Genetic Diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Colonizing Sweet Potato and Cassava in South Sudan
Abstract
1. Introduction
2. Materials and Methods
2.1. Whitefly Sampling
2.2. DNA Extraction
2.3. Mitochondrial COI (MtCOI) PCR Amplification and Sequencing
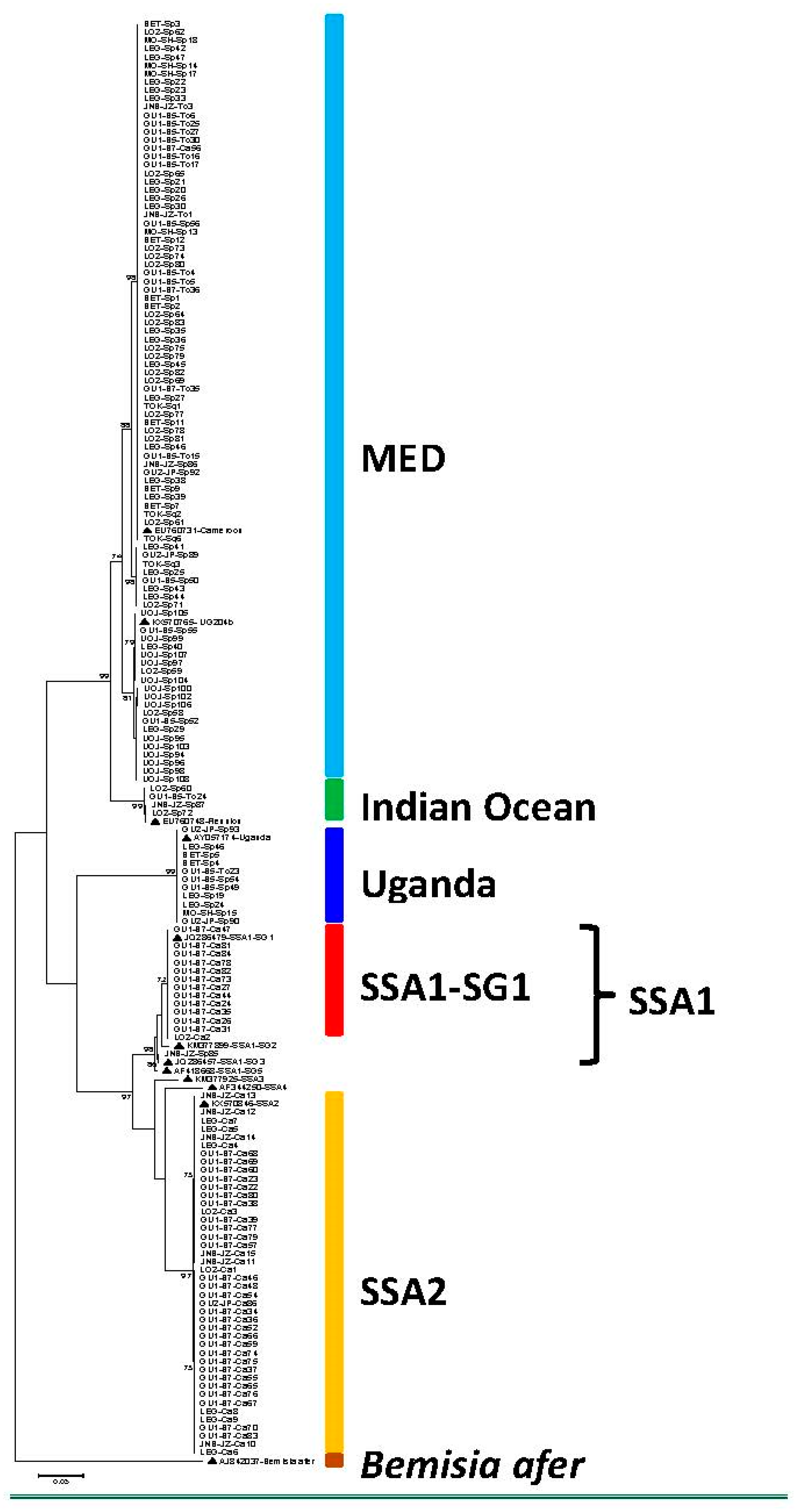
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Claessens, L.; Stoorvogel, J.J.; Antle, J.M. Exante assessment of dual-purpose sweet potato in the crop–livestock system of western Kenya: A minimum-data approach. Agric. Syst. 2009, 99, 13–22. [Google Scholar] [CrossRef]
- Hillocks, R.J.; Thresh, J.; Bellotti, A.E. (Eds.) Cassava: Biology, Production and Utilization; CABI: Wallingford, UK, 2002. [Google Scholar]
- Jarvis, A.; Ramirez-Villegas, J.; Campo, B.V.H.; Navarro-Racines, C. Is cassava the answer to African climate change adaptation? Trop. Plant Biol. 2012, 5, 9–29. [Google Scholar] [CrossRef]
- Low, J.; Lynam, J.; Lemaga, B.; Crissman, C.; Barker, I.; Thiele, G.; Namanda, S.; Wheatley, C.; Andrade, M. Sweetpotato in Sub-Saharan Africa. In The Sweetpotato; Loebenstein, G., Thottappilly, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 359–390. [Google Scholar]
- FAOSTAT. Food and Agricultural Organization of the United Nations. 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 25 April 2019).
