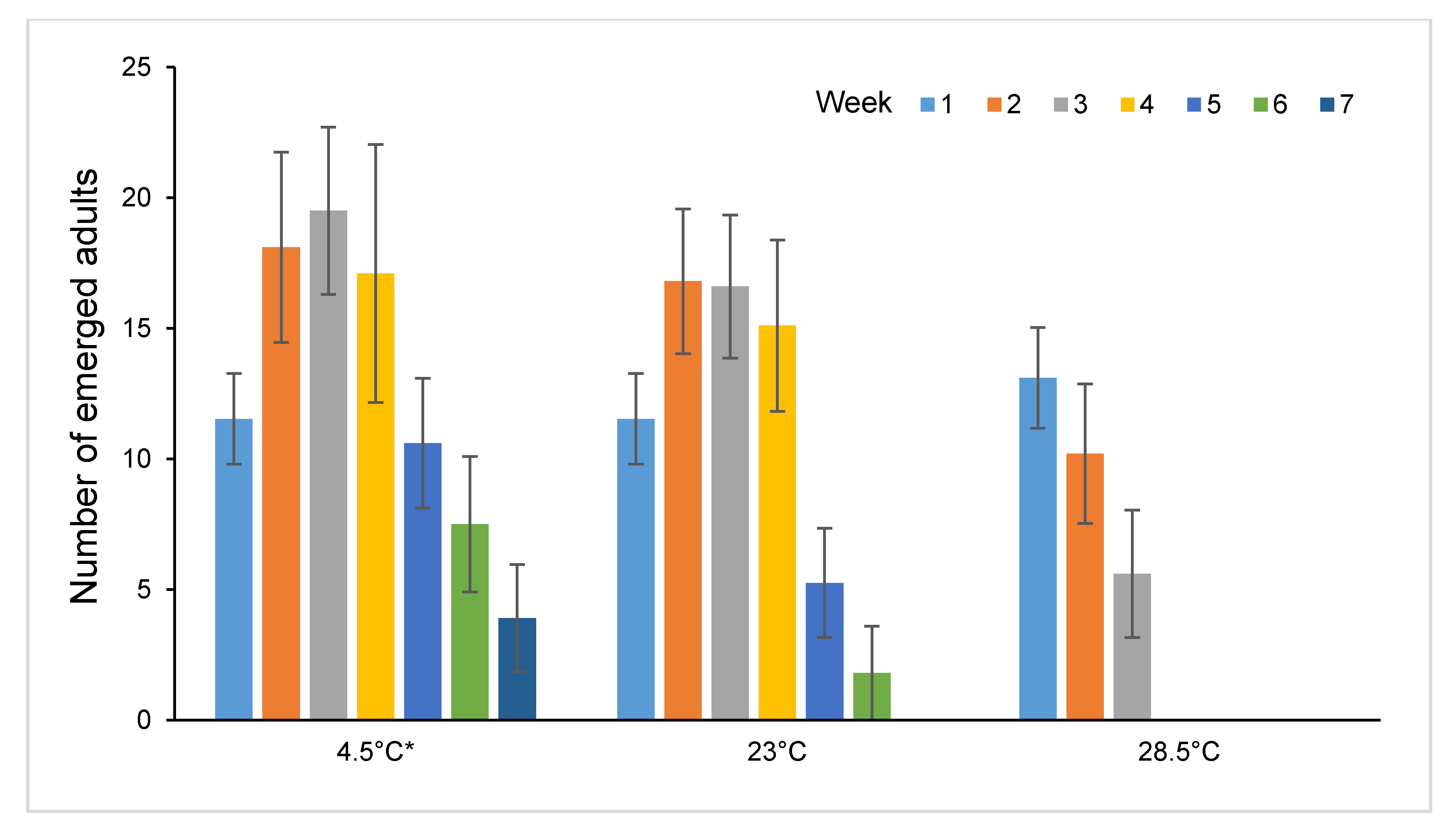
3.3. Longevity of Sclerodermus brevicornis Females Stored at Different Temperatures
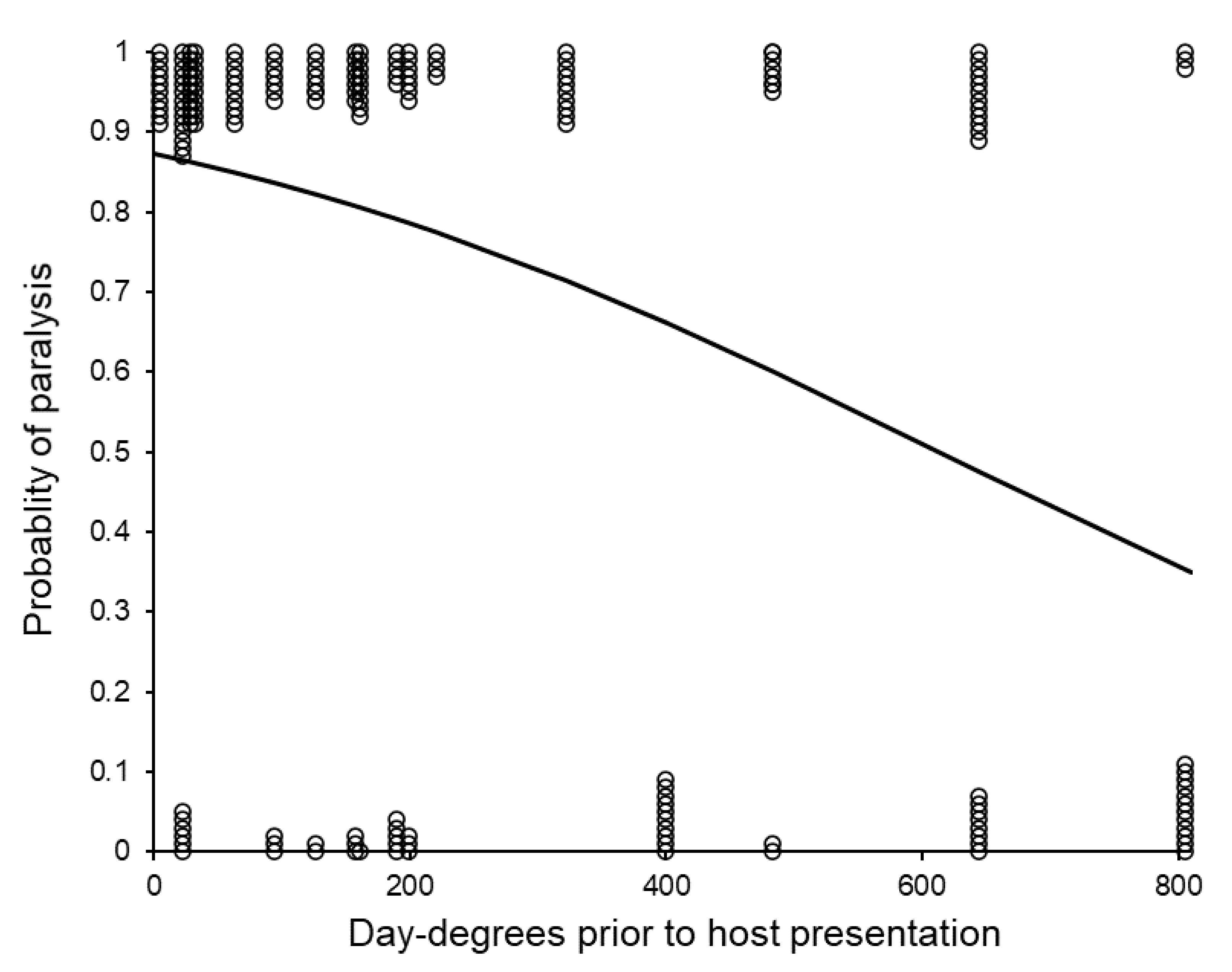
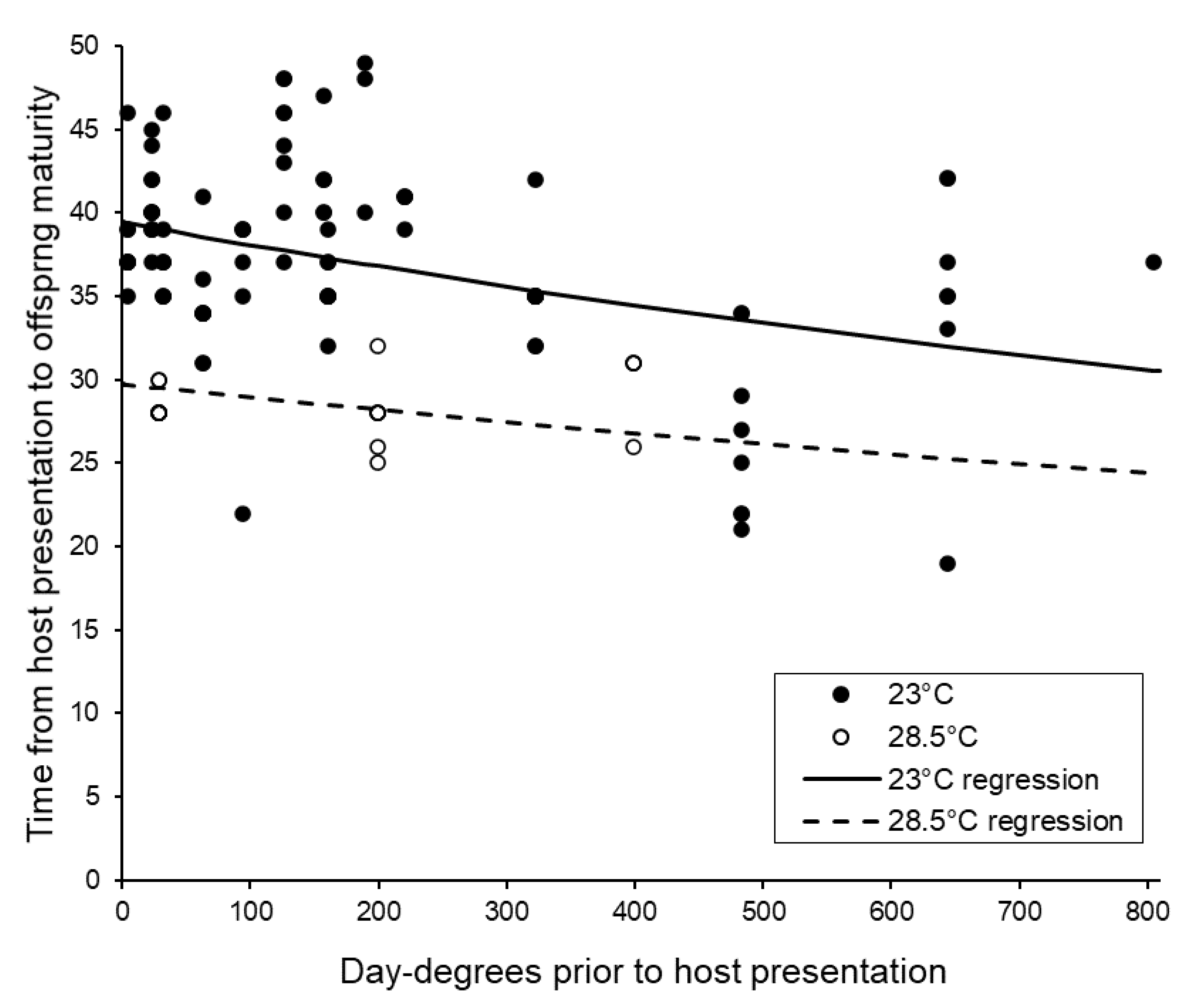
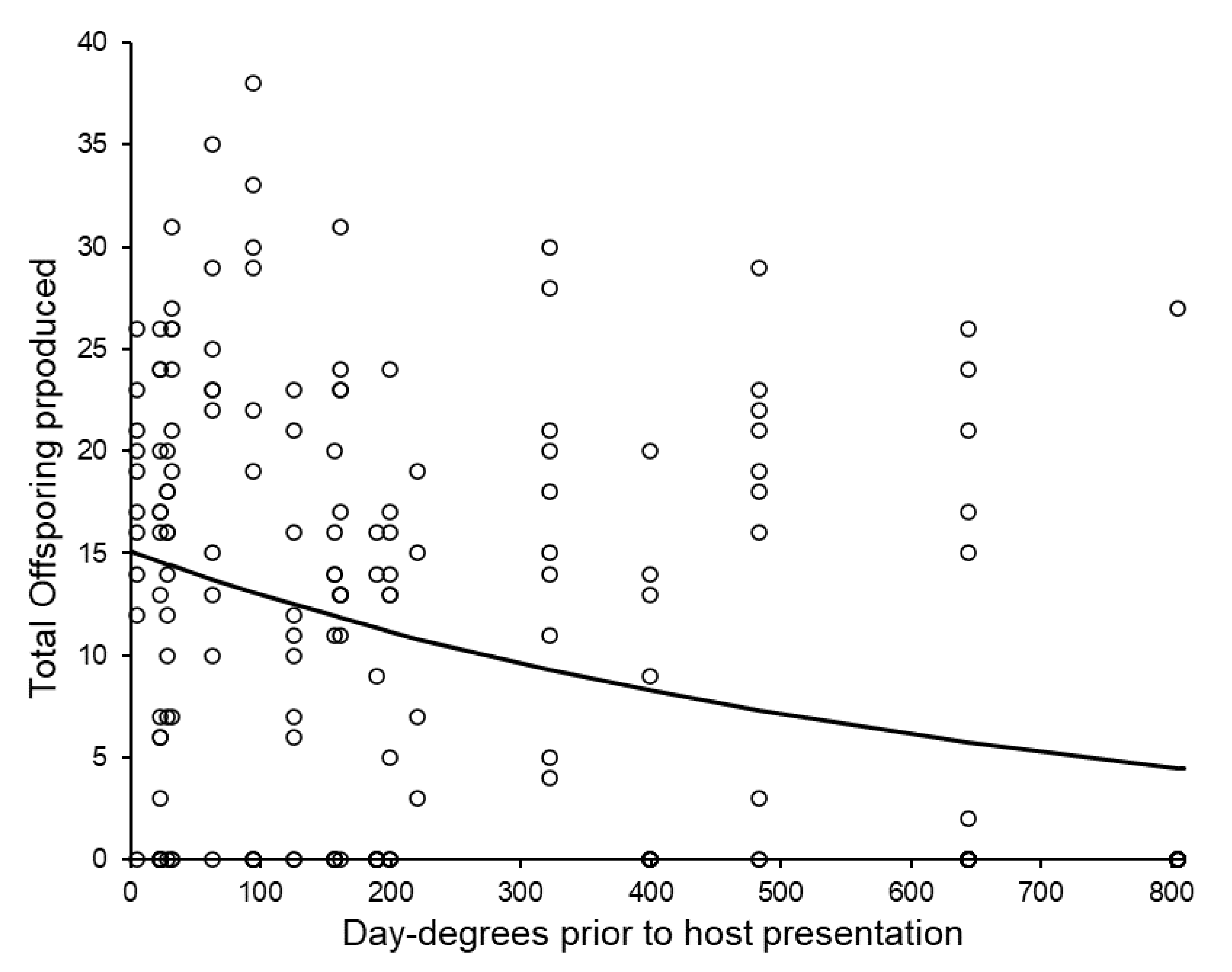
3.4. Post-Storage Reproductive Performance of Sclerodermus brevicornis Females
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jucker, C.; Lupi, D. Exotic Insects in Italy: An Overview on Their Environmental Impact. In The Importance of Biological Interactions in the Study of Biodiversity; Lopez Pujol, J., Ed.; InTech: London, UK, 2011; pp. 51–74. ISBN 978-953-307-751-2. [Google Scholar]
- Rassati, D.; Toffolo, E.P.; Roques, A.; Battisti, A.; Faccoli, M. Trapping wood boring beetles in Italian ports: A pilot study. J. Pest Sci. 2014, 87, 61–69. [Google Scholar] [CrossRef]
- Allen, E.; Noseworthy, M.; Ormsby, M. Phytosanitary measures to reduce the movement of forest pests with the international trade of wood products. Biol. Invasions 2017, 19, 3365–3376. [Google Scholar] [CrossRef]
- Yalcin, M.; Akcay, C.; Tascioglu, C.; Yuksel, B.; Ozbayram, A.K. Damage severity of wood-destroying insects according to the Bevan damage classification system in log depots of Northwest Turkey. Sci. Rep. 2020, 10, 13705. [Google Scholar] [CrossRef] [PubMed]
- Cocquempot, C. Alien longhorned beetles (Coleoptera: Cerambycidae): Original interceptions and introductions in Europe, mainly in France, and notes about recently imported species. Redia 2007, 89, 35–50. [Google Scholar]
