Proximate Drivers of Migration and Dispersal in Wing-Monomorphic Insects
Abstract
1. Introduction
Definitions
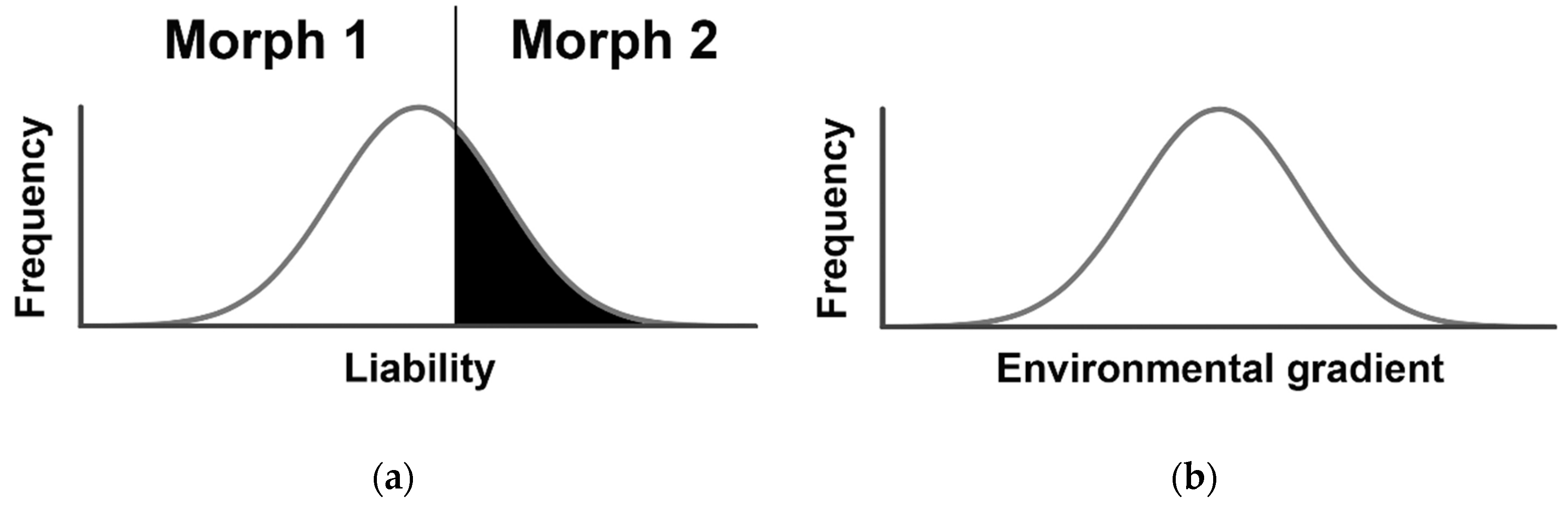
2. Threshold Genetic Models: Back to the Future
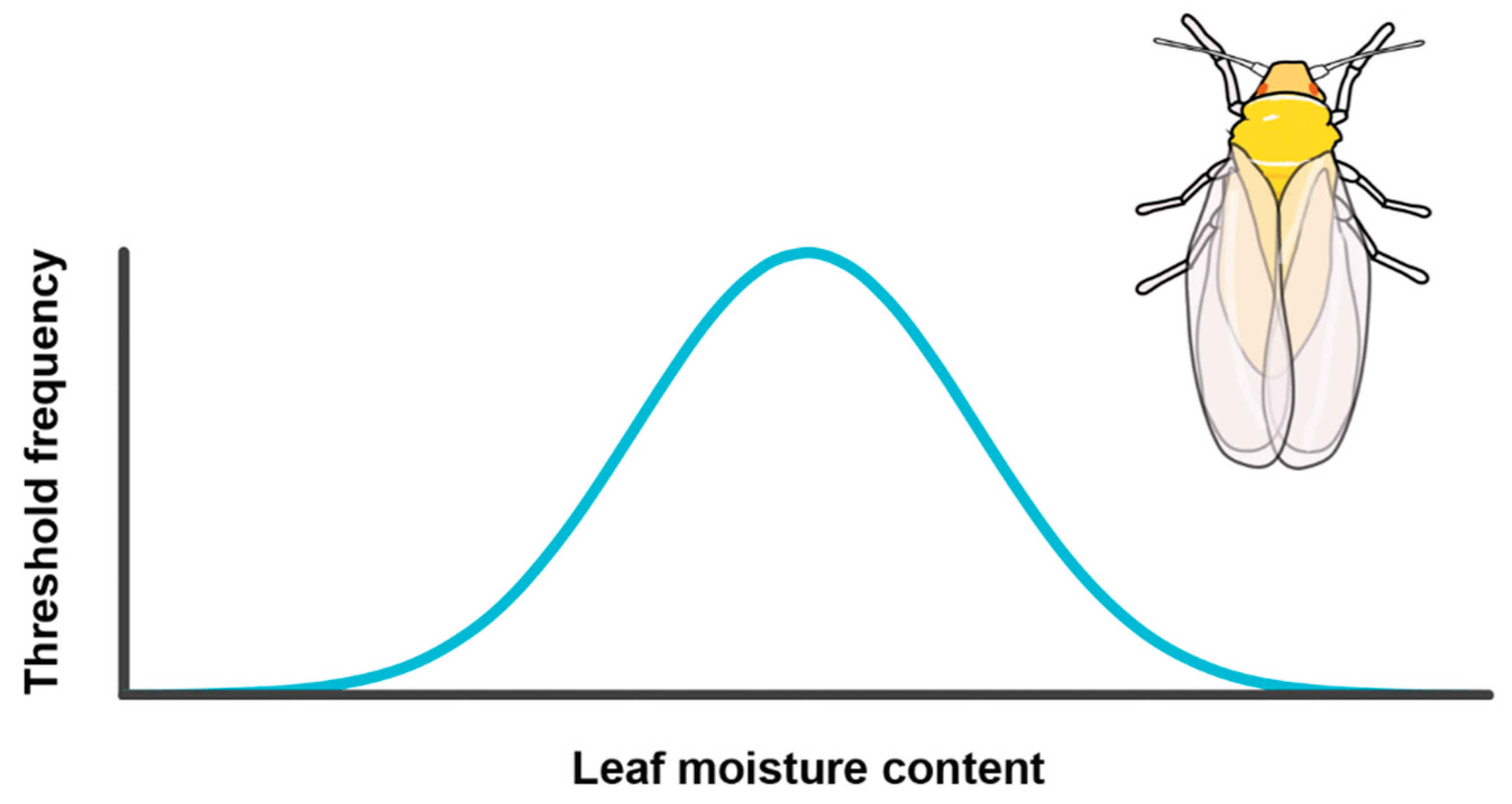
Testing the Environmental Threshold Model
3. The Oogenesis-Flight Relationship: It’s Complicated
3.1. Alternative Adaptive Hypotheses in the Oogenesis-Flight Relationship
3.2. Oogenesis and Flight May not Compete for Resources
3.3. Masking of the Trade-Off between Oogenesis and Flight
4. Natal Dispersal and Sex Determination in Hymenoptera: Alphabet Soup
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Asplen, M.K.; Hardin, J.A.; Byrne, D.N. The relationship between pre-oviposition flight behaviour and reproductive timing in whitefly parasitoids. Physiol. Entomol. 2009, 34, 350–358. [Google Scholar] [CrossRef]
- Asplen, M.K. Dispersal strategies in terrestrial insects. Curr. Opin. Insect Sci. 2018, 27, 16–20. [Google Scholar] [CrossRef]
- Bonte, D.; Dahirel, M. Dispersal: A central and independent trait in life history. Oikos 2016, 126, 472–479. [Google Scholar] [CrossRef]
- Stinner, R.E.; Barfield, C.S.; Stimac, J.L.; Dohse, L. Dispersal and movement of insect pests. Annu. Rev. Entomol. 1983, 28, 319–335. [Google Scholar] [CrossRef]
- Chapman, J.W.; Drake, V.A.; Reynolds, D.R. Recent insights from radar studies of insect flight. Annu. Rev. Entomol. 2011, 56, 337–356. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Asplen, M.K. A ‘Goldilocks’ hypothesis for dispersal of biological control agents. BioControl 2011, 56, 441–450. [Google Scholar] [CrossRef]
- Renault, D.; Laparie, M.; McCauley, S.J.; Bonte, D. Environmental adaptations, ecological filtering, and dispersal central to insect invasions. Annu. Rev. Entomol. 2018, 63, 345–368. [Google Scholar] [CrossRef]
- Roff, D.A. The evolution of wing dimorphism in insects. Evolution 1986, 40, 1009–1020. [Google Scholar] [CrossRef]
- Zera, A.J.; Denno, R.F. Physiology and ecology of dispersal polymorphism in insects. Annu. Rev. Entomol. 1997, 42, 207–230. [Google Scholar] [CrossRef]
- Werren, J.H. Sex ratio adaptations to local mate competition in a parasitic wasp. Science 1980, 208, 1157–1159. [Google Scholar] [CrossRef]
- Herre, E.A. Sex ratio adjustment in fig wasps. Science 1985, 228, 896–898. [Google Scholar] [CrossRef]
- Thayer, M.K. Discovery of sexual wing dimorphism in Staphylinidae (Coleoptera): “Omalium” flavidum, and a discussion of wing dimorphism in insects. J. New York Entomol. Soc. 1992, 100, 540–573. [Google Scholar]
- Roff, D.A.; Fairbairn, D.J. Wing dimomorphisms and the evolution of migratory polymorphisms among the Insecta. Amer. Zool. 1991, 31, 243–251. [Google Scholar] [CrossRef]
