Comparison of Twelve Ant Species and Their Susceptibility to Fungal Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Housing
2.2. Procedures: Species-Specific Survival
2.3. Procedures: Behavioral Measurements
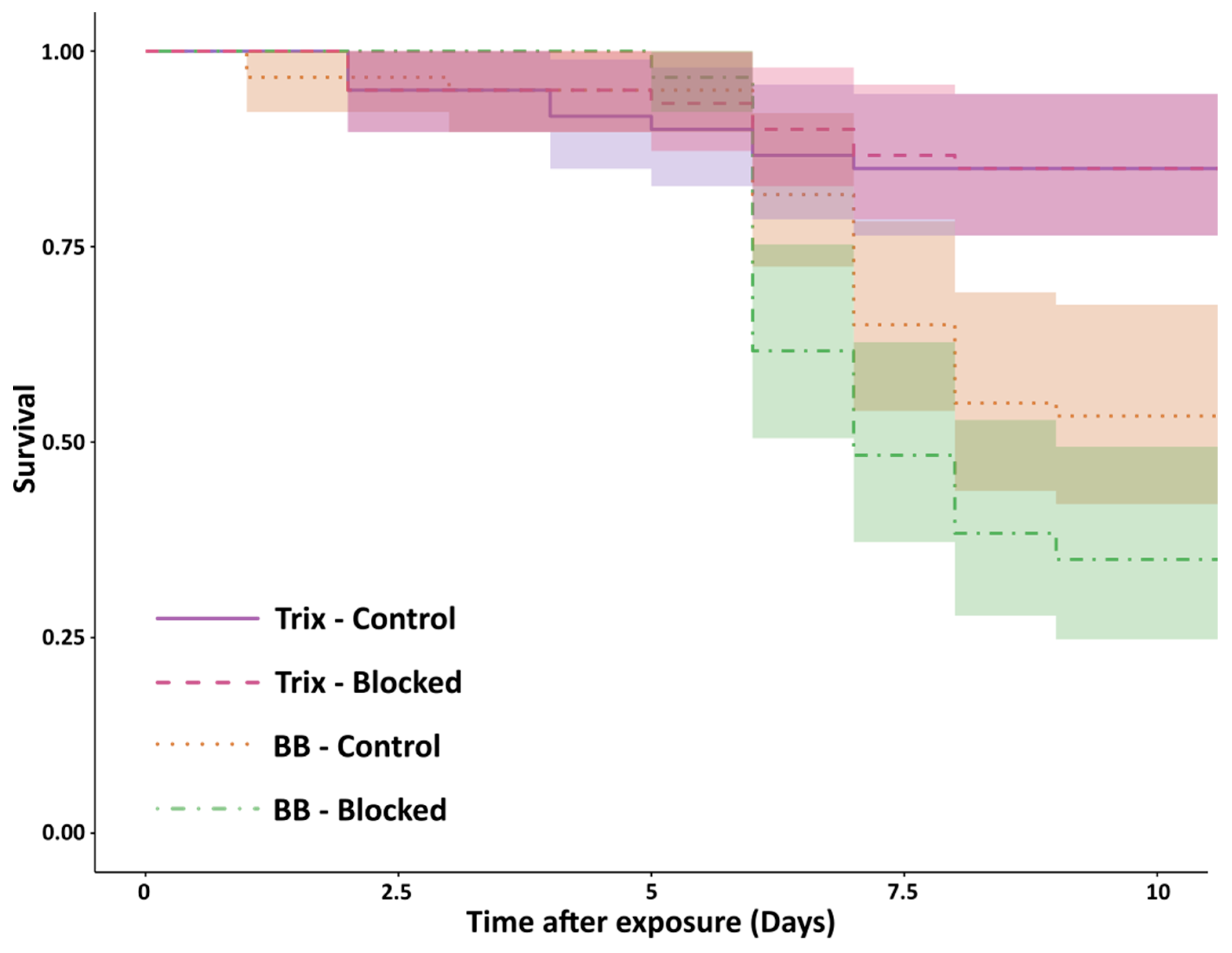
2.4. Procedures: Effect of the Metapleural Gland on Survival
2.5. Statistical Analysis
2.5.1. Survival
2.5.2. Behavior
2.5.3. Metapleural Gland
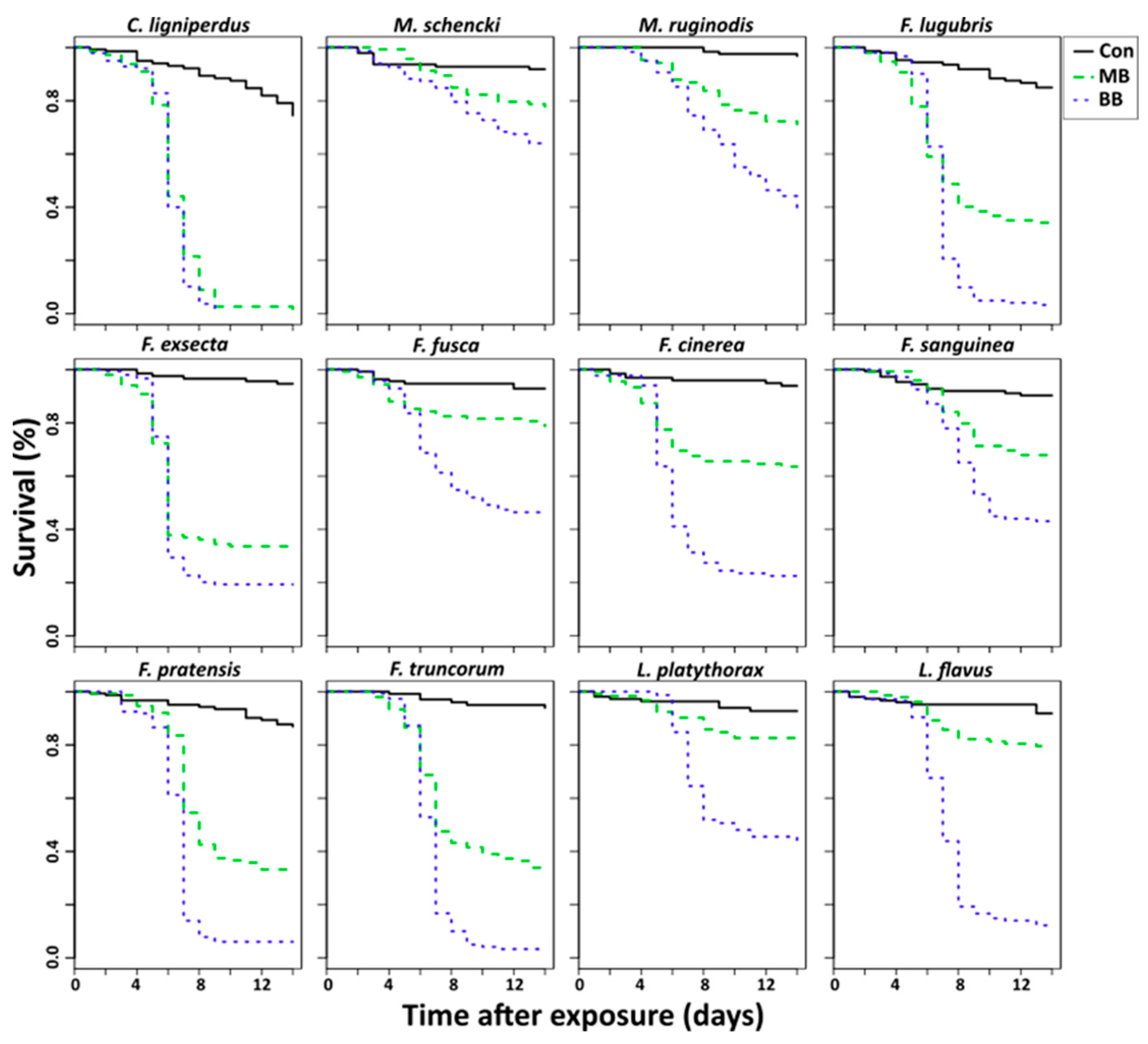
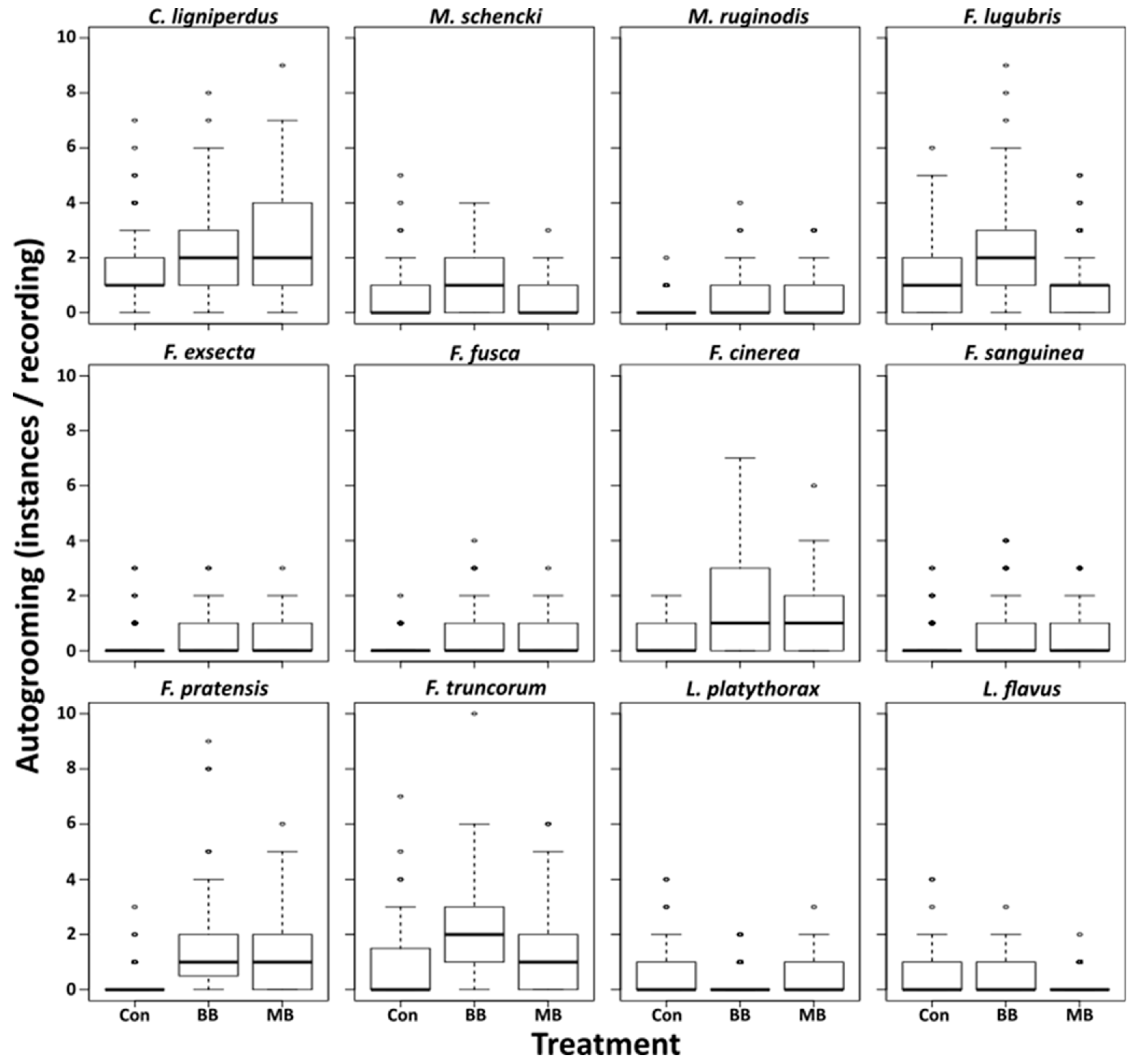
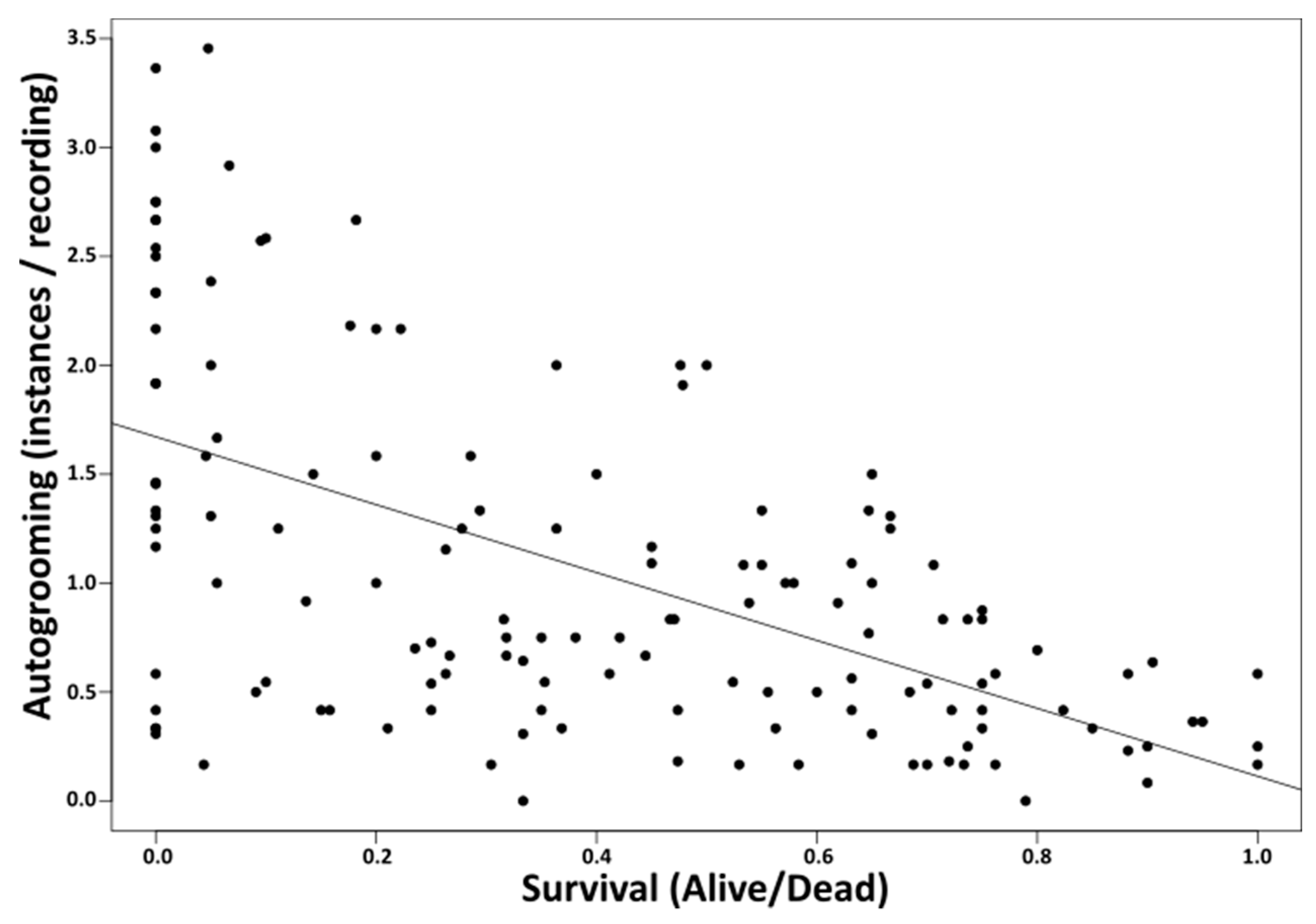
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap Press of Harvard University Press: Cambridge, UK, 1990; ISBN 978-0-674-04075-5. [Google Scholar]
- Fefferman, N.H.; Traniello, J.F.A. Social insects as models in epidemiology: Establishing the foundation for an interdisciplinary approach to disease and sociality. In Organization of Insect Societies: From Genome to Sociocomplexity; Harvard University Press: Cambridge, UK, 2009. [Google Scholar]
- Cremer, S.; Armitage, S.A.O.; Schmid−Hempel, P. Social Immunity. Curr. Biol. 2007, 17, R693–R702. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.O.H.; Boomsma, J.J. Genetic diversity and disease resistance in leaf-cutting ant societies. Evolution 2004, 58, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.H.; Rothman, L.E. Virulence and transmission of infectious diseases in humans and insects: Evolutionary and demographic patterns. Trends Ecol. Evol. 1995, 10, 194–198. [Google Scholar] [CrossRef]