- FAO/WFP. FAO/WFP Crop and Food Security Mission to South Sudan. 2016. Available online: http://www.fao.org/emergencies/resources/documents/resources-detail/en/c/409602/ (accessed on 26 March 2019).
- Ntawuruhunga, P.; Legg, J.; Okidi, J.; Okao-Okuja, G.; Tadu, G.; Remington, T. Southern Sudan, Equatoria Region, Cassava Baseline Survey Technical Report; IITA: Ibadan, Nigeria, 2007; 65p, Available online: https://www.researchgate.net/profile/James_Legg/publication/242233867_Southern_Sudan_Equatoria_Region_Cassava_Baseline_Survey_Technical_Report/links/00b7d52d3fb5ed3386000000.pdf (accessed on 26 March 2019).
- Alabi, O.J.; Ogbe, F.O.; Bandyopadhyay, R.; Kumar, P.L.; Dixon, A.G.; Hughes, J.d.A.; Naidu, R.A. Alternate hosts of African cassava mosaic virus and East African cassava mosaic Cameroon virus in Nigeria. Arch. Virol. 2008, 153, 1743–1747. [Google Scholar] [CrossRef]
- Legg, J.; Owor, B.; Sseruwagi, P.; Ndunguru, J. Cassava mosaic virus disease in East and Central Africa: Epidemiology and management of a regional pandemic. Adv. Virus Res. 2006, 67, 355–418. [Google Scholar] [PubMed]
- Legg, J.P.; Sseruwagi, P.; Boniface, S.; Okao-Okuja, G.; Shirima, R.; Bigirimana, S.; Gashaka, G.; Herrmann, H.-W.; Jeremiah, S.; Obiero, H.; et al. Spatio-temporal patterns of genetic change amongst populations of cassava Bemisia tabaci whiteflies driving virus pandemics in East and Central Africa. Virus Res. 2014, 186, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Mukasa, S.; Rubaihayo, P.; Valkonen, J. Sequence variability within the 3′-proximal part of the Sweet potato mild mottle virus genome. Arch. Virol. 2003, 148, 487–496. [Google Scholar] [CrossRef]
- Mukasa, S.B.; Rubaihayo, P.R.; Valkonen, J.P.T. Interactions between a crinivirus, an ipomovirus and a potyvirus in coinfected sweetpotato plants. Plant Pathol. 2006, 55, 458–467. [Google Scholar] [CrossRef]
- Ngailo, S.; Shimelis, H.A.; Sibiya, J.; Mtunda, K. Assessment of sweetpotato farming systems, production constraints and breeding priorities in eastern Tanzania. S. Afr. J. Plant Soil 2016, 33, 105–112. [Google Scholar] [CrossRef]
- Hong, Y.; Robinson, D.; Harrison, B. Nucleotide sequence evidence for the occurrence of three distinct whitefly-transmitted geminiviruses in cassava. J. Gen. Virol. 1993, 74, 2437–2443. [Google Scholar] [CrossRef]
- Legg, J.P.; Fauquet, C.M. Cassava mosaic geminiviruses in Africa. Plant Mol. Biol. 2004, 56, 585–599. [Google Scholar] [CrossRef]
- Winter, S.; Koerbler, M.; Stein, B.; Pietruszka, A.; Paape, M.; Butgereitt, A. Analysis of cassava brown streak viruses reveals the presence of distinct virus species causing cassava brown streak disease in East Africa. J. Gen. Virol. 2010, 91, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Owor, B.; Legg, J.P.; Okao-Okuja, G.; Obonyo, R.; Ogenga-Latigo, M.W. The effect of cassava mosaic geminiviruses on symptom severity, growth and root yield of a cassava mosaic virus disease-susceptible cultivar in Uganda. Ann. Appl. Biol. 2004, 145, 331–337. [Google Scholar] [CrossRef]
- Dafalla, G.; Ahmed, M. Whiteflies as pests and vectors of viruses in vegetable and legume mixed cropping systems in Eastern and Southern Africa. In Whitefly and Whitefly-Borne Viruses in the Tropics: Building a Knowledge Base for Global Action; Publication No. 341; Anderson, P.K., Morales, F.J., Eds.; Centro Internacional de Agricultura Tropical (CIAT): Cali, Colombia, 2005; pp. 118–128. [Google Scholar]
- Tadu, G.; Winter, S.; Gadelseed, A.; Dafalla, G. Association of East African cassava mosaic virus-Uganda (EACMV-UG) with cassava mosaic disease in Sudan. Plant Pathol. 2006, 55, 287. [Google Scholar] [CrossRef]
