Modeling Potential Habitat for Amblyomma Tick Species in California
Abstract
1. Introduction
2. Materials and Methods
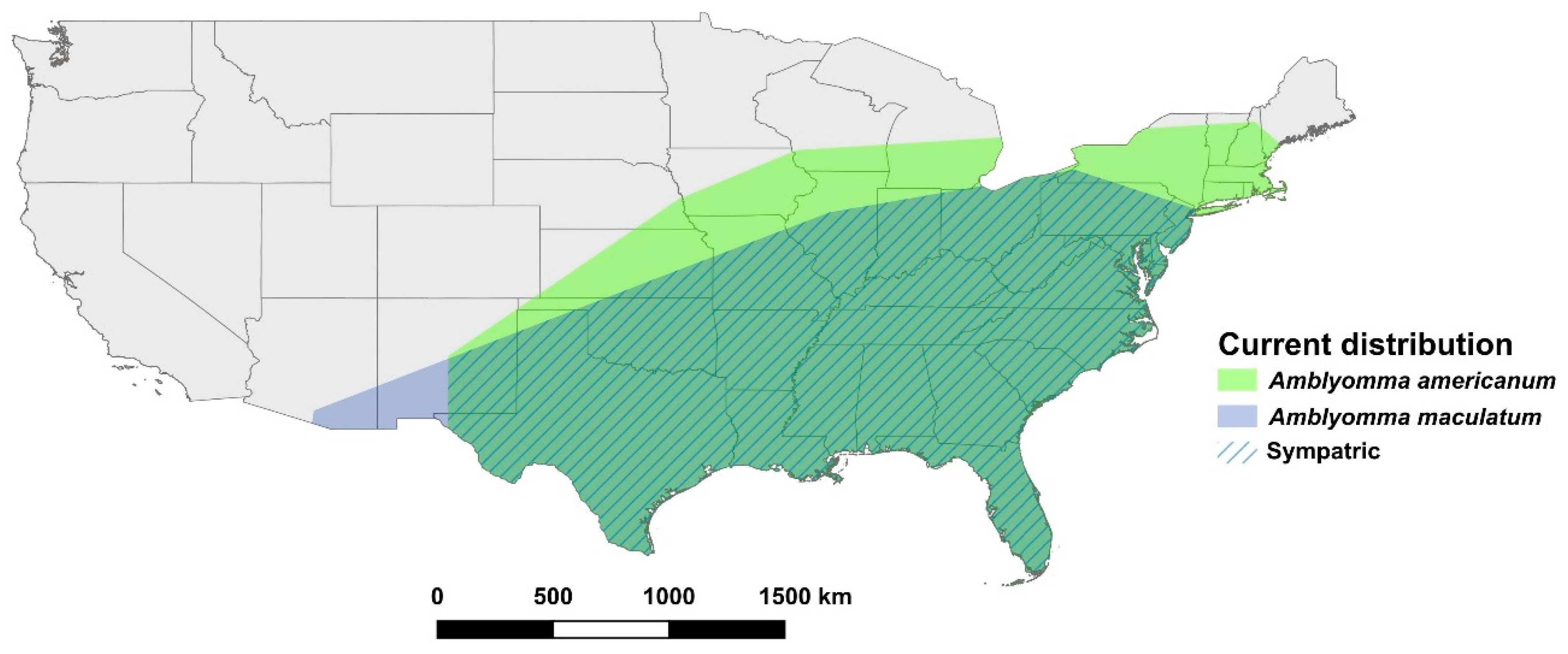
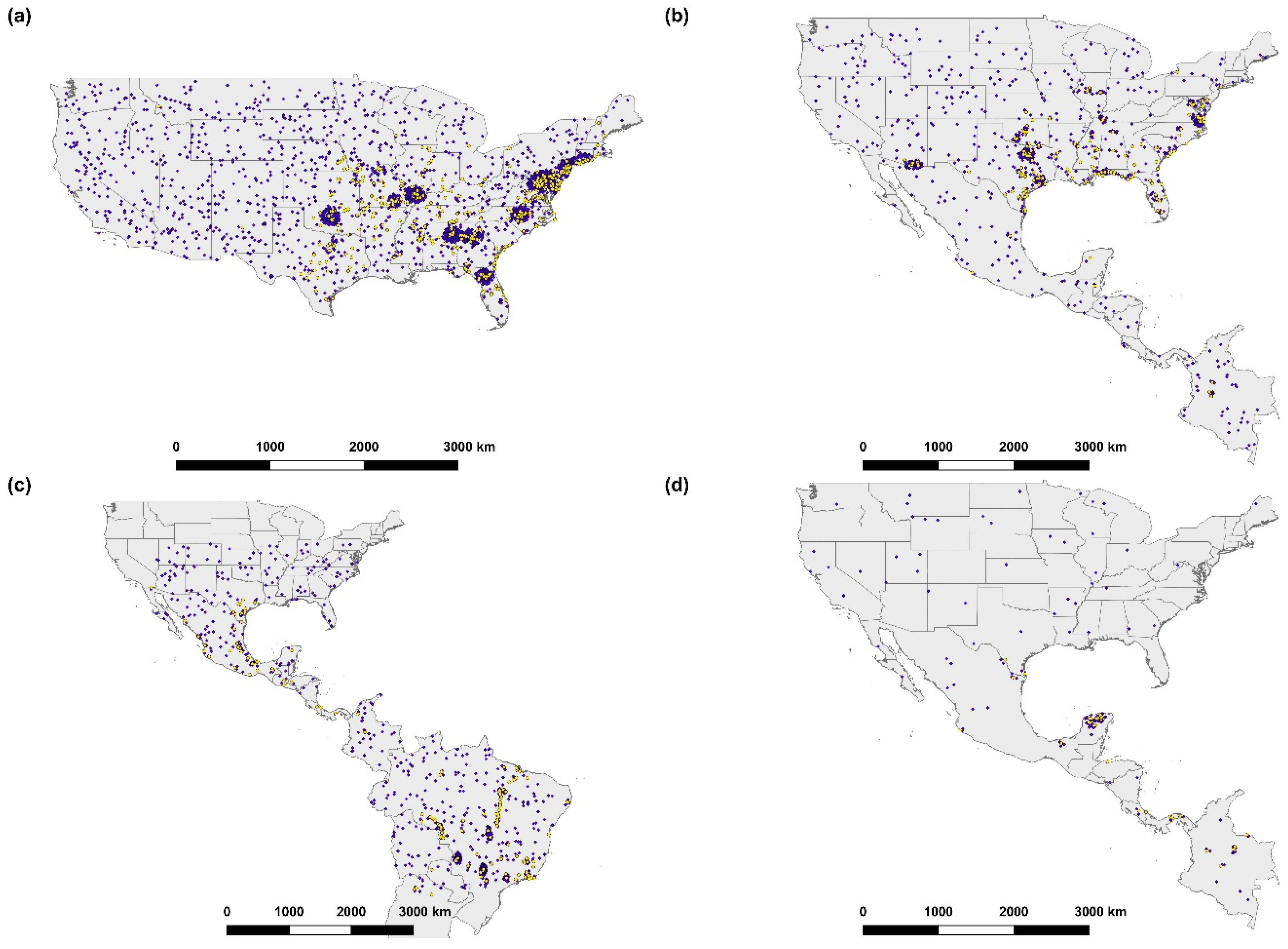
2.1. Amblyomma spp. Presence Data
2.2. Environmental Predictor Variables
2.3. Modeling of Habitat Suitability
2.4. Model Evaluation and Visualization
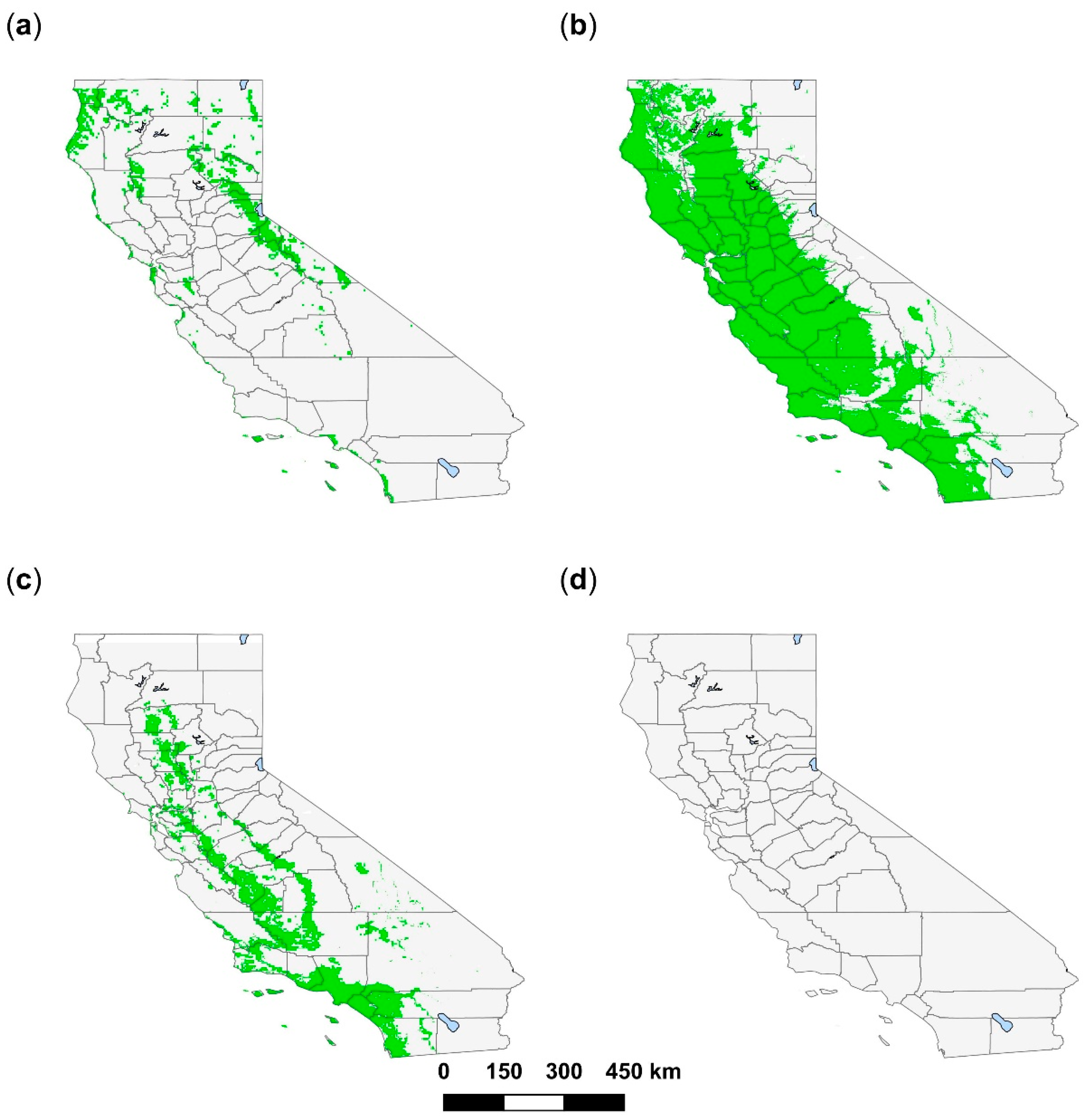
3. Results