- Ugelvig, L.V.; Kronauer, D.J.C.; Schrempf, A.; Heinze, J.; Cremer, S. Rapid anti-Pathogen response in ant societies relies on high genetic diversity. Proc. R. Soc. B Biol. Sci. 2010, 277, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Cremer, S.; Pull, C.D.; Fürst, M.A. Social Immunity: Emergence and Evolution of Colony-Level Disease Protection. Annu. Rev. Entomol. 2018, 63, 105–123. [Google Scholar] [CrossRef]
- Cremer, S.; Sixt, M. Analogies in the evolution of individual and social immunity. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J. Social immunity and the evolution of group living in insects. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140102. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, X.Y.; Tang, Q.B.; Lei, C.L.; Huang, Q.Y. The mechanisms of social immunity against fungal infections in eusocial insects. Toxins 2019, 11, 244. [Google Scholar] [CrossRef]
- Drees, B.M.; Miller, R.W.; Vinson, B.S.; Georgis, R. Susceptibility and behavioral response of Red Imported Fire Ant (Hymenoptera: Formicidae) to selected entomogenous nematodes (Rhabditida: Steinernematidae & Heterorhabditidae). J. Econ. Entomol. 1992, 85, 365–370. [Google Scholar]
- Mburu, D.M.; Ochola, L.; Maniania, N.K.; Njagi, P.G.N.; Gitonga, L.M.; Ndung’u, M.W.; Wanjoya, A.K.; Hassanali, A. Relationship between virulence and repellency of entomopathogenic isolates of Metarhizium anisopliae and Beauveria bassiana to the termite Macrotermes michaelseni. J. Insect Physiol 2009, 55, 774–780. [Google Scholar] [CrossRef]