- Lin, X.; Lavine, L.C. Endocrine regulation of a dispersal polymorphism in winged insects: A short review. Curr. Opin. Insect Sci. 2018, 25, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Herman, W.S.; Tatar, M. Juvenile hormone regulation of longevity in the migratory monarch butterfly. Proc. Biol. Sci. 2001, 268, 2509–2514. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Zhang, W.; Niitepõld, K.; Hsu, J.; Fernández Haeger, J.; Zalucki, M.P.; Altizer, S.; de Roode, J.C.; Reppert, S.M.; Kronforst, M.R. The genetics of monarch butterfly migration and warning coloration. Nature 2014, 514, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Edelsparre, A.H.; Vesterberg, A.; Lim, J.H.; Anwari, M.; Fitzpatrick, M.J. Alleles underlying larval foraging behaviour influence adult dispersal in nature. Ecol. Lett. 2014, 17, 333–339. [Google Scholar] [CrossRef]
- Muñoz, D.; Jimenez, A.; Marinotti, O.; James, A.A. The AeAct-4 gene is expressed in the developing flight muscles of female Aedes aegypti. Insect Mol. Biol. 2004, 13, 563–568. [Google Scholar] [CrossRef]
- Jones, C.M.; Papanicolaou, A.; Mironidis, G.K.; Vontas, J.; Yang, Y.; Lim, K.S.; Oakeshott, J.G.; Bass, C.; Chapman, J.W. Genomewide transcriptional signatures of migratory flight activity in a globally invasive insect pest. Mol. Ecol. 2015, 24, 4901–4911. [Google Scholar] [CrossRef]
- Dingle, H.; Drake, V.A. What is migration? BioScience 2007, 57, 113–121. [Google Scholar] [CrossRef]
- Kennedy, J.S. Migration: Behavioral and ecological. In Migration: Mechanisms and Adaptive Significance, Contributions in Marine Science 27 (suppl.); Rankin, M.A., Ed.; Marine Science Institute, University of Texas at Austin: Port Aransas, TX, USA, 1985; pp. 5–26. [Google Scholar]
- De Belle, J.S.; Sokolowski, M.B. Heredity of rover/sitter: Alternative foraging strategies of Drosophila melanogaster larvae. Heredity 1987, 59, 73–83. [Google Scholar] [CrossRef]
- Fitzpatrick, M.J.; Feder, E.; Rowe, L.; Sokolowski, M.B. Maintaining a behaviour polymorphism by frequency-dependent selection on a single gene. Nature 2007, 447, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Ehrman, L.; Parsons, P.A. The Genetics of Behavior; Sinauer: Sunderland, MA, USA, 1976. [Google Scholar]
- Lande, R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 1982, 34, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.A.; Reppert, S.A. Coldness triggers northward flight in remigrant monarch butterflies. Curr. Biol. 2013, 23, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Merlin, C.; Liedvogel, M. The genetics and epigenetics of animal migration and orientation: Birds, butterflies and beyond. J. Exp. Biol. 2019, 222. [Google Scholar] [CrossRef] [PubMed]
- Roff, D.A. The evolution of threshold traits in animals. Q. Rev. Biol. 1996, 71, 3–35. [Google Scholar] [CrossRef]
- Roff, D.A. Dimorphisms and threshold traits. Nat. Educ. 2008, 1, 211. [Google Scholar]
- Pulido, F. Evolutionary genetics of partial migration—The threshold model of migration revis (it) ed. Oikos 2011, 120, 1776–1783. [Google Scholar] [CrossRef]