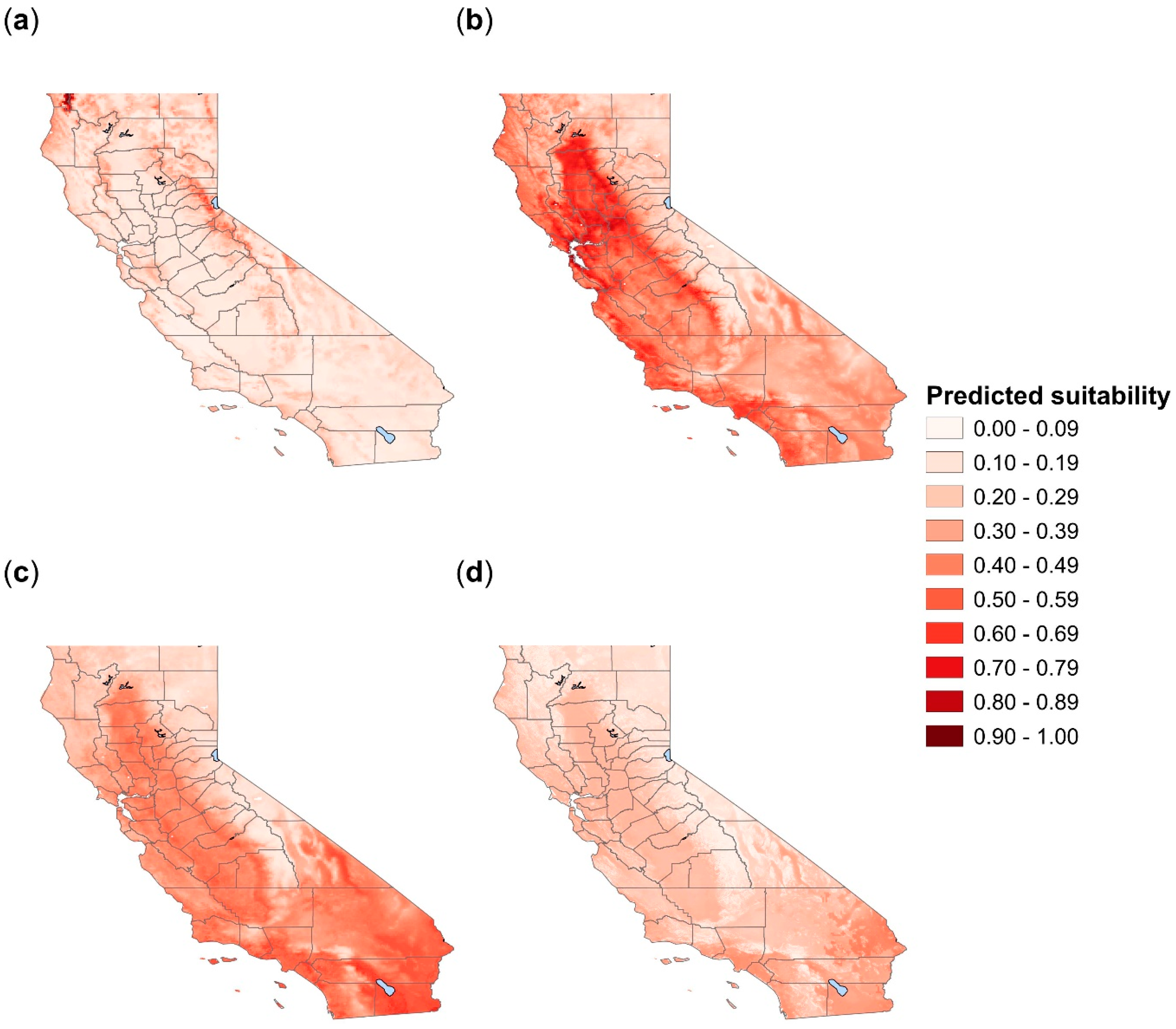
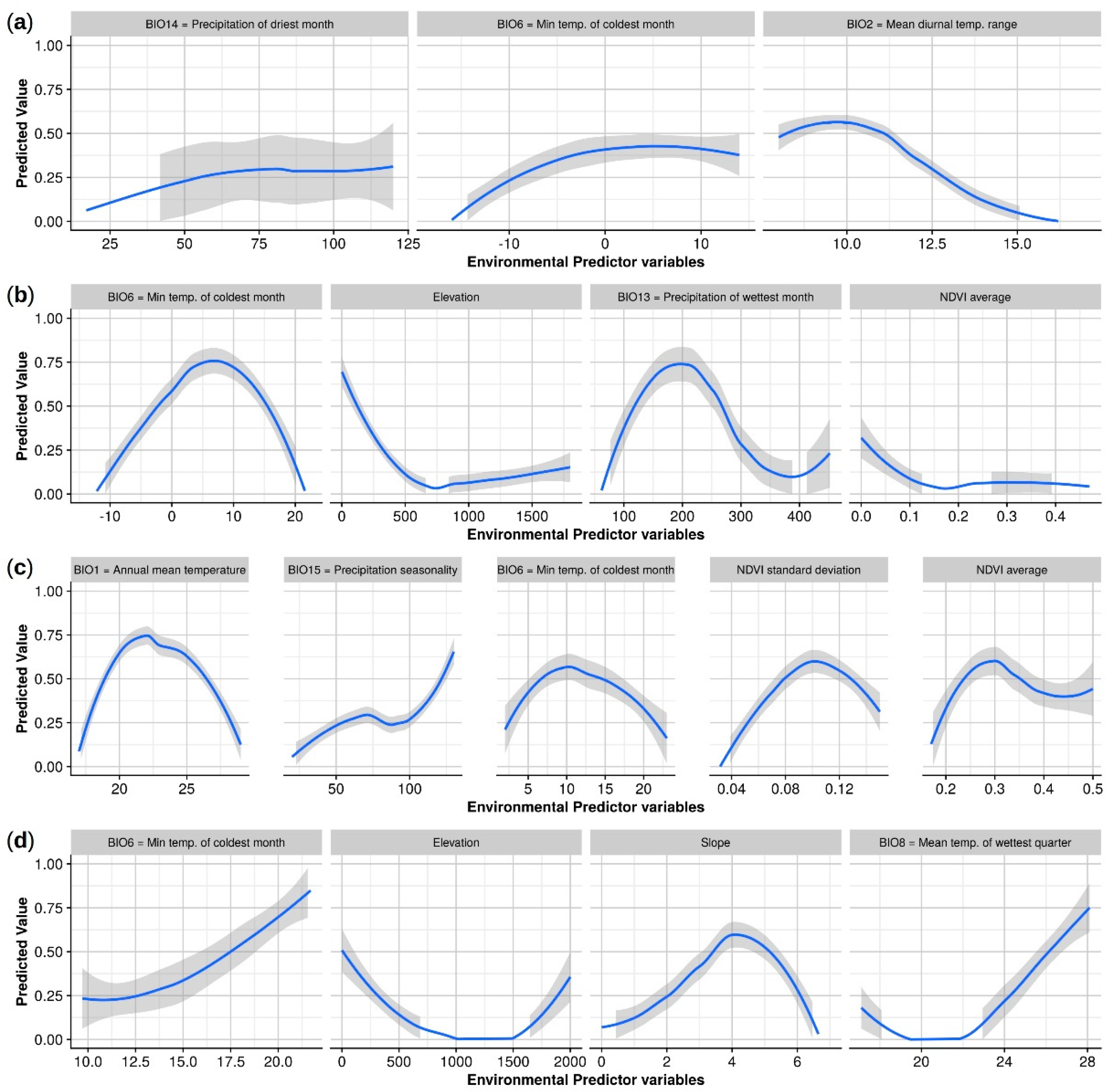
3.1. Amblyomma americanum
3.2. Amblyomma maculatum
3.3. Amblyomma cajennense
3.4. Amblyomma mixtum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Bolte, J.R.; Hair, J.A.; Fletcher, J. White-tailed deer mortality following tissue destruction induced by lone star ticks. J. Wildl. Manag. 1970, 34, 546–552. [Google Scholar] [CrossRef]