- Rassati, D.; Faccoli, M.; Petrucco Toffolo, E.; Battisti, A.; Marini, L. Improving the early detection of alien wood-boring beetles in ports and surrounding forests. J. Appl. Ecol. 2015, 52, 50–58. [Google Scholar] [CrossRef]
- Haack, R.A.; Britton, K.O.; Brockerhof, E.G.; Cavey, J.F.; Garrett, L.J.; Kimberley, M.; Lowenstein, F.; Nuding, A.; Olson, L.J.; Turner, J.; et al. Effectiveness of the international phytosanitary standard ISPM no. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. PLoS ONE 2014, 9, e96611. [Google Scholar] [CrossRef]
- Eschen, R.; Rigaux, L.; Sukovata, L.; Vettraino, A.M.; Marzano, M.; Grégoire, J.C. Phytosanitary inspection of woody plants for planting at European Union entry points: A practical enquiry. Biol. Invasions 2015, 17, 2403–2413. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Liebhold, A.M. Ecology of forest insect invasions. Biol. Invasions 2017, 19, 3141–3159. [Google Scholar] [CrossRef]
- Levine, J.M.; D’Antonio, C.M. Forecasting biological invasions with increasing international trade. Conserv. Biol. 2003, 17, 322–326. [Google Scholar] [CrossRef]
- Bebber, D.P.; Holmes, T.; Smith, D.; Gurr, S.J. Economic and physical determinants of the global distributions of crop pests and pathogens. New Phytol. 2014, 202, 901–910. [Google Scholar] [CrossRef]