- Reber, A.; Purcell, J.; Buechel, S.D.; Buri, P.; Chapuisat, M. The expression and impact of antifungal grooming in ants. J. Evol. Biol. 2011, 24, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Yek, S.H.; Mueller, U.G. The metapleural gland of ants. Biol. Rev. 2011, 86, 774–791. [Google Scholar] [CrossRef]
- Tragust, S. External immune defence in ant societies (Hymenoptera: Formicidae): The role of antimicrobial venom and metapleural gland secretion. Myrmecol. News 2016, 23, 119–128. [Google Scholar]
- Fernández-Marín, H.; Zimmerman, J.K.; Rehner, S.A.; Wcislo, W.T. Active use of the metapleural glands by ants in controlling fungal infection. Proc. R. Soc. B Biol. Sci. 2006, 273, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Brütsch, T.; Jaffuel, G.; Vallat, A.; Turlings, T.C.J.; Chapuisat, M. Wood ants produce a potent antimicrobial agent by applying formic acid on tree-Collected resin. Ecol. Evol. 2017, 7, 2249–2254. [Google Scholar] [CrossRef]
- Castella, G.; Chapuisat, M.; Christe, P. Prophylaxis with resin in wood ants. Anim. Behav. 2008, 75, 1591–1596. [Google Scholar] [CrossRef]
- Christe, P.; Oppliger, A.; Bancalà, F.; Castella, G.; Chapuisat, M. Evidence for collective medication in ants. Ecol. Lett. 2003, 6, 19–22. [Google Scholar] [CrossRef]