- Sahashi, G.; Morita, K. Migration costs drive convergence of threshold traits for migratory tactics. Proc. R. Soc. B 2013, 280. [Google Scholar] [CrossRef]
- Roff, D.A.; Fairbairn, D.J. The evolution and genetics of migration in insects. BioScience 2007, 57, 155–164. [Google Scholar] [CrossRef]
- Hardie, J. Flight behavior in migrating insects. J. Agric. Entomol. 1993, 10, 239–245. [Google Scholar]
- Blackmer, J.L.; Naranjo, S.E.; Williams III, L.H. Tethered and untethered flight by Lygus hesperus and Lygus lineolaris (Heteroptera: Miridae). Environ. Entomol. 2004, 33, 1389–1400. [Google Scholar] [CrossRef]
- Taylor, R.A.J.; Bauer, L.S.; Poland, T.M.; Windell, K.N. Flight performance of Agrilus planipennis (Coloeptera: Buprestidae) on a flight mill and in free flight. J. Insect Behav. 2010, 23, 128–148. [Google Scholar] [CrossRef]
- Hazel, W.N.; Smock, R.; Johnson, M.D. A polygenic model for the evolution and maintenance of conditional strategies. Proc. R. Soc. Lond. B 1990, 242, 181–187. [Google Scholar]
- Hazel, W.N.; Smock, R.; Lively, C.M. The ecological genetics of conditional strategies. Am. Nat. 2004, 163, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.N.; Bellows, T.S., Jr. Whitefly biology. Annu. Rev. Entomol. 1991, 36, 431–457. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Brown, J.K. Current status of Bemisia tabaci as a plant pest and virus vector in agroecosystems worldwide. FAO Plant Prot. Bull. 1994, 42, 3–32. [Google Scholar]
- De Barro, P.J. Bemisia tabaci biotype B: A review of its biology, distribution and control. CSIRO Aust. Div. Entomol. Tech. Paper 1995, 36, 1–58. [Google Scholar]
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef]
- Blackmer, J.L.; Byrne, D.N. Flight behaviour of Bemisia tabaci in a vertical flight chamber: Effect of time of day, sex, age and host quality. Physiol. Entomol. 1993, 18, 223–232. [Google Scholar] [CrossRef]
- Blackmer, J.L.; Byrne, D.N. Environmental and physiological factors influencing phototactic flight of Bemisia tabaci. Physiol. Entomol. 1993, 18, 336–342. [Google Scholar] [CrossRef]
- Byrne, D.N.; Rathman, R.J.; Orum, T.V.; Palumbo, J.C. Localized migration and dispersal by the sweet potato whitefly. Bemisia tabaci. Oecologia 1996, 105, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, R.; Byrne, D.N. Aerial distribution, flight behaviour and eggload: Their inter-relationship during dispersal by the sweetpotato whitefly. J. Anim. Ecol. 1998, 67, 741–750. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Booth, C.O. Free flight of aphids in the laboratory. J. Exp. Biol. 1963, 40, 67–85. [Google Scholar]
- Blackmer, J.L.; Byrne, D.N.; Tu, Z. Behavioral, morphological, and physiological traits associated with migratory Bemisia tabaci (Homoptera: Aleyrodidae). J. Insect Behav. 1994, 8, 251–267. [Google Scholar] [CrossRef]
- Byrne, D.N. Migration and dispersal by the sweet potato whitefly, Bemisia tabaci. Agric. For. Meterol. 1999, 97, 309–316. [Google Scholar] [CrossRef]