- Cuellar, W.J.; Galvez, M.; Fuentes, S.; Tugume, J.; Kreuze, J. Synergistic interactions of begomoviruses with Sweet potato chlorotic stunt virus (genus Crinivirus) in sweet potato (Ipomoea batatas L.). Mol. Plant Pathol. 2015, 16, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Clark, C. Interactions among Sweet potato chlorotic stunt virus and different potyviruses and potyvirus strains infecting sweetpotato in the United States. Plant Dis. 2006, 90, 1347–1352. [Google Scholar] [CrossRef]
- Untiveros, M.; Fuentes, S.; Salazar, L.F. Synergistic interaction of Sweet potato chlorotic stunt virus (Crinivirus) with Carla-, Cucumo-, Ipomo-, and Potyviruses infecting sweet potato. Plant Dis. 2007, 91, 669–676. [Google Scholar] [CrossRef]
- Aritua, V.; Adipala, E. Characteristics and diversity in sweetpotato-infecting viruses in Africa. Acta Hortic. 2006, 703, 175–182. [Google Scholar] [CrossRef]
- Gutierrez, D.; Fuentes, S.; Salazar, L. Sweet potato virus disease (SPVD): Distribution, incidence, and effect on sweetpotato yield in Peru. Plant Dis. 2003, 87, 297–302. [Google Scholar] [CrossRef]
- Karyeija, R.; Kreuze, J.; Gibson, R.; Valkonen, J. Synergistic interactions of a potyvirus and a phloem-limited crinivirus in sweet potato plants. Virology 2000, 269, 26–36. [Google Scholar] [CrossRef]
- Ndunguru, J.; Kapinga, R.; Sseruwagi, P.; Sayi, B.; Mwanga, R.; Tumwegamire, S.; Rugutu, C. Assessing the sweetpotato virus disease and its associated vectors in northwestern Tanzania and central Uganda. Afr. J. Agric. Res. 2009, 4, 334–343. [Google Scholar]
- Njeru, R.; Mburu, M.; Cheramgoi, E.; Gibson, R.; Kiburi, Z.; Obudho, E.; Yobera, D. Studies on the physiological effects of viruses on sweet potato yield in Kenya. Ann. Appl. Biol. 2004, 145, 71–76. [Google Scholar] [CrossRef]
- Misaka, B.C.; Legg, J.P. Survey and detection of viruses infecting sweet potato (Ipomoea batatas (L.) Lam) in South Sudan. Viruses. Manuscript in preparation.
- Byrne, D.N.; Bellows, J.T.S. Whitefly Biology. Annu. Rev. Entomol. 1991, 36, 431–458. [Google Scholar] [CrossRef]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef]
- Butler, G.D.; Rimon, D., Jr.; Henneberry, T.J. Bemisia tabaci (Homoptera: Aleyrodidae): Populations on different cotton varieties and cotton stickiness in Israel. Crop Prot. 1988, 7, 43–47. [Google Scholar] [CrossRef]
- Liburd, O.; Nyoike, T.; Razze, J. Biology and Management of Whiteflies in Sustainable Field Production of Cucurbits; ENY-848/IN762, IFAS Extension; University of Florida: Gainesville, FL, USA, 2015; Available online: https://www.researchgate.net/profile/Janine_Spies/publication/301867528_Biology_and_Management_of_Whiteflies_in_Sustainable_Field_Production_of_Cucurbits/links/572a5b5d08ae057b0a079069/Biology-and-Management-of-Whiteflies-in-Sustainable-Field-Production-of-Cucurbits.pdf (accessed on 13 March 2019).
- Miyazaki, J.; Stiller, W.N.; Wilson, L.J. Identification of host plant resistance to silverleaf whitefly in cotton: Implications for breeding. Field Crops Res. 2013, 154, 145–152. [Google Scholar] [CrossRef]
- Polston, J.E.; De Barro, P.; Boykin, L.M. Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Manag. Sci. 2014, 70, 1547–1552. [Google Scholar] [CrossRef]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging virus diseases transmitted by whiteflies. Annu. Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Verbeek, M.; van Bekkum, P.J.; Dullemans, A.M.; van der Vlugt, R.A.A. Torradoviruses are transmitted in a semi-persistent and stylet-borne manner by three whitefly vectors. Virus Res. 2014, 186, 55–60. [Google Scholar] [CrossRef]
- Legg, J.P. Epidemiology of a whitefly-transmitted cassava mosaic geminivirus pandemic in Africa. In Bemisia: Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 233–257. [Google Scholar]