- Rassati, D.; Lieutier, F.; Faccoli, M. Alien wood-boring beetles in Mediterranean regions. In Insects and Diseases of Mediterranean Forest Systems; Springer: Cham, Switzerland, 2016; pp. 293–327. [Google Scholar]
- Maspero, M.; Jucker, C.; Colombo, M. First record of Anoplophora glabripennis (Motschulsky) (Coleoptera Cerambycidae Lamiinae Lamiini) in Italy. Boll. Zool. Agr. Bachic. 2007, 37, 161–164. [Google Scholar]
- Brockerhoff, E.G.; Liebhold, A.M.; Jactel, H. The ecology of forest insect invasions and advances in their management. Can. J. For. Res. 2006, 36, 263–268. [Google Scholar] [CrossRef]
- Haack, R.A. Exotic bark-and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Eyre, D.; Haack, R.A. Invasive cerambycid pests and biosecurity measures. In Cerambycidae of the World: Biology and Pest Management; Wang, Q., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 563–607. [Google Scholar]
- Faccoli, M.; Favaro, R.; Smith, M.T.; Wu, J. Life history of the Asian longhorn beetle Anoplophora glabripennis (Coleoptera Cerambycidae) in southern Europe. Agric. For. Entomol. 2015, 17, 188–196. [Google Scholar] [CrossRef]
- Hérard, F.; Maspero, M. History of discoveries and management of the citrus longhorned beetle, Anoplophora chinensis, in Europe. J. Pest Sci. 2019, 92, 117–130. [Google Scholar] [CrossRef]
- Eppo. 2020. Available online: https://gd.eppo.int/taxon/ANOLCN/distribution/IT (accessed on 20 August 2020).