- Dingle, H. Animal migration: Is there a common migratory syndrome? J. Ornithol. 2006, 147, 212–220. [Google Scholar] [CrossRef]
- Byrne, D.N.; Houck, M.A. Morphometric identification of wing polymorphism in Bemisia tabaci (Homoptera: Aleyrodidae). Ann. Entomol. Soc. Am. 1990, 83, 487–493. [Google Scholar] [CrossRef]
- Johnson, C.G. Migration and Dispersal of Insects by Flight; Methuen: London, UK, 1969. [Google Scholar]
- Rankin, M.A.; Hampton, E.N.; Summy, K.R. Investigations of the oogenesis-flight syndrome in Anthonomus grandis (Coloptera: Curculionidae) using tethered flight tests. J. Insect Behav. 1994, 7, 795–810. [Google Scholar] [CrossRef]
- Dingle, H. Migration: The Biology of Life on the Move; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Desender, K. Flight muscle development and dispersal in the life cycle of carabid beetles: Patterns and processes. Bull. L’Institut R. Sci. Naturelles Belgique 2000, 70, 13–31. [Google Scholar]
- Lorenz, M.W. Oogenesis-flight syndrome in crickets: Age-dependent egg production, flight performance, and biochemical composition of the flight muscles in adult female Gryllus bimaculatus. J. Insect Physiol. 2007, 53, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Sappington, T.W.; Showers, W.B. Reproductive maturity, mating status, and long-duration flight behavior of Agrotis ipsilon (Lepidoptera: Noctuidae) and the conceptual misuse of the oogenesis flight syndrome by entomologists. Environ. Entomol. 1992, 21, 677–688. [Google Scholar]
- Stewart, S.D.; Gaylor, M.J. Effects of age, sex, and reproductive status on flight by the tarnished plant bug (Heteroptera: Miridae). Environ. Entomol. 1994, 23, 80–84. [Google Scholar] [CrossRef]
- Jiang, X.F.; Luo, L.Z.; Zhang, L.; Sappington, T.W.; Hu, Y. Regulation of migration in Mythimma separate (Walker) in China: A review integrating environmental, physiological, hormal, genetic, and molecular factors. Environ. Entomol. 2010, 40, 516–533. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, P.; Sappington, T.W.; Lu, W.; Luo, L.; Jiang, X. Accelerated and synchronized oviposition induced by flight of young females may intensify larval outbreaks of the rice leaf roller. PLoS ONE 2015, 10, e0121821. [Google Scholar] [CrossRef]
- Tigreros, N.; Davidowitz, G. Flight-fecundity tradeoffs in wing-monomorphic insects. Adv. Insect Physiol. 2019, 56, 1–41. [Google Scholar]
- Jervis, M.A.; Heimpel, G.E.; Ferns, P.N.; Harvey, J.A.; Kidd, N.A.C. Life-history strategies in parasitoid wasps: A comparative analysis of ‘ovigeny’. J. Anim. Ecol. 2001, 70, 442–458. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ferns, P.N. The timing of egg maturation in insects: Ovigeny index and initial egg load as measures of fitness and of resource allocation. Oikos 2004, 107, 449–461. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ellers, J.; Harvey, J.A. Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 2008, 53, 361–385. [Google Scholar] [CrossRef]