- Theis, F.J.; Ugelvig, L.V.; Marr, C.; Cremer, S. Opposing effects of allogrooming on disease transmission in ant societies. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140108. [Google Scholar] [CrossRef]
- Walker, T.N.; Hughes, W.O.H. Adaptive social immunity in leaf-cutting ants. Biol. Lett. 2009, 5, 446–448. [Google Scholar] [CrossRef]
- Hamilton, C.; Lejeune, B.T.; Rosengaus, R.B. Trophallaxis and prophylaxis: Social immunity in the carpenter ant Camponotus pennsylvanicus. Biol. Lett. 2011, 7, 89–92. [Google Scholar] [CrossRef]
- Heinze, J.; Walter, B. Moribund Ants Leave Their Nests to Die in Social Isolation. Curr. Biol. 2010, 20, 249–252. [Google Scholar] [CrossRef]
- Bos, N.; Lefèvre, T.; Jensen, A.B.; D’Ettorre, P. Sick ants become unsociable: Sick ants become unsociable. J. Evol. Biol. 2012, 25, 342–351. [Google Scholar] [CrossRef]
- Pull, C.D.; Ugelvig, L.V.; Wiesenhofer, F.; Grasse, A.V.; Tragust, S.; Schmitt, T.; Brown, M.J.; Cremer, S. Destructive disinfection of infected brood prevents systemic disease spread in ant colonies. Elife 2018, 7, 29. [Google Scholar] [CrossRef]
- Qiu, H.L.; Lu, L.H.; Shi, Q.X.; Tu, C.C.; Lin, T.; He, Y.R. Differential necrophoric behaviour of the ant Solenopsis invicta towards fungal-infected corpses of workers and pupae. Bull. Entomol. Res. 2015, 105, 607–614. [Google Scholar] [CrossRef]
- Diez, L.; Lejeune, P.; Detrain, C. Keep the nest clean: Survival advantages of corpse removal in ants. Biol. Lett. 2014, 10, 20140306. [Google Scholar] [CrossRef]