- Lupi, D.; Jucker, C.; Colombo, M. Distribution and biology of the yellow-spotted longicorn beetle Psacothea hilaris hilaris (Pascoe) in Italy. OEPP/EPPO Bull. 2013, 43, 316–322. [Google Scholar] [CrossRef]
- Scrivener, A.M.; Watanabe, H.; Noda, H. Diet and carbohydrate digestion in the yellow-spotted longicorn beetle Psacothea hilaris. J. Insect Physiol. 1997, 43, 1039–1052. [Google Scholar] [CrossRef]
- Watari, Y.; Yamanaka, T.; Asano, W.; Ishikawa, Y. Prediction of the life cycle of the west Japan type yellow-spotted longicorn beetle, Psacothea hilaris (Coleoptera: Cerambycidae) by numerical simulations. Appl. Entomol. Zool. 2002, 37, 559–569. [Google Scholar] [CrossRef]
- Akbulut, S.; Stamps, W.T. Insect vectors of the pinewood nematode: A review of the biology and ecology of Monochamus species. For. Pathol. 2012, 42, 89–99. [Google Scholar] [CrossRef]
- Jucker, C.; Loni, A.; Calzolari, M.; Belokobylskij, S.; Lupi, D. Accidental introduction in Italy of the parasitoid Spathius vulnificus Wilkinson (Hymenoptera Braconidae Doryctinae). Redia 2008, 101, 189–192. [Google Scholar] [CrossRef]
- Brabbs, T.; Collins, D.; Hérard, F.; Maspero, M.; Eyre, D. Prospects for the use of biological control agents against Anoplophora in Europe. Pest Manag. Sci. 2015, 71, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Loni, A.; Jucker, C.; Belokobylskij, S.; Lupi, D. First record of Rhoptrocentrus piceus Marshall (Hymenoptera, Braconidae, Doryctinae) as parasitoid of Psacothea hilaris hilaris (Pascoe) (Coleoptera, Cerambycidae). ZooKeys 2015, 482, 1–8. [Google Scholar]
- Wingfield, M.J.; Brockerhoff, E.G.; Wingfield, B.D.; Slippers, B. Planted forest health: The need for a global strategy. Science 2015, 349, 832–836. [Google Scholar] [CrossRef]
- Hajek, A.E.; Hurley, B.P.; Kenis, M.; Garnas, J.R.; Bush, S.J.; Wingfield, M.J.; van Lenteren, J.C.; Cock, M.J.W. Exotic biological control agents: A solution or contribution to arthropod invasions? Biol. Invasions 2016, 18, 953–969. [Google Scholar] [CrossRef]
- Mazzetto, F.; Marchetti, E.; Amiresmaeili, N.; Sacco, D.; Francati, S.; Jucker, C.; Dindo, M.L.; Lupi, D.; Tavella, L. Drosophila parasitoids in northern Italy and their potential to attack the exotic pest Drosophila suzukii. J. Pest Sci. 2016, 89, 837–850. [Google Scholar] [CrossRef]
- Amiresmaeili, N.; Jucker, C.; Savoldelli, S.; Lupi, D. Understanding Trichopria drosophilae performance in laboratory conditions. Bull. Insectol. 2018, 71, 251–256. [Google Scholar]
- Ferracini, C.; Bertolino, S.; Bernardo, U.; Bonsignore, C.P.; Faccoli, M.; Ferrari, E.; Lupi, D.; Maini, S.; Mazzon, L.; Nugnes, F.; et al. Do Torymus sinensis (Hymenoptera: Torymidae) and agroforestry system affect native parasitoids associated with the Asian chestnut gall wasp? Biol. Control 2018, 121, 36–43. [Google Scholar] [CrossRef]
- Lotfalizadeh, H. Review of chalcidoid parasitoids (Hymenoptera: Chalcidoidea) of xylophagous beetles. Mun. Entomol. Zool. 2012, 7, 309–333. [Google Scholar]
- Naves, P.; Kenis, M.; Sousa, E. Parasitoids associated with Monochamus galloprovincialis (Oliv.) (Coleoptera: Cerambycidae) within the pine wilt nematode-affected zone in Portugal. J. Pest Sci. 2005, 78, 57–62. [Google Scholar] [CrossRef]
- Delvare, G.; Bon, M.C.; Hérard, F.; Cocquempot, C.; Maspero, M.; Colombo, M. Description of Aprostocetus anoplophorae n. sp. (Hymenoptera: Eulophidae), a new egg parasitoid of the invasive pest Anoplophora chinensis (Förster) (Coleoptera: Cerambycidae). Ann. Soc. Entomol. Fr. 2004, 40, 227–233. [Google Scholar] [CrossRef]
- Siscaro, G. Avetianella longoi sp. n. (Hymenoptera Encyrtidae) egg parasitoid of Phoracantha semipunctata F. (Coleoptera Cerambycidae). Bollettino di Zoologia Agraria e Bachicoltura Milano 1992, 24, 205–212. [Google Scholar]
- Trjapitzin, V.A.; Volkovitsh, M.G. A review of species of the genus Oobius Trjapitzin, 1963 (Hymenoptera, Encyrtidae) Egg parasitoids of jewel beetles, longicorn beetles (Coleoptera, Buprestidae, Cerambycidae), and robber flies (Diptera, Asilidae). Entomol. Rev. 2011, 91, 670. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Tavakoli Korghond, G.R.; Mianbandi, K.; Mahmoodi, H.; Mohammadipour, K.; Noyes, J. Ooencyrtus ferdowsii sp. n. (Hymenoptera: Encyrtidae), an egg parasitoid of Osphranteria coerulescens (Coleoptera: Cerambycidae) in Iran. Zool. Middle East 2015, 61, 45–49. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Cao, L.M.; Zhang, Y.L.; Wang, X.Y.; Zhan, M.K. A new egg parasitoid species (Hymenoptera: Pteromalidae) of Monochamus alternatus (Coleoptera: Cerambycidae), with notes on its biology. Ann. Entomol. Soc. Am. 2014, 107, 407–412. [Google Scholar] [CrossRef]
- Austin, A.D.; Quicke, D.L.J.; Marsh, P.M. The hymenopterous parasitoids of eucalypt longicorn beetles, Phoracantha spp. (Coleoptera: Cerambycidae) in Australia. Bull. Entomol. Res. 1994, 84, 145–174. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G.; Paine, T.D.; Wang, Q.; Paine, E.O. Patterns of host utilization by two parasitoids (Hymenoptera: Braconidae) of the eucalyptus longhorned borer (Coleoptera: Cerambycidae). Biol. Control 2001, 21, 152–159. [Google Scholar] [CrossRef]