- Jervis, M.A.; Boggs, C.L.; Ferns, P.N. Egg maturation strategy and survival trade-offs in holometabolous insects: A comparative approach. Biol. J. Linn. Soc. 2007, 90, 293–302. [Google Scholar] [CrossRef]
- Stevens, V.M.; Trochet, A.; Blanchet, S.; Moulherat, S.; Clobert, J.; Baguette, M. Dispersal syndromes and the use of life-histories to predict dispersal. Evol. Appl. 2013, 6, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R.; Casas, J. Parasitoid and host nutritional physiology in behavioral ecology. In Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications; Wajnberg, E., Bernstein, C., van Alphen, J., Eds.; Blackwell and Oxford University Press: Malden, MA, USA; New York, NY, USA, 2008; pp. 113–128. [Google Scholar]
- Amat, I.; Besnard, S.; Foray, V.; Pelosse, P.; Bernstein, C.; Desouhant, E. Fuelling flight in a parasitic wasp: Which energetic substrate to use? Ecol. Entomol. 2012, 37, 480–489. [Google Scholar] [CrossRef]
- Heimpel, G.E. Linking parasitoid nectar feeding and dispersal in conservation biological control. Biol. Control 2019, 132, 36–41. [Google Scholar] [CrossRef]
- Visser, B.; Ellers, J. Lack of lipogenesis in parasitoids: A review of physiological mechanisms and evolutionary implications. J. Insect Physiol. 2008, 54, 1315–1322. [Google Scholar] [CrossRef]
- Visser, B.; Le Lann, C.; den Blanken, F.J.; Harvey, J.A.; van Alphen, J.J.M.; Ellers, J. Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proc. Natl. Acad. Sci. USA 2010, 107, 8677–8682. [Google Scholar] [CrossRef]
- Casas, J.; Pincebourde, S.; Mandon, N.; Vannier, F.; Poujol, R.; Giron, D. Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecology 2005, 86, 545–554. [Google Scholar] [CrossRef][Green Version]
- De Jong, G.; van Noordwijk, A.J. Acquisition and allocation of resources: Genetic (co) variances, selection, and life histories. Am. Nat. 1992, 139, 749–770. [Google Scholar] [CrossRef]
- Asplen, M.K.; Bruns, E.; David, A.S.; Denison, R.F.; Epstein, B.; Kaiser, M.C.; Kaser, J.M.; Lacroix, C.; Mohl, E.K.; Quiram, G.; et al. Do trade-offs have explanatory power for the evolution of organismal interactions? Evolution 2012, 66, 1297–1307. [Google Scholar] [CrossRef]
- Herre, E.A.; West, S.A. Conflict of interest in a mutualism: Documenting the elusive fig wasp/seed trade-off. Proc. R. Soc. Lond. B 1997, 264, 1501–1507. [Google Scholar] [CrossRef]
- Forbes, A.A.; Bagley, R.K.; Beer, M.A.; Hippee, A.C.; Widmayer, H.A. Quantifying the unquantifiable: Why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol. 2018, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.J.; Harvey, P.H. The natal and breeding dispersal of birds. Ann. Rev. Ecol. Syst. 1982, 13, 1–21. [Google Scholar] [CrossRef]
- Pusey, A.E. Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol. Evol. 1987, 2, 295–299. [Google Scholar] [CrossRef]