- Legg, J.P.; Kumar, P.L.; Makeshkumar, T.; Tripathi, L.; Ferguson, M.; Kanju, E.; Ntawuruhunga, P.; Cuellar, W. Cassava virus diseases: Biology, epidemiology, and management. Adv. Virus Res. 2015, 91, 85–142. [Google Scholar] [PubMed]
- Maruthi, M.N.; Jeremiah, S.C.; Mohammed, I.U.; Legg, J.P. The role of the whitefly, Bemisia tabaci (Gennadius), and farmer practices in the spread of cassava brown streak ipomoviruses. J. Phytopathol. 2017, 165, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, H.A.; Fuentes, S.; Morales, F.J.; Glover, R.; Malumphy, C.; Barker, I. Bemisia afer sensu lato, a vector of Sweet potato chlorotic stunt virus. Plant Dis. 2010, 94, 510–514. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sim, J.; Valverde, R.; Clark, C. Whitefly transmission of Sweetpotato chlorotic stunt virus. Plant Dis. 2000, 84, 1250. [Google Scholar] [CrossRef]
- Valverde, R.A.; Sim, J.; Lotrakul, P. Whitefly transmission of sweet potato viruses. Virus Res. 2004, 100, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Reingold, V.; Antignus, Y. Ipomovirus—An atypical genus in the family Potyviridae transmitted by whiteflies. Pest Manag. Sci. 2014, 70, 1553–1567. [Google Scholar] [CrossRef]
- Hassan, I.; Orilio, A.F.; Fiallo-Olive, E.; Briddon, R.W.; Navas-Castillo, J. Infectivity, effects on helper viruses and whitefly transmission of the deltasatellites associated with sweepoviruses (genus Begomovirus, family Geminiviridae). Sci. Rep. 2016, 6, 30204. [Google Scholar] [CrossRef]
- Ling, K.-S.; Harrison, H.F.; Simmons, A.M.; Zhang, S.C.; Jackson, D.M. Experimental host range and natural reservoir of Sweet potato leaf curl virus in the United States. Crop Prot. 2011, 30, 1055–1062. [Google Scholar] [CrossRef]
- Simmons, A.M.; Ling, K.-S.; Harrison, H.F.; Jackson, D.M. Sweet potato leaf curl virus: Efficiency of acquisition, retention and transmission by Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Prot. 2009, 28, 1007–1011. [Google Scholar] [CrossRef]
- Maruthi, M.; Hillocks, R.; Mtunda, K.; Raya, M.; Muhanna, M.; Kiozia, H.; Rekha, A.; Colvin, J.; Thresh, J. Transmission of Cassava brown streak virus by Bemisia tabaci (Gennadius). J. Phytopathol. 2005, 153, 307–312. [Google Scholar] [CrossRef]
- Aritua, V.; Adipala, E.; Carey, E.; Gibson, R. The incidence of sweet potato virus disease and virus resistance of sweet potato grown in Uganda. Ann. Appl. Biol. 1998, 132, 399–411. [Google Scholar] [CrossRef]
- Byamukama, E.; Gibson, R.; Aritua, V.; Adipala, E. Within-crop spread of sweet potato virus disease and the population dynamics of its whitefly and aphid vectors. Crop Prot. 2004, 23, 109–116. [Google Scholar] [CrossRef]
- Cohen, J.; Franck, A.; Vetten, H.; Lesemann, D.; Loebenstein, G. Purification and properties of closterovirus-like particles associated with a whitefly-transmitted disease of sweet potato. Ann. Appl. Biol. 1992, 121, 257–268. [Google Scholar] [CrossRef]
- Boykin, L.M.; Armstrong, K.F.; Kubatko, L.; De Barro, P. Species delimitation and global biosecurity. Evol. Bioinform. 2012, 8, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K. Molecular markers for the identification and global tracking of whitefly vector–Begomovirus complexes. Virus Res. 2000, 71, 233–260. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.-S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- De Barro, P.J. The Bemisia tabaci Species Complex: Questions to Guide Future Research. J. Integr. Agric. 2012, 11, 187–196. [Google Scholar] [CrossRef]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.; De Barro, P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- Tay, W.T.; Evans, G.A.; Boykin, L.M.; De Barro, P.J. Will the real Bemisia tabaci please stand up? PLoS ONE 2012, 7, e50550. [Google Scholar] [CrossRef] [PubMed]