- Millar, J.G.; Paine, T.D.; Campbell, C.D.; Hanks, L.M. Methods for rearing Syngaster lepidus and Jarra phoracantha (Hymenoptera: Braconidae), larval parasitoids of the phloem-colonizing longhorned beetles Phoracantha semipunctata and P. recurva (Coleoptera: Cerambycidae). Bull. Entomol. Res. 2002, 92, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Hilszczanski, J. Natural enemies of Cerambycidae and Buprestidae infesting living trees. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Springer: Dordrecht, The Netherlands, 2007; pp. 475–498. [Google Scholar]
- Tsankov, G.; Georgiev, G. Records on parasitoids of smaller poplar borer, Saperda populnea [Coleoptera, Cerambycidae] along the Danube in Bulgaria. Entomophaga 1991, 36, 493–498. [Google Scholar] [CrossRef]
- Duan, J.J.; Aparicio, E.; Tatman, D.; Smith, M.T.; Luster, D.G. Potential new associations of North American parasitoids with the invasive Asian longhorned beetle (Coleoptera: Cerambycidae) for biological control. J. Econ. Entomol. 2016, 109, 699–704. [Google Scholar] [CrossRef]
- Lupi, D.; Favaro, R.; Jucker, C.; Azevedo, C.O.; Hardy, I.C.W.; Faccoli, M. Reproductive biology of Sclerodermus brevicornis, a European parasitoid developing on three species of invasive longhorn beetles. Biol. Control 2017, 105, 40–48. [Google Scholar] [CrossRef]
- Golec, J.R.; Duan, J.J.; Aparicio, E.; Hough-Goldstein, J. Life history, reproductive biology, and larval development of Ontsira mellipes (Hymenoptera: Braconidae), a newly associated parasitoid of the invasive Asian longhorned beetle (Coleoptera: Cerambycidae). J. Econ. Entomol. 2016, 109, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Q.; Wang, X.Y.; Zhang, Y.N. Recent advances in biological control of important native and invasive forest pests in China. Biol. Control 2014, 68, 117–128. [Google Scholar] [CrossRef]
- Li, L.; Sun, J. Host suitability of a gregarious parasitoid on beetle hosts: Flexibility between fitness of adult and offspring. PLoS ONE 2011, 6, e18563. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhao, X.; Li, Y.; Liu, X.; Zhang, Q. Maternal care in the parasitoid Sclerodermus harmandi (Hymenoptera: Bethylidae). PLoS ONE 2012, 7, e51246. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Q.; Wang, X.Y.; Yao, Y.X.; Gould, J.R.; Cao, L.M. A new species of Sclerodermus (Hymenoptera: Bethylidae) parasitizing Agrilus planipennis (Coleoptera: Buprestidae) from China, with a key to Chinese species in the genus. Ann. Entomol. Soc. Am. 2012, 105, 619–627. [Google Scholar] [CrossRef]
- Men, J.; Zhao, B.; Cao, D.D.; Wang, W.C.; Wei, J.R. Evaluating host location in three native Sclerodermus species and their ability to cause mortality in the wood borer Aromia bungii (Coleoptera: Cerambycidae) in laboratory. Biol. Control 2019, 134, 95–102. [Google Scholar] [CrossRef]
- Kühne, H.; Becker, G. Zur Biologie und Ökologie von Scleroderma domesticum Latreille (Bethylidae, Hymenoptera), eine Parasiten holzzerstörender Insektenlarven. Zeitschrift für Angew. Entomol. 1974, 76, 278–303. [Google Scholar] [CrossRef]
- Evans, H.E. The Bethylidae of America North of Mexico. Mem. Am. Entomol. Inst. 1978, 27, 1–332. [Google Scholar]
- Baena, M.; Zuzarte, A.J. Contribución al estudio de los Bostríquidos de Portugal y actualización del catálogo de la fauna ibérica (Coleoptera, Bostrichidae). Zool. Baetica 2013, 24, 25–51. [Google Scholar]
- Jiang, Y.; Yang, Z.; Wang, X.; Hou, Y. Molecular identification of sibling species of Sclerodermus (Hymenoptera: Bethylidae) that parasitize buprestid and cerambycid beetles by using partial sequences of mitochondrial DNA cytochrome oxidase subunit 1 and 28S ribosomal RNA gene. PLoS ONE 2015, 10, e0119573. [Google Scholar] [CrossRef]
- Tang, X.; Meng, L.; Kapranas, A.; Xu, F.; Hardy, I.C.W.; Li, B. Mutually beneficial host exploitation and ultra-biased sex ratios in quasisocial parasitoids. Nat. Commun. 2014, 5, 4942. [Google Scholar] [PubMed]
- Zhang, L.Q.; Song, S.H.; Huang, H.H.; Li, X.Q.; Qui, L. Biological Control of a Wood Borer in China. IPM Pract. 1989, 11, 5–7. [Google Scholar]
- Liu, Z.; Xu, B.; Li, L.; Sun, J. Host-size mediated trade-off in a parasitoid Sclerodermus harmandi. PLoS ONE 2011, 6, e23260. [Google Scholar]
- Wang, X.; Wei, K.; Yang, Z.; Jennings, D.E.; Duan, J.J. Effects of biotic and abiotic factors on phenotypic partitioning of wing morphology and development in Sclerodermus pupariae (Hymenoptera: Bethylidae). Sci. Rep. 2016, 6, 26408. [Google Scholar] [PubMed]
- Costa, J.T. The other insect societies: Overview and new directions. Curr. Opin. Insect Sci. 2018, 28, 40–49. [Google Scholar] [PubMed]