- Perrin, N.; Goudet, J. Inbreeding, kinship, and the evolution of natal dispersal. In Dispersal; Clobert, J., Danchin, E., Dhondt, A.A., Nichols, J.D., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 123–142. [Google Scholar]
- Matthysen, E. Multicausality of dispersal: A review. In Dispersal Ecology and Evolution; Clobert, J., Baguette, M., Benton, T.G., Bullock, J.M., Eds.; Oxford University Press: New York, NY, USA, 2012; pp. 3–12. [Google Scholar]
- Asplen, M.K.; Chacón, J.M.; Heimpel, G.E. Sex-specific dispersal by a parasitoid wasp in the field. Entomol. Exp. Appl. 2016, 159, 252–259. [Google Scholar] [CrossRef]
- Hamilton, W.D. Extraordinary sex ratios. Science 1967, 28, 477–488. [Google Scholar] [CrossRef]
- Henter, H.J. Inbreeding depression and haplodiploidy: Experimental measures in a parasitoid and comparisons across diploid and haplodiploid insect taxa. Evolution 2003, 57, 1793–1803. [Google Scholar] [CrossRef]
- Whiting, P.W. Multiple alleles in complementary sex determination of Habrobracon. Genetics 1943, 28, 365–382. [Google Scholar]
- Van Wilgenburg, E.; Driessen, G.; Beukeboom, L.W. Single locus complementary sex determination in Hymenoptera: An “unintelligent” design? Front. Zool. 2006, 3, e1. [Google Scholar] [CrossRef]
- Heimpel, G.E.; de Boer, J.G. Sex determination in the Hymenoptera. Annu. Rev. Entomol. 2008, 53, 209–230. [Google Scholar] [CrossRef]
- Ode, P.J.; Antolin, M.F.; Strand, M.R. Differential dispersal and female-biased sex allocation in a parasitic wasp. Ecol. Entomol. 1998, 23, 314–318. [Google Scholar] [CrossRef]
- Ruf, D.; Dorn, S.; Mazzi, D. Females leave home for sex: Natal dispersal in a parasitoid with complementary sex determination. Anim. Behav. 2011, 81, 1083–1089. [Google Scholar] [CrossRef]
- Asplen, M.K.; Whitfield, J.B.; de Boer, J.G.; Heimpel, G.E. Ancestral state reconstruction analysis of hymenopteran sex determination mechanisms. J. Evol. Biol. 2009, 22, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, E.C.; Beukeboom, L.W.; van de Zande, L. Maternal control of haplodiploid sex determination in the wasp Nasonia. Science 2010, 328, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.M.; Compton, S.G.; Herre, E.A.; West, S.A. Alternative mating tactics and extreme male dimorphism in fig wasps. Proc. R. Soc. Lond. B 1997, 264, 747–754. [Google Scholar] [CrossRef]
- Loehlin, D.W.; Enders, L.S.; Werren, J.H. Evolution of sex-specific wing shape at the widerwing locus in four species of Nasonia. Heredity 2010, 104, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, X.-Y.; Yang, Z.-Q.; Duan, J.J. Effects of photoperiod and light intensity on wing dimorphism and development in the parasitoid Sclerodermus pupariae (Hymenoptera: Bethylidae). Biol. Control 2019, 133, 117–122. [Google Scholar] [CrossRef]
- Schrempf, A.; Aron, S.; Heinze, J. Sex determination and inbreeding in an ant with regular sib-mating. Heredity 2006, 97, 75–80. [Google Scholar] [CrossRef]