- Legg, J.; French, R.; Rogan, D.; Okao-Okuja, G.; Brown, J. A distinct Bemisia tabaci (Gennadius) (Hemiptera: Sternorrhyncha: Aleyrodidae) genotype cluster is associated with the epidemic of severe cassava mosaic virus disease in Uganda. Mol. Ecol. 2002, 11, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Romba, R.; Gnankine, O.; Drabo, S.F.; Tiendrebeogo, F.; Henri, H.; Mouton, L.; Vavre, F. Abundance of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) and its parasitoids on vegetables and cassava plants in Burkina Faso (West Africa). Ecol. Evol. 2018, 8, 6091–6103. [Google Scholar] [CrossRef] [PubMed]
- Sseruwagi, P.; Legg, J.; Maruthi, M.; Colvin, J.; Rey, M.; Brown, J.K. Genetic diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) populations and presence of the B biotype and a non-B biotype that can induce silverleaf symptoms in squash, in Uganda. Ann. Appl. Biol. 2005, 147, 253–265. [Google Scholar] [CrossRef]
- Tocko-Marabena, B.K.; Silla, S.; Simiand, C.; Zinga, I.; Legg, J.; Reynaud, B.; Delatte, H. Genetic diversity of Bemisia tabaci species colonizing cassava in Central African Republic characterized by analysis of cytochrome c oxidase subunit I. PLoS ONE 2017, 12, e0182749. [Google Scholar] [CrossRef] [PubMed]
- Esterhuizen, L.L.; Mabasa, K.G.; Van Heerden, S.W.; Czosnek, H.; Brown, J.K.; Van Heerden, H.; Rey, M.E. Genetic identification of members of the Bemisia tabaci cryptic species complex from South Africa reveals native and introduced haplotypes. J. Appl. Entomol. 2013, 137, 122–135. [Google Scholar] [CrossRef]
- Ghosh, S.; Bouvaine, S.; Maruthi, M. Prevalence and genetic diversity of endosymbiotic bacteria infecting cassava whiteflies in Africa. BMC Microbiol. 2015, 15, 93. [Google Scholar] [CrossRef]
- Gnankine, O.; Mouton, L.; Henri, H.; Terraz, G.; Houndeté, T.; Martin, T.; Vavre, F.; Fleury, F. Distribution of Bemisia tabaci (Homoptera: Aleyrodidae) biotypes and their associated symbiotic bacteria on host plants in West Africa. Insect Conserv. Divers. 2013, 6, 411–421. [Google Scholar] [CrossRef]
- Mugerwa, H.; Rey, M.E.; Alicai, T.; Ateka, E.; Atuncha, H.; Ndunguru, J.; Sseruwagi, P. Genetic diversity and geographic distribution of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) genotypes associated with cassava in East Africa. Ecol. Evol. 2012, 2, 2749–2762. [Google Scholar] [CrossRef]
- Brown, J.K.; Coats, S.A.; Bedford, I.D.; Markham, P.G.; Bird, J.; Frohlich, D.R. Characterization and distribution of esterase electromorphs in the whitefly, Bemisia tabaci (Genn.) (Homoptera: Aleyrodidae). Biochem. Genet. 1995, 33, 205–214. [Google Scholar]
- Hadjistylli, M.; Brown, J.K.; Roderick, G.K. Tools and recent progress in studying gene flow and population genetics of the Bemisia tabaci sibling species group. In Bemisia: Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 69–103. [Google Scholar]
- Brown, J.K. Phylogenetic biology of the Bemisia tabaci sibling species group. In Bemisia: Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 31–67. [Google Scholar]
- Ally, H.M.; El Hamss, H.; Simiand, C.; Maruthi, M.N.; Colvin, J.; Omongo, C.A.; Delatte, H. What has changed in the outbreaking populations of the severe crop pest whitefly species in cassava in two decades? Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Hadjistylli, M.; Roderick, G.K.; Brown, J.K. Global Population Structure of a Worldwide Pest and Virus Vector: Genetic Diversity and Population History of the Bemisia tabaci Sibling Species Group. PLoS ONE 2016, 11, e0165105. [Google Scholar] [CrossRef]