- Abdi, M.K.; Lupi, D.; Jucker, C.; Hardy, I.C.W. Kinship effects in quasi-social parasitoids I: Co-foundress number and relatedness affect suppression of dangerous hosts. Biol. J. Linn. Soc. 2020, 130, 627–641. [Google Scholar]
- Abdi, M.K.; Hardy, I.C.W.; Jucker, C.; Lupi, D. Kinship effects in quasi-social parasitoids II: Co-foundress relatedness and host dangerousness interactively affect host exploitation. Biol. J. Linn. Soc. 2020, 130, 642–660. [Google Scholar]
- Chen, J.; Cheng, H. Advances in applied research on Sclerodermus spp. Chin. J. Biol. Control 2000, 16, 166–170. [Google Scholar]
- Kapranas, A.; Hardy, I.C.W.; Tang, X.; Gardner, A.; Li, B. Sex ratios, virginity, and local resource enhancement in a quasi-social parasitoid. Entomol. Exp. Appl. 2016, 159, 243–251. [Google Scholar]
- Abdi, M.K.; Jucker, C.; de Marchi, B.; Hardy, I.C.W.; Lupi, D. Performance of Sclerodermus brevicornis, a parasitoid of invasive longhorn beetles, when reared on rice moth larvae. Entomol. Exp. Appl. 2020. [Google Scholar] [CrossRef]
- Hu, S.; Wang, X.Y.; Yang, Z.Q.; Duan, J.J. Effects of photoperiod and light intensity on wing dimorphism and development in the parasitoid Sclerodermus pupariae (Hymenoptera: Bethylidae). Biol. Control 2019, 133, 117–122. [Google Scholar] [CrossRef]
- Zera, A.J.; Denno, R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997, 42, 207–230. [Google Scholar] [CrossRef] [PubMed]
- Hardy, I.C.W.; Mayhew, P.J. Sex ratio, sexual dimorphism and mating structure in bethylid wasps. Behav. Ecol. Sociobiol. 1998, 42, 383–395. [Google Scholar] [CrossRef]
- Chen, R.; Tang, Y.; Tang, H.; Wang, X.; Yang, Z. Effects of larvae body size of Thyestilla gebleri on oviposition decision of Sclerodermus alternatusi. Chin. J. Biol. Control 2019, 35, 848–854. [Google Scholar]
- Chen, R.; Tang, Y.; Tang, H.; Wang, X.; Yang, Z. Effect of Temperature on development and reproduction of Sclerodermus alternatusi. For. Res. 2019, 32, 114–119. [Google Scholar]
- Yang, Z.Q. Advance in bio-control researches of the important forest insect pests with natural enemies in China. Chin. J. Biol. Control 2004, 20, 221–227. [Google Scholar]
- Kaishu, D.U. Experiment in the control of Anoplophora chinensis (Foster) with Scleroderma sichuanensis Xaio. J. Anhui Agric. Sci. 2006, 34, 3104. [Google Scholar]
- Tang, Y.L.; Wang, X.Y.; Yang, Z.Q.; Jiang, J.; Wang, X.H.; Lu, J. Alternative hosts of Sclerodermus pupariae (Hymenoptera: Bethylidae), a larval parasitoid of the longhorn beetle Massicus raddei (Coleoptera: Cerambycidae). Acta Entomol. Sin. 2012, 55, 55–62. [Google Scholar]
- Lai, Y.X.; Wang, Y.H.; Wang, X.Y.; Yang, Z.; Tang, Y.; Qin, R.; Zhang, Y.N.; Zhang, Y.F. A field test of controlling the pine wilt disease with the technique of isolating Monochamus alternatus on forest spot and releasing parasitoid Sclerodermus guani. Chin. J. Biol. Control 2012, 28, 460–465. [Google Scholar]
- Zhang, Y.; Yang, Z.; Wang, X.; Zhang, Y.; Wu, C.; Ma, S.; Lu, Z. Functional response of the parasitoid Sclerodermus sp. (Hymenoptera: Bethylidae) to the third instar larvae of host Monochamus alternatus (Coleoptera: Cerambycidae). Acta Entomol. Sin. 2012, 55, 426–434. [Google Scholar]
- Wu, H.; Wang, X.; Li, M.; Yang, Z.; Zeng, F.; Wang, H.; Sun, J. Biology and mass rearing of Sclerodermus pupariae Yang et Yao (Hymenoptera: Bethylidae), an important ectoparasitoid of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae) in China. Acta Entomol. Sin. 2008, 51, 46–54. [Google Scholar]
- Wang, X.; Yang, Z.; Tang, Y.; Jiang, J.; Gao, C.; Liu, Y.; Zhang, X. Parasitism of Sclerodermus pupariae (Hymenoptera: Bethylidae) on the young larvae of Massicus raddei (Coleoptera: Cerambycidae). Acta Entomol. Sin. 2010, 53, 675–682. [Google Scholar]
- Maspero, M.; Jucker, C.; Colombo, M.; Herard, F.; Ciampitti, M.; Cavagna, B. News about CLB and ALB in Italy. Forstschutz Aktuell 2008, 44, 25–26. [Google Scholar]
- Jucker, C.; Tantardini, A.; Colombo, M. First record of Psacothea hilaris (Pascoe) in Europe (Coleoptera Cerambycidae Lamiinae Lamiini). Boll. Zool. Agric. Bachic. 2006, 38, 187–191. [Google Scholar]
- Lupi, D.; Jucker, C.; Rocco, A.; Harrison, R.; Colombo, M. Notes on biometric variability in invasive species: The case of Psacothea hilaris hilaris (Pascoe) (Coleoptera, Cerambycidae, Lamiinae). Bull. Insectol. 2015, 68, 135–145. [Google Scholar]
- Favaro, R.; Lupi, D.; Jucker, C.; Cappellozza, S.; Faccoli, M. An artificial diet for rearing Asian longhorn beetles (Coleoptera, Cerambycidae) invasive to Europe. Bull. Insectol. 2017, 70, 91–99. [Google Scholar]
- Lupi, D.; Jucker, C.; Rocco, A.; Cappellozza, S.; Colombo, M. Diet Effect on Psacothea hilaris hilaris (Coleoptera: Cerambycidae) Performance under Laboratory Conditions. Int. J. Agric. Innov. Res. 2015, 4, 97–104. [Google Scholar]
- Locatelli, D.P.; Savoldelli, S.; Girgenti, P.; Lucchini, G.A.; Limonta, L. Can environmental dust from silo area allow the development of stored product insects? J. Stored Prod. Res. 2017, 71, 41–46. [Google Scholar] [CrossRef]