- Schrempf, A.; Reber, C.; Tinaut, A.; Heinze, J. Inbreeding and local mate competition in the ant Cardiocondyla batesii. Behav. Ecol. Sociobiol. 2005, 57, 502–510. [Google Scholar] [CrossRef]
- Cremer, S.; Heinze, J. Stress grows wings: Environmental induction of winged dispersal males in Cardiocondyla ants. Curr. Biol. 2003, 13, 219–223. [Google Scholar] [CrossRef]
- Yamauchi, K.; Asano, Y.; Lautenschläger, B.; Trindl, A.; Heinze, J. A new type of male dimorphism with ergatoid and winged males in Cardiocondyla cf. kagutsuchi. Insectes Soc. 2005, 52, 274–281. [Google Scholar] [CrossRef]
- Niyibigira, E.I.; Overholt, W.A.; Stouthamer, R. Cotesia flavipes (Hymenoptera: Braconidae) does not exhibit complementary sex determination. (ii) Evidence from laboratory experiments. Appl. Entomol. Zool. 2004, 39, 717–725. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, H.; Dorn, S. Single-locus sex determination in the parasitoid wasp Cotesia glomerata (Hymenoptera: Braconidae). Heredity 2006, 96, 487–492. [Google Scholar] [CrossRef] [PubMed]
- De Boer, J.G.; Ode, P.J.; Rendahl, A.K.; Vet, L.E.M.; Whitfield, J.B.; Heimpel, G.E. Experimental support for multiple-locus complementary sex determination in the parasitoid Cotesia vestalis. Genetics 2008, 180, 1525–1535. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Hypothesis | Focal Taxa | Test |
|---|---|---|
| ETM 1 explains whitefly migration, with leaf moisture content as driving factor | Bemisia tabaci | Vertical flight chamber studies on individuals faced with different levels of plant water stress, followed by disruptive selection |
| Positive relationship between OI 2 and flight propensity, duration and distance | Parastioids of whiteflies (Amitus spp.; Encarsia spp.) and aphids (aphidiine braconids; Aphelinus spp.) | Vertical flight chamber studies on individuals from representative species of each parasitoid guild with high and low OIs |
| Hymenopterans with sl-CSD 3 will exhibit higher levels of natal dispersal than those with other sex determination mechanisms | Cotesia spp. | Vertical flight chamber and/or tethered flight mill studies of unmated individuals from species with sl-CSD, ml-CSD 4 or no CSD |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asplen, M.K. Proximate Drivers of Migration and Dispersal in Wing-Monomorphic Insects. Insects 2020, 11, 61. https://doi.org/10.3390/insects11010061
Asplen MK. Proximate Drivers of Migration and Dispersal in Wing-Monomorphic Insects. Insects. 2020; 11(1):61. https://doi.org/10.3390/insects11010061
Chicago/Turabian StyleAsplen, Mark K. 2020. "Proximate Drivers of Migration and Dispersal in Wing-Monomorphic Insects" Insects 11, no. 1: 61. https://doi.org/10.3390/insects11010061
APA StyleAsplen, M. K. (2020). Proximate Drivers of Migration and Dispersal in Wing-Monomorphic Insects. Insects, 11(1), 61. https://doi.org/10.3390/insects11010061