- Wosula, E.N.; Chen, W.; Fei, Z.; Legg, J.P. Unravelling the Genetic Diversity among Cassava Bemisia tabaci Whiteflies Using NextRAD Sequencing. Genome Biol. Evol. 2017, 9, 2958–2973. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wosula, E.N.; Hasegawa, D.K.; Casinga, C.; Shirima, R.R.; Fiaboe, K.K.M.; Hanna, R.; Fosto, A.; Goergen, G.; Tamò, M.; et al. Genome of the African cassava whitefly Bemisia tabaci and distribution and genetic diversity of cassava-colonizing whiteflies in Africa. Insect Biochem. Mol. Biol. 2019, 110, 112–120. [Google Scholar] [CrossRef] [PubMed]
- de Moya, R.S.; Brown, J.K.; Sweet, A.D.; Walden, K.K.; Paredes-Montero, J.R.; Waterhouse, R.M.; Johnson, K.P. Nuclear orthologs Derived from whole genome sequencing indicate cryptic diversity in the Bemisia tabaci (Insecta: Aleyrodidae) complex of whiteflies. Diversity 2019, 11, 151. [Google Scholar] [CrossRef]
- Boykin, L.M.; Shatters, R.G.; Rosell, R.C.; McKenzie, C.L.; Bagnall, R.A.; De Barro, P.; Frohlich, D.R. Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol. Phylogenet. Evol. 2007, 44, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.D.; Fondong, V.N.; Rey, C.; Rogan, D.; Fauquet, C.M.; Brown, J.K. Molecular evidence for five distinct Bemisia tabaci (Homoptera: Aleyrodidae) geographic haplotypes associated with cassava plants in sub-Saharan Africa. Ann. Entomol. Soc. Am. 2004, 97, 852–859. [Google Scholar] [CrossRef]
- Hu, J.; De Barro, P.; Zhao, H.; Wang, J.; Nardi, F.; Liu, S.-S. An extensive field survey combined with a phylogenetic analysis reveals rapid and widespread invasion of two alien whiteflies in China. PLoS ONE 2011, 6, e16061. [Google Scholar] [CrossRef]
- Islam, W.; Lin, W.; Qasim, M.; Islam, S.U.; Ali, H.; Adnan, M.; Arif, M.; Du, Z.; Wu, Z. A nation-wide genetic survey revealed a complex population structure of Bemisia tabaci in Pakistan. Acta Trop. 2018, 183, 119–125. [Google Scholar]
- Khatun, M.; Jahan, S.; Lee, S.; Lee, K.-Y. Genetic diversity and geographic distribution of the Bemisia tabaci species complex in Bangladesh. Acta Trop. 2018, 187, 28–36. [Google Scholar]
- Mugerwa, H.; Seal, S.; Wang, H.-L.; Patel, M.V.; Kabaalu, R.; Omongo, C.A.; Alicai, T.; Tairo, F.; Ndunguru, J.; Sseruwagi, P.; et al. African ancestry of New World, Bemisia tabaci-whitefly species. Sci. Rep. 2018, 8, 2734. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Horowitz, A.; Denholm, I.; Gorman, K.; Cenis, J.; Kontsedalov, S.; Ishaaya, I. Biotype Q of Bemisia tabaci identified in Israel. Phytoparasitica 2003, 31, 94–98. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ishaaya, I. Dynamics of biotypes B and Q of the whitefly Bemisia tabaci and its impact on insecticide resistance. Pest Manag. Sci. 2014, 70, 1568–1572. [Google Scholar] [CrossRef]
- Roditakis, E.; Grispou, M.; Morou, E.; Kristoffersen, J.B.; Roditakis, N.; Nauen, R.; Vontas, J.; Tsagkarakou, A. Current status of insecticide resistance in Q biotype Bemisia tabaci populations from Crete. Pest Manag. Sci. 2009, 65, 313–322. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, H.; Yang, Y.; Wu, Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci from China. Pest Manag. Sci. 2010, 66, 1360–1366. [Google Scholar] [CrossRef]
- Zhang, D.X.; Hewitt, G.M. Nuclear integrations: Challenges for mitochondrial DNA markers. Trends Ecol. Evol. 1996, 11, 247–251. [Google Scholar] [CrossRef]
- Tay, W.T.; Elfekih, S.; Court, L.N.; Gordon, K.H.; Delatte, H.; De Barro, P.J. The trouble with MEAM2: Implications of pseudogenes on species delimitation in the globally invasive Bemisia tabaci (Hemiptera: Aleyrodidae) cryptic species complex. Genome Biol. Evol. 2017, 9, 2732–2738. [Google Scholar] [CrossRef][Green Version]