- Lupi, D.; Bernardo, U.; Bonsignore, C.P.; Colombo, M.; Dindo, M.L.; Faccoli, M.; Ferracini, C.; Gualtieri, L.; Marullo, R.; Mazzon, L.; et al. Insects and globalization: Sustainable control of exotic species in Italian agro-forestry ecosystems. Landscape management for functional biodiversity. IOBC/WPRS Bull. 2014, 100, 87–90. [Google Scholar]
- Jervis, M.A.; Copland, M.J.W.; Harvey, J.A. The life-cycle. In Insects as Natural Enemies: A Practical Perspective; Jervis, M.A., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 73–166. [Google Scholar]
- Crawley, M.J. GLIM for Ecologists; Blackwell Scientific Publications: Oxford, UK, 1993. [Google Scholar]
- Faraway, J.J. Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–312. [Google Scholar]
- Wilson, K.; Hardy, I.C.W. Statistical analysis of sex ratios: An introduction. In Sex Ratios: Concepts and Research Methods; Hardy, I.C.W., Ed.; Cambridge University Press: Cambridge, UK, 2002; pp. 48–92. [Google Scholar]
- Aitkin, M.; Anderson, D.; Francis, B.; Hinde, J. Statistical Modelling in GLIM; Clarendon Press: Oxford, UK, 1989; pp. 1–374. [Google Scholar]
- van Lenteren, J.C. Quality Control and Production of Biological Control Agents: Theory and Practice; CABI Publishing: Wallingford, UK, 2003; pp. 1–352. [Google Scholar]
- Wei, K.; Tang, Y.L.; Wang, X.Y.; Cao, L.M.; Yang, Z.G. The developmental strategies and related profitability of an idiobiont ectoparasitoid Sclerodermus pupariae vary with host size. Ecol. Entomol. 2014, 39, 101–108. [Google Scholar]
- Colinet, H.; Hance, T. Interspecific variation in the response to low temperature storage in different aphid parasitoids. Annal. Appl. Biol. 2010, 156, 147–156. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Tommasini, M.G. Mass production, storage, shipment and quality control of natural enemies. In Integrated Pest and Disease Management in Greenhouse Crops; Springer: Dordrecht, The Netherlands, 1999; pp. 276–294. [Google Scholar]
- Dindo, M.L.; Grenier, S. Production of dipteran parasitoids. In Mass Production of Beneficial Organisms; Academic Press: Cambridge, MA, USA, 2014; pp. 101–143. [Google Scholar]
- Mahi, H.; Rasekh, A.; Michaud, J.P.; Shishehbor, P. Biology of Lysiphlebus fabarum following cold storage of larvae and pupae. Entomol. Exp. Appl. 2014, 153, 10–19. [Google Scholar]
- Glenister, C.S.; Hoffmann, M.P. Mass-reared natural enemies: Scientific technological, and informational needs and considerations. In Mass-Reared Natural Enemies: Application, Regulation, and Needs; Ridgway, R., Hoffmann, M.P., Inscoe, M.N., Glenister, C.S., Eds.; Entomological Society of America: Lanham, MD, USA, 1998; pp. 242–247. [Google Scholar]
- Pitcher, S.A.; Hoffmann, M.P.; Gardner, J.; Wright, M.G.; Kuhar, T.P. Cold storage of Trichogramma ostriniae reared on Sitotroga cerealella eggs. Biocontrol 2002, 47, 525–535. [Google Scholar]
- McDonald, R.C.; Kok, L.T. Post refrigeration viability of Pteromalus puparum (Hymenoptera: Pteromalidae) prepupae within host chrysalids. J. Entomol. Sci. 1990, 25, 409–413. [Google Scholar] [CrossRef]
- Venkatesan, T.; Singh, S.P.; Jalali, S.K. Rearing of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) on semi-synthetic diet and its predatory efficiency against cotton pests. Entomon 2000, 25, 81–89. [Google Scholar]
- Colinet, H.; Boivin, G. Insect parasitoids cold storage: A comprehensive review of factors of variability and consequences. Biol. Control 2011, 58, 83–95. [Google Scholar] [CrossRef]
- Benelli, M.; Ponton, F.; Lallu, U.; Mitchell, K.A.; Taylor, P.W. Cool storage of Queensland fruit fly pupae for improved management of mass production schedules. Pest Manag. Sci. 2019, 75, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Hance, T.; van Baaren, J.; Vernon, P.; Boivin, G. Impact of Extreme Temperatures on Parasitoids in a Climate Change Perspective. Ann. Rev. Entomol. 2007, 52, 107–126. [Google Scholar] [CrossRef]
- Hong, J.; Koh, S.; Chung, Y.; Shin, S.; Kim, G.; Choi, K. Biological characteristics of Sclerodermus harmandi (Hymenoptera: Bethylidae) parasitized on Cerambycid. Korean J. Appl. Entomol. 2008, 47, 133–139. [Google Scholar]
- Ode, P.J.; Hardy, I.C.W. Parasitoid sex ratios and biological control. In Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications; Wajnberg, E., Bernstein, C., van Alphen, J.J.M., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 253–291. [Google Scholar]

| Estimated Parameter | Host | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | |
| Cumulative probabilities | ||||
| Mean | 0.933 | 0.767 | 0.167 | 0.133 |
| +S.E. | 0.034 | 0.068 | 0.080 | 0.075 |
| −S.E. | 0.063 | 0.086 | 0.058 | 0.051 |
| Separate probabilities | ||||
| Mean | 0.933 | 0.821 | 0.227 | 0.801 |
| +S.E. | 0.034 | 0.061 | 0.101 | 0.124 |
| −S.E. | 0.063 | 0.084 | 0.077 | 0.233 |
| Time to oviposition | ||||
| Mean (days) | 19.928 | 25.52 | 25.400 | 25.753 |
| +S.E. | 2.549 | 1.683 | 2.729 | 5.236 |
| −S.E. | 2.027 | 1.4815 | 2.246 | 3.722 |