- Diez, L.; Deneubourg, J.L.; Detrain, C. Social prophylaxis through distant corpse removal in ants. Naturwissenschaften 2012, 99, 833–842. [Google Scholar] [CrossRef]
- Renucci, M.; Tirard, A.; Provost, E. Complex undertaking behavior in Temnothorax lichtensteini ant colonies: From corpse-burying behavior to necrophoric behavior. Insectes Sociaux 2011, 58, 9–16. [Google Scholar] [CrossRef]
- Rosengaus, R.; Traniello, J. Disease susceptibility and the adaptive nature of colony demography in the dampwood termite Zootermopsis angusticollis. Behav. Ecol. Sociobiol. 2001, 50, 546–556. [Google Scholar] [CrossRef]
- Bot, A.N.M.; Currie, C.R.; Hart, A.G.; Boomsma, J.J. Waste management in leaf-cutting ants. Ethol. Ecol. Evol. 2001, 13, 225–237. [Google Scholar] [CrossRef]
- Hart, A.G.; Ratnieks, F.L.W. Task partitioning, division of labour and nest compartmentalisation collectively isolate hazardous waste in the leafcutting ant Atta cephalotes. Behav. Ecol. Sociobiol. 2001, 49, 387–392. [Google Scholar] [CrossRef]
- Yanagawa, A.; Fujiwara-Tsujii, N.; Akino, T.; Yoshimura, T.; Yanagawa, T.; Shimizu, S. Behavioral changes in the termite, Coptotermes formosanus (Isoptera), inoculated with six fungal isolates. J. Invertebr. Pathol. 2011, 107, 100–106. [Google Scholar] [CrossRef]
- Lindström, S.; Timonen, S.; Sundström, L.; Johansson, H. Ants reign over a distinct microbiome in their nests. Soil Biol. Biochem. in press.