- Gueguen, G.; Vavre, F.; Gnankine, O.; Peterschmitt, M.; Charif, D.; Chiel, E.; Gottlieb, Y.; Ghanim, M.; Zchori-Fein, E.; Fleury, F. Endosymbiont metacommunities, mtDNA diversity and the evolution of the Bemisia tabaci (Hemiptera: Aleyrodidae) species complex. Mol. Ecol. 2010, 19, 4365–4376. [Google Scholar] [CrossRef]
- Delatte, H.; Holota, H.; Warren, B.H.; Becker, N.; Thierry, M.; Reynaud, B. Genetic diversity, geographical range and origin of Bemisia tabaci (Hemiptera: Aleyrodidae) Indian Ocean Ms. Bull. Entomol. Res. 2011, 101, 487–497. [Google Scholar] [CrossRef]
- Wainana, J.M. Phylogenomics of Viruses and the Whiteflies: Bemisia tabaci Gennadius) (Hemiptera: Aleyrodidae) and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) Found within Heterogeneous Agro-Ecosystems of the Western Highlands of Kenya. Ph.D. Thesis, University of Western Australia, Perth, Australia, 2019. [Google Scholar]
- Sseruwagi, P.; Maruthi, M.; Colvin, J.; Rey, M.; Brown, J.K.; Legg, J. Colonization of non-cassava plant species by cassava whiteflies (Bemisia tabaci) in Uganda. Entomol. Exp. Appl. 2006, 119, 145–153. [Google Scholar] [CrossRef]
- Manani, D.; Ateka, E.; Nyanjom, S.; Boykin, L. Phylogenetic relationships among whiteflies in the Bemisia tabaci (Gennadius) species complex from major cassava growing areas in Kenya. Insects 2017, 8, 25. [Google Scholar] [CrossRef]
- Legg, J.P. Host-associated strains within Ugandan populations of the whitefly Bemisia tabaci (Genn.), (Hom., Aleyrodidae). J. Appl. Entomol. 1996, 120, 523–527. [Google Scholar] [CrossRef]
- Milenovic, M.; Wosula, E.N.; Rapisarda, C.; Legg, J.P. Impact of host plant species and whitefly species on feeding behavior of Bemisia tabaci. Front. Plant Sci. 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.S.; Kumar, L.; Shabani, F.; Picanço, M.C. Mapping global risk levels of Bemisia tabaci in areas of suitability for open field tomato cultivation under current and future climates. PLoS ONE 2018, 13, e0198925. [Google Scholar] [CrossRef]
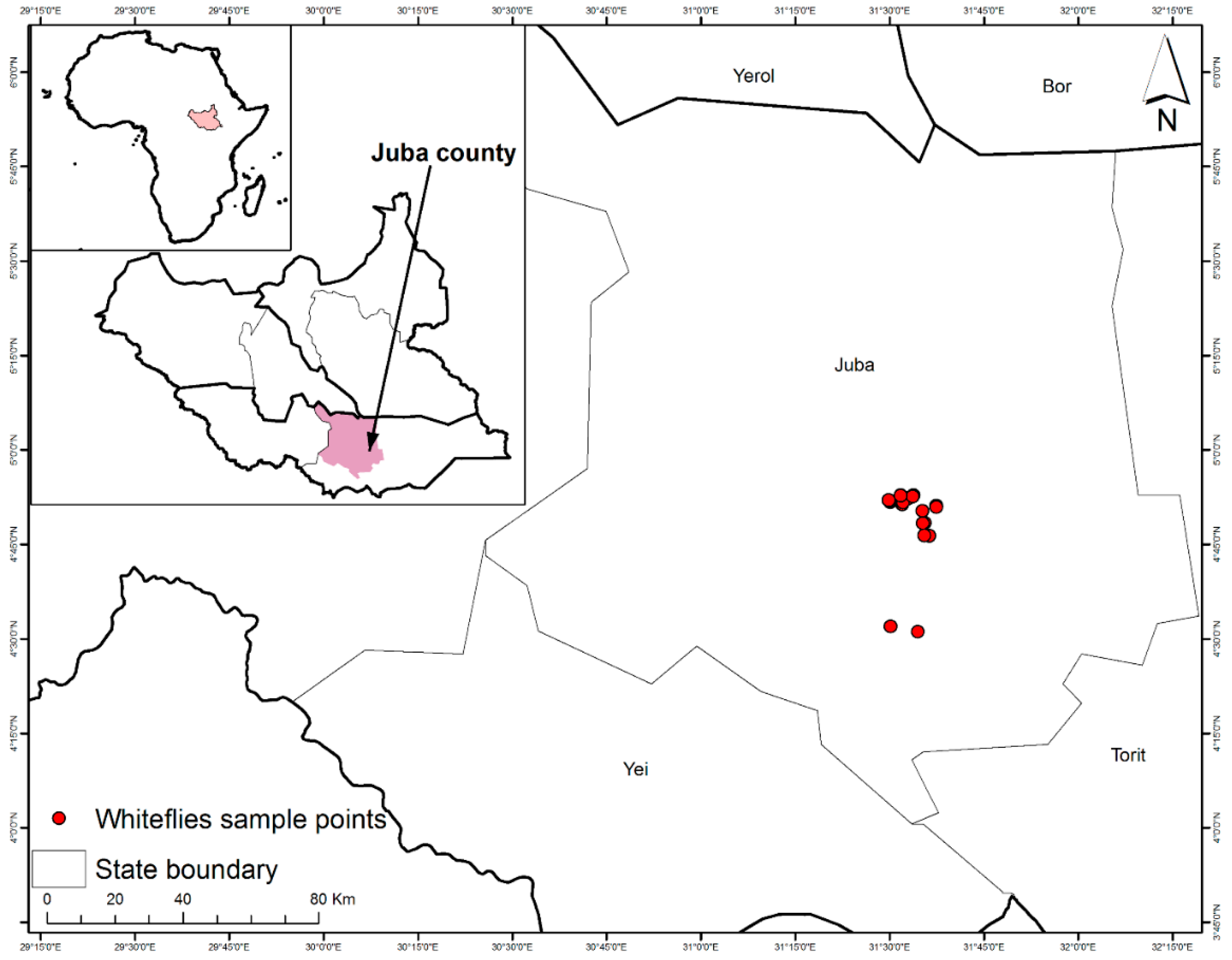
| Host Plant | Area/Payam | Sampling Site | Latitude (°N) | Longitude (°E) | Elevation (masl) | Date |
|---|---|---|---|---|---|---|
| Cassava | Rajaf West | Lologo 2 | 04°48.456′ | 031°35.463′ | 468 | 31.07.2018 |
| Northern Bari | Lemon Gaba | 04°52.051′ | 031°29.873′ | 487 | 03.08.2018 | |
| Kondokoro | Juba Na Bari-Jezira | 04°51.167′ | 031°37.430′ | 457 | 04.08.2018 | |
| Munuki | Gudele 1 Block 7 | 04°52.383′ | 031°33.025′ | 478 | 04.08.2018 | |
| Northern Bari | Gudele 2 Jopa | 04°52.852′ | 031°31.742′ | 472 | 04.08.2018 | |
| Tomato | Kondokoro | Juba Na Bari-Jezira | 04°50.945′ | 031°37.392′ | 460 | 04.08.2018 |
| Munuki | Gudele 1 Block 5 | 04°52.849′ | 031°33.770′ | 466 | 27.07.2018 | |
| Munuki | Gudele 1 Block 7 | 04°52.383′ | 031°33.025′ | 478 | 04.08.2018 | |
| Squash | Rajaf East | Tokiman | 04°46.396′ | 031°36.334′ | 460 | 04.08.2018 |
| Sweet potato | Rajaf West | Beitery | 04°51.323′ | 031°32.009′ | 508 | 19.07.2018 |
| Munuki | Mouna-Suk Hajer | 04°31.130′ | 031°34.483′ | 490 | 24.07.2018 | |
| Northern Bari | Lemon Gaba | 04°51.688′ | 031°30.104′ | 506 | 25.07.0218 | |
| Northern Bari | Lemon Gaba | 04°31.965′ | 031°30.140′ | 490 | 27.07.2018 | |
| Munuki | Gudele 1 Block 5 | 04°52.849′ | 031°33.770′ | 466 | 27.07.2018 | |
| Northern Bari | Lemon Gaba | 04°51.850′ | 031°30.083′ | 498 | 28.07.2018 | |
| Rajaf West | Beitery | 04°51.669′ | 031°32.117′ | 488 | 28.07.2018 | |
| Rajaf West | Lologo 2 | 04°48.443′ | 031°35.564′ | 473 | 30.08.3018 | |
| Rajaf West | Lologo 2 | 04°46.447′ | 031°35.496′ | 476 | 31.07.2018 | |
| Rajaf West | Lologo 2 | 04°48.344′ | 031°35.302′ | 474 | 01.08.2018 | |