| Estimated Parameter | Host | |||
|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | |
| Cumulative probabilities | ||||
| Mean | 0.267 | 0.092 | 0.008 | 0.008 |
| +S.E. | 0.042 | 0.030 | 0.014 | 0.014 |
| −S.E. | 0.038 | 0.023 | 0.005 | 0.005 |
| Separate probabilities | ||||
| Mean | 0.267 | 0.297 | 0.111 | 1.00 † |
| +S.E. | 0.042 | 0.080 | 0.154 | |
| −S.E. | 0.038 | 0.069 | 0.070 | |
| Time to transfer to next host | ||||
| Mean (days) | 32.134 | 29.896 | 26.00 † | - |
| +S.E. | 1.568 | 2.131 | ||
| −S.E. | 1.392 | 2.487 | ||
| Storage Temperature (±1 °C) | Maximum Longevity (Days) | Mean Longevity (Days ± S.E.) | DR50 (Days Until ½ the Females Died) |
|---|---|---|---|
| 4.5 | 98 | 49.81 ± 1.67 a | 43 |
| 23 | 58 | 36.10 ± 0.95 b | 36 |
| 28.5 | 35 | 17.18 ± 0.46 c | 17 |
| 34 | 9 | 6.54 ± 0.15 d | 5 |
| Reproductive Stage | Storage Day-Degrees | Post-Storage Temperature | Interaction |
|---|---|---|---|
| Host presentation to paralysis | Faster after more day-degrees F1,129 = 11.05, p < 0.001 | Faster at higher temperature F1,129 = 5.22, p = 0.024 | N.S. F1,128 = 0.24, p = 0.625 |
| Host presentation to oviposition | Faster after more day-degrees F1,125 = 9.38, p = 0.003 | Faster at higher temperature F1,125 = 20.53, p < 0.001 | N.S. F1,128 = 0.38, p = 0.541 |
| Paralysis to oviposition | N.S. F1,119 = 0.01, p = 0.906 | Faster at higher temperature F1,120 = 5.96, p = 0.016 | N.S. F1,118 = 0.15, p = 0.700 |
| Oviposition to larvae | Marginally N.S. F1,120 = 3.74, p = 0.056 | Faster at higher temperature F1,121 = 17.06, p < 0.001 | N.S. F1,119 = 0.07, p = 0.797 |
| Larvae to pupation | N.S. F1,118 = 1.57, p = 0.212 | N.S. F1,117 = 0.31, p = 0.580 | N.S. F1,116 = 0.38, p = 0.536 |
| Pupation to adult emergence | Slower after more day-degrees F1,112 = 12.02, p < 0.001 | Faster at higher temperature F1,112 = 80.64, p < 0.001 | Development slowed more greatly by day-degrees accumulated when temperature higher F1,112 = 7.91, p = 0.006 |
| Overall host presentation to emergence | Faster after more day-degrees F1,115 = 19.03, p < 0.001 | Faster at higher temperature F1,115 = 54.31, p < 0.001 | N.S. F1,114 = 2.58, p = 0.111 |
| Experimental Conditions | Timing of Reproductive Stages (Days) | ||||||
|---|---|---|---|---|---|---|---|
| Foundress Storage Temperature (±1 °C) | Offspring Production Temperature (±1 °C) | Host Presentation to Paralysis | Paralysis to Oviposition | Oviposition to Hatching | Hatching to Pupation | Pupation to Emergence | Oviposition to Emergence |
| 4.5 | 23 | 4.47 ± 0.39 a | 4.24 ± 0.27 ab | 6.51 ± 0.4 a | 8.04 ± 0.47 a | 16.54 ± 0.44 a | 30.49 ± 0.56 a |
| 23 | 23 | 3.24 ± 0.28 ab | 4.77 ± 0.21 a | 5.11 ± 0.28 b | 6.96 ± 0.44 a | 17.11 ± 0.39 a | 28.82 ± 0.51 a |
| 28.5 | 28.5 | 2.90 ± 0.34 b | 3.45 ± 0.30 b | 3.71 ± 0.31 c | 7.25 ± 0.34 a | 11.35 ± 0.52 b | 22.30 ± 0.41 b |
| Storage Temperature (±1 °C) | Post-Storage Temperature (±1 °C) | Replicates with Reproductive Success (%) | Total Offspring Production (Including Zeros) (Mean ± 1 S.E.) | Brood Size (Excluding Zeros) (Mean ± 1 S.E.) |
|---|---|---|---|---|
| 4.5 | 23 | 54% | 13.36 ± 1.33 a | 19.48 ± 1.11 a |
| 23 | 23 | 69% | 9.35 ± 1.12 b | 17.28 ± 1.15 ab |
| 28.5 | 28.5 | 67% | 9.63 ± 1.44 ab | 14.45 ± 1.03 b |
| Sex | Total Offspring | Alate | Apterous |
|---|---|---|---|
| Male | 163 | 160 (98.16%) | 3 (1.84%) |
| Female | 1901 | 12 (0.63%) | 1889 (99.37%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jucker, C.; Hardy, I.C.W.; Malabusini, S.; de Milato, S.; Zen, G.; Savoldelli, S.; Lupi, D. Factors Affecting the Reproduction and Mass-Rearing of Sclerodermus brevicornis (Hymenoptera: Bethylidae), a Natural Enemy of Exotic Flat-Faced Longhorn Beetles (Coleoptera: Cerambycidae: Lamiinae). Insects 2020, 11, 657. https://doi.org/10.3390/insects11100657
Jucker C, Hardy ICW, Malabusini S, de Milato S, Zen G, Savoldelli S, Lupi D. Factors Affecting the Reproduction and Mass-Rearing of Sclerodermus brevicornis (Hymenoptera: Bethylidae), a Natural Enemy of Exotic Flat-Faced Longhorn Beetles (Coleoptera: Cerambycidae: Lamiinae). Insects. 2020; 11(10):657. https://doi.org/10.3390/insects11100657
Chicago/Turabian StyleJucker, Costanza, Ian C.W. Hardy, Serena Malabusini, Silvia de Milato, Giacomo Zen, Sara Savoldelli, and Daniela Lupi. 2020. "Factors Affecting the Reproduction and Mass-Rearing of Sclerodermus brevicornis (Hymenoptera: Bethylidae), a Natural Enemy of Exotic Flat-Faced Longhorn Beetles (Coleoptera: Cerambycidae: Lamiinae)" Insects 11, no. 10: 657. https://doi.org/10.3390/insects11100657
APA StyleJucker, C., Hardy, I. C. W., Malabusini, S., de Milato, S., Zen, G., Savoldelli, S., & Lupi, D. (2020). Factors Affecting the Reproduction and Mass-Rearing of Sclerodermus brevicornis (Hymenoptera: Bethylidae), a Natural Enemy of Exotic Flat-Faced Longhorn Beetles (Coleoptera: Cerambycidae: Lamiinae). Insects, 11(10), 657. https://doi.org/10.3390/insects11100657