- Lindström, S.; Rowe, O.; Timonen, S.; Sundström, L.; Johansson, H. Trends in bacterial and fungal communities in ant nests observed with Terminal-Restriction Fragment Length Polymorphism (T−RFLP) and Next Generation Sequencing (NGS) techniques—Validity and compatibility in ecological studies. Peer J. 2018, 6, e5289. [Google Scholar] [CrossRef]
- Aubert, A.; Richard, F.J. Social management of LPS−induced inflammation in Formica polyctena ants. Brain. Behav. Immun. 2008, 22, 833–837. [Google Scholar] [CrossRef]
- Konrad, M.; Vyleta, M.L.; Theis, F.J.; Stock, M.; Tragust, S.; Klatt, M.; Drescher, V.; Marr, C.; Ugelvig, L.V.; Cremer, S. Social transfer of pathogenic fungus promotes active immunisation in ant colonies. PLoS Biol. 2012, 10, e1001300. [Google Scholar] [CrossRef]
- Loreto, R.G.; Hughes, D.P. Disease dynamics in ants. Adv. Genet. 2016, 94, 287–306. [Google Scholar]
- Małagocka, J.; Jensen, A.B.; Eilenberg, J. Pandora formicae, a specialist ant pathogenic fungus: New insights into biology and taxonomy. J. Invertebr. Pathol. 2017, 143, 108–114. [Google Scholar] [CrossRef]
- Seifert, B. The Ants of Central and North Europe; Lutra: Boxberg, Germany, 2018; ISBN 978-3-936412-07-9. [Google Scholar]
- Czechowski, W.; Radchenko, A.; Czechowska, W.; Vepsäläinen, K. The Ants of Poland: With Reference to the Myrmecofauna of Europe; Natura optima dux Foundation: Warszawa, Poland, 2012; ISBN 978-83-930773-4-2. [Google Scholar]
- Ivens, A.B.; Kronauer, D.J.; Pen, I.; Weissing, F.J.; Boomsma, J.J. Ants farm subterranean aphids mostly in single clone groups—an example of prudent husbandry for carbohydrates and proteins? BMC Evol. Biol. 2012, 12, 106. [Google Scholar] [CrossRef]
- Bhatkar, A.; Whitcomb, W.H. Artificial Diet for Rearing Various Species of Ants. Fla. Entomol. 1970, 53, 229. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- R Studio Team. R Studio: Integrated Development for R; R Studio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox model. In Statistics for Biology and Health; Springer: New York, NY, USA, 2000. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D.; Venables, W.N. Modern Applied Statistics with S. In Statistics and Computing, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95457-8. [Google Scholar]
- Robinson, D.; Hayes, A. Broom: Convert Statistical Analysis Objects into tidy Tibbles. The Comprehensive R Archive Network. Available online: https://cran.r-project.org/web/packages/broom/ (accessed on 26 August 2019).
- Fox, J.; Weisberg, S.; Fox, J. An R Companion to Applied Regression, 2nd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2011; ISBN 978-1-4129-7514-8. [Google Scholar]
- Meyer, D.; Zeileis, A.; Hornik, K. Vcd: Visualizing Categorical Data. The Comprehensive R Archive Network. Available online: https://cran.r-project.org/web/packages/vcd/index.html (accessed on 26 August 2019).
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Goropashnaya, A.V.; Fedorov, V.B.; Seifert, B.; Pamilo, P. Phylogenetic relationships of Palaearctic Formica species (Hymenoptera, Formicidae) based on mitochondrial cytochrome b sequences. PLoS ONE 2012, 7, e41697. [Google Scholar] [CrossRef][Green Version]
- Walker, T.N.; Hughes, W.O.H. Arboreality and the evolution of disease resistance in ants. Ecol. Entomol. 2011, 36, 588–595. [Google Scholar] [CrossRef]
- Rosengaus, R.B.; Maxmen, A.B.; Coates, L.E.; Traniello, J.F.A. Disease resistance: A benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Behav. Ecol. Sociobiol. 1998, 44, 125–134. [Google Scholar] [CrossRef]
- Okuno, M.; Tsuji, K.; Sato, H.; Fujisaki, K. Plasticity of grooming behavior against entomopathogenic fungus Metarhizium anisopliae in the ant Lasius japonicus. J. Ethol. 2012, 30, 23–27. [Google Scholar] [CrossRef]