| Munuki | Gudele 1 Block 5 | 04°52.662′ | 031°33.690′ | 469 | 02.08.2018 | |
| Northern Bari | Lemon Gaba | 04°52.051′ | 031°29.873′ | 487 | 03.08.2018 | |
| Kondokoro | Juba Na Bari-Jezira | 04°50.945′ | 031°37.392′ | 460 | 04.08.2018 | |
| Northern Bari | Gudele 2 Jopa | 04°52.766′ | 031°31.757′ | 470 | 04.08.2018 | |
| Juba | University of Juba (Green house) | 04°50.327′ | 031°35.225′ | 494 | 10.08.2017 |
| Location | Host Plant | ||||
|---|---|---|---|---|---|
| Sweet Potato (Sp) | Cassava (Ca) | Tomato (To) | Squash (Sq) | Total | |
| Tokiman (TOK) | - | - | - | 4 | 4 |
| Lologo 2 (LO2) | 20 | 3 | - | - | 23 |
| Beitery (BET) | 9 | - | - | - | 9 |
| Juba Na Bari-Jezira (JNB-JZ) | 3 | 6 | 2 | - | 11 |
| Mouna-Suk Hager (MO-SH) | 5 | - | - | - | 5 |
| Gudele 1 Block 5 (GU1-B5) | 6 | - | 11 | - | 17 |
| Gudele 1 Block 7 (GU1-B7) | - | 41 | 2 | - | 43 |
| Gudele 2 Jopa (GU2-JP) | 4 | 1 | - | - | 5 |
| Lemon Gaba (LEG) | 25 | 6 | - | - | 31 |
| Univeristy of Juba (UOJ) | 14 | - | - | - | 14 |
| Total | 86 | 57 | 15 | 4 | 162 |
| B. tabaci Haplotypes | Host Plants | ||||
|---|---|---|---|---|---|
| Sweet Potato | Cassava | Tomato | Squash | Total | |
| MED | 72 (44.4%) | 1 (0.6%) | 13 (8.0%) | 4 (2.5%) | 90 (55.5%) |
| Indian Ocean | 3 (1.9%) | - | 1 (0.6%) | - | 4 (2.5%) |
| Uganda | 10 (6.2%) | - | 1 (0.6%) | - | 11 (6.8%) |
| SSA1-SG1 | - | 13 (8.0%) | - | - | 13 (8.0%) |
| SSA1-SG3 | 1(0.6%) | 1(0.6%) | |||
| SSA2 | - | 43 (26.5%) | - | - | 43 (26.5%) |
| Total | 86 | 57 | 15 | 4 | 162 |
| Parameter | All | MED | SSA2 | SSA1-SG1 | Uganda | Indian Ocean | SSA1-SG3 |
|---|---|---|---|---|---|---|---|
| Sample size | 162 | 90 | 43 | 13 | 11 | 4 | 1 |
| Number of haplotypes | 13 | 6 | 2 | 1 | 1 | 2 | 1 |
| Polymorphic sites (S) | 212 | 28 | 3 | 0 | 0 | 1 | - |
| Average number of nucleotide differences (k) | 73.45702 | 9.26367 | 1.52824 | 0.000 | 0.000 | 0.66667 | - |
| Nucleotide diversity (Pi) | 0.09873 | 0.01245 | 0.00205 | 0.000 | 0.000 | 0.00090 | - |
| Haplotype diversity (Hd) | 0.804 | 0.506 | 0.509 | 0.000 | 0.000 | 0.667 | - |
| Variance of Hd | 0.00054 | 0.00356 | 0.00039 | 0.000 | 0.000 | 0.04167 | - |
| Standard deviation of Hd | 0.023 | 0.060 | 0.020 | 0.000 | 0.000 | 0.204 | - |
| Theta per sequence | 45.21592 | 5.52109 | 0.69336 | - | - | 0.54545 | - |
| Theta per site | 0.06077 | 0.00742 | 0.00093 | 0.000 | 0.000 | 0.00073 | - |
| Fu’s Fs statistic | 96.201 | 16.737 | 5.448 | - | - | 0.540 | - |
| Tajima’s D p | 2.01806 0.10 > p > 0.05 | 2.07283 p < 0.05 | 2.57824 p < 0.05 | - | - | 1.63299 p > 0.10 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misaka, B.C.; Wosula, E.N.; Marchelo-d’Ragga, P.W.; Hvoslef-Eide, T.; Legg, J.P. Genetic Diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Colonizing Sweet Potato and Cassava in South Sudan. Insects 2020, 11, 58. https://doi.org/10.3390/insects11010058
Misaka BC, Wosula EN, Marchelo-d’Ragga PW, Hvoslef-Eide T, Legg JP. Genetic Diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Colonizing Sweet Potato and Cassava in South Sudan. Insects. 2020; 11(1):58. https://doi.org/10.3390/insects11010058
Chicago/Turabian StyleMisaka, Beatrice C., Everlyne N. Wosula, Philip W. Marchelo-d’Ragga, Trine Hvoslef-Eide, and James P. Legg. 2020. "Genetic Diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Colonizing Sweet Potato and Cassava in South Sudan" Insects 11, no. 1: 58. https://doi.org/10.3390/insects11010058
APA StyleMisaka, B. C., Wosula, E. N., Marchelo-d’Ragga, P. W., Hvoslef-Eide, T., & Legg, J. P. (2020). Genetic Diversity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) Colonizing Sweet Potato and Cassava in South Sudan. Insects, 11(1), 58. https://doi.org/10.3390/insects11010058