- Greenfield, B.P.J.; Lord, A.M.; Dudley, E.; Butt, T.M. Conidia of the insect pathogenic fungus, Metarhizium anisopliae, fail to adhere to mosquito larval cuticle. R. Soc. Open Sci. 2014, 1, 140193. [Google Scholar] [CrossRef]
- Sun, J.; Fuxa, J.R.; Henderson, G. Effects of virulence, sporulation, and temperature on Metarhizium anisopliae and Beauveria bassiana laboratory transmission in Coptotermes formosanus. J. Invertebr. Pathol. 2003, 84, 38–46. [Google Scholar] [CrossRef]
- Feldhaar, H.; Gross, R. Immune reactions of insects on bacterial pathogens and mutualists. Microbes Infect. 2008, 10, 1082–1088. [Google Scholar] [CrossRef]
- Sinotte, V.; Freedman, S.; Ugelvig, L.; Seid, M. Camponotus floridanus ants incur a trade-off between phenotypic development and pathogen susceptibility from their mutualistic endosymbiont Blochmannia. Insects 2018, 9, 58. [Google Scholar] [CrossRef]
- Vitikainen, E.; Sundström, L. Inbreeding and caste−specific variation in immune defence in the ant Formica exsecta. Behav. Ecol. Sociobiol. 2011, 65, 899–907. [Google Scholar] [CrossRef]
- Hughes, W.O.H.; Thomsen, L.; Eilenberg, J.; Boomsma, J.J. Diversity of entomopathogenic fungi near leaf−Cutting ant nests in a neotropical forest, with particular reference to Metarhizium anisopliae var. anisopliae. J. Invertebr. Pathol. 2004, 85, 46–53. [Google Scholar] [CrossRef]
- Keller, S.; Kessler, P.; Schweizer, C. Distribution of insect pathogenic soil fungi in Switzerland with special reference to Beauveria brongniartii and Metharhizium anisopliae. BioControl 2003, 48, 307–319. [Google Scholar] [CrossRef]
- Tranter, C.; Hughes, W.O.H. Acid, silk and grooming: Alternative strategies in social immunity in ants? Behav. Ecol. Sociobiol. 2015, 69, 1687–1699. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.D.; Spivak, M. Increased resin collection after parasite challenge: A case of self-medication in honey bees? PLoS ONE 2012, 7, e34601. [Google Scholar] [CrossRef]
| Species | Term | Estimate | Std. Error | Z | p-value |
|---|---|---|---|---|---|
| C. ligniperdus | Con-MB | −0.993 | 0.064 | −15.513 | <0.001 |
| C. ligniperdus | Con-BB | −1.092 | 0.062 | −17.493 | <0.001 |
| C. ligniperdus | MB-BB | −0.099 | 0.040 | −2.489 | 0.013 |
| M. schencki | Con-MB | −0.609 | 0.254 | −2.399 | 0.016 |
| M. schencki | Con-BB | −1.035 | 0.249 | −4.158 | <0.001 |
| M. schencki | MB-BB | −0.426 | 0.175 | −2.441 | 0.015 |
| M. ruginodis | Con-MB | −1.098 | 0.261 | −4.203 | <0.001 |
| M. ruginodis | Con-BB | −1.571 | 0.269 | −5.834 | <0.001 |
| M. ruginodis | MB-BB | −0.473 | 0.110 | −4.288 | <0.001 |
| F. lugubris | Con-MB | −0.876 | 0.110 | −7.981 | <0.001 |
| F. lugubris | Con-BB | −1.317 | 0.107 | −12.317 | <0.001 |
| F. lugubris | MB-BB | −0.441 | 0.059 | −7.465 | <0.001 |
| F. exsecta | Con-MB | −1.556 | 0.215 | −7.239 | <0.001 |
| F. exsecta | Con-BB | −1.905 | 0.213 | −8.928 | <0.001 |
| F. exsecta | MB-BB | −0.348 | 0.073 | −4.778 | <0.001 |
| F. fusca | Con-MB | −0.896 | 0.290 | −3.092 | 0.002 |
| F. fusca | Con-BB | −1.642 | 0.288 | −5.695 | <0.001 |
| F. fusca | MB-BB | −0.747 | 0.177 | −4.223 | <0.001 |
| F. cinerea | Con-MB | −1.316 | 0.274 | −4.798 | <0.001 |
| F. cinerea | Con-BB | −2.097 | 0.277 | −7.575 | <0.001 |
| F. cinerea | MB-BB | −0.781 | 0.127 | −6.165 | <0.001 |
| F. sanguinea | Con-MB | −0.656 | 0.169 | −3.875 | <0.001 |
| F. sanguinea | Con-BB | −1.075 | 0.170 | −6.329 | <0.001 |
| F. sanguinea | MB-BB | −0.419 | 0.107 | −3.930 | <0.001 |
| F. pratensis | Con-MB | −0.877 | 0.113 | −7.785 | <0.001 |
| F. pratensis | Con-BB | −1.384 | 0.112 | −12.410 | <0.001 |
| F. pratensis | MB-BB | −0.507 | 0.061 | −8.373 | <0.001 |
| F. truncorum | Con-MB | −1.116 | 0.157 | −7.125 | <0.001 |
| F. truncorum | Con-BB | −1.596 | 0.156 | −10.260 | <0.001 |
| F. truncorum | MB-BB | −0.479 | 0.051 | −9.382 | <0.001 |
| L. platythorax | Con-MB | −0.505 | 0.274 | −1.844 | 0.065 |
| L. platythorax | Con-BB | −1.290 | 0.275 | −4.698 | <0.001 |
| L. platythorax | MB-BB | −0.784 | 0.184 | −4.273 | <0.001 |
| L. flavus | Con-MB | −0.411 | 0.162 | −2.540 | 0.011 |
| L. flavus | Con-BB | −1.515 | 0.156 | −9.679 | <0.001 |
| L. flavus | MB-BB | −1.104 | 0.109 | −10.092 | <0.001 |
| Species | Term | Estimate | Std. Error | Z | p |
|---|---|---|---|---|---|
| C. ligniperdus | Con-MB | 0.329 | 0.145 | 2.261 | 0.024 |
| C. ligniperdus | Con-BB | 0.351 | 0.145 | 2.417 | 0.016 |
| C. ligniperdus | MB-BB | −0.023 | 0.136 | −0.166 | 0.868 |
| M. schencki | Con-MB | −0.045 | 0.229 | −0.196 | 0.844 |
| M. schencki | Con-BB | 0.506 | 0.207 | 2.451 | 0.014 |
| M. schencki | MB-BB | 0.551 | 0.209 | 2.643 | 0.008 |
| M. ruginodis | Con-MB | 1.243 | 0.358 | 3.470 | <0.001 |
| M. ruginodis | Con-BB | 1.277 | 0.357 | 3.581 | <0.001 |
| M. ruginodis | MB-BB | 0.034 | 0.250 | 0.135 | 0.892 |
| F. lugubris | Con-MB | −0.479 | 0.178 | −2.690 | 0.007 |
| F. lugubris | Con-BB | 0.308 | 0.157 | 1.958 | 0.05 |
| F. lugubris | MB-BB | 0.787 | 0.173 | 4.543 | <0.001 |
| F. exsecta | Con-MB | 0.712 | 0.261 | 2.725 | 0.006 |
| F. exsecta | Con-BB | 0.541 | 0.269 | 2.010 | 0.044 |
| F. exsecta | MB-BB | −0.170 | 0.223 | −0.764 | 0.445 |
| F. fusca | Con-MB | 1.646 | 0.400 | 4.113 | <0.001 |
| F. fusca | Con-BB | 1.439 | 0.407 | 3.535 | <0.001 |
| F. fusca | MB-BB | −0.207 | 0.253 | −0.818 | 0.414 |
| F. cinerea | Con-MB | 1.071 | 0.209 | 5.126 | <0.001 |
| F. cinerea | Con-BB | 1.365 | 0.203 | 6.715 | <0.001 |
| F. cinerea | MB-BB | 0.294 | 0.146 | 2.020 | 0.043 |
| F. sanguinea | Con-MB | 0.752 | 0.313 | 2.397 | 0.017 |
| F. sanguinea | Con-BB | 0.834 | 0.309 | 2.701 | 0.007 |
| F. sanguinea | MB-BB | 0.082 | 0.271 | 0.304 | 0.761 |
| F. pratensis | Con-MB | 1.946 | 0.303 | 6.413 | <0.001 |
| F. pratensis | Con-BB | 2.159 | 0.301 | 7.180 | <0.001 |
| F. pratensis | MB-BB | 0.212 | 0.167 | 1.267 | 0.205 |
| F. truncorum | Con-MB | 0.459 | 0.179 | 2.567 | 0.01 |
| F. truncorum | Con-BB | 0.751 | 0.171 | 4.392 | <0.001 |
| F. truncorum | MB-BB | 0.292 | 0.155 | 1.886 | 0.059 |
| L. platythorax | Con-MB | −0.242 | 0.267 | −0.908 | 0.364 |
| L. platythorax | Con-BB | −0.630 | 0.297 | −2.118 | 0.034 |
| L. platythorax | MB-BB | −0.388 | 0.307 | −1.263 | 0.207 |
| L. flavus | Con-MB | −0.974 | 0.283 | −3.441 | 0.001 |
| L. flavus | Con-BB | −0.307 | 0.228 | −1.349 | 0.177 |
| L. flavus | MB-BB | 0.667 | 0.299 | 2.233 | 0.026 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bos, N.; Kankaanpää-Kukkonen, V.; Freitak, D.; Stucki, D.; Sundström, L. Comparison of Twelve Ant Species and Their Susceptibility to Fungal Infection. Insects 2019, 10, 271. https://doi.org/10.3390/insects10090271
Bos N, Kankaanpää-Kukkonen V, Freitak D, Stucki D, Sundström L. Comparison of Twelve Ant Species and Their Susceptibility to Fungal Infection. Insects. 2019; 10(9):271. https://doi.org/10.3390/insects10090271
Chicago/Turabian StyleBos, Nick, Viljami Kankaanpää-Kukkonen, Dalial Freitak, Dimitri Stucki, and Liselotte Sundström. 2019. "Comparison of Twelve Ant Species and Their Susceptibility to Fungal Infection" Insects 10, no. 9: 271. https://doi.org/10.3390/insects10090271
APA StyleBos, N., Kankaanpää-Kukkonen, V., Freitak, D., Stucki, D., & Sundström, L. (2019). Comparison of Twelve Ant Species and Their Susceptibility to Fungal Infection. Insects, 10(9), 271. https://doi.org/10.3390/insects10090271