- Barnard, D.R. Injury thresholds and production loss functions for the lone star tick, Amblyomma americanum (Acari: Ixodidae), on pastured, preweaner beef cattle, Bos taurus. J. Econ. Entomol. 1985, 78, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Merten, H.A.; Durden, L.A. A state-by-state survey of ticks recorded from humans in the United States. J. Vector Ecol. 2000, 25, 102–113. [Google Scholar] [PubMed]
- Dawson, J.E.; Ewing, S.A.; Davidson, W.R.; Childs, J.E.; Little, S.E.; Standaert, S.M. Human monocytic ehrlichiosis. In Tick-borne Diseases of Humans; Goodman, J.L., Dennis, D.T., Sonenshine, D.E., Eds.; ASM Press: Washington, DC, USA, 2005; pp. 239–257. ISBN 978-1-55581-238-6. [Google Scholar]
- Hayes, E.B. Tularemia. In Tick-borne Diseases of Humans; Goodman, J.L., Dennis, D.T., Sonenshine, D.E., Eds.; ASM Press: Washington, DC, USA, 2005; pp. 143–149, 207–217. ISBN 978-1-55581-238-6. [Google Scholar]
- Paddock, C.D.; Liddell, A.M.; Storch, G.A. Human monocytic ehrlichiosis. In Tick-borne Diseases of Humans; Goodman, J.L., Dennis, D.T., Sonenshine, D.E., Eds.; ASM Press: Washington, DC, USA, 2005; pp. 258–267. ISBN 978-1-55581-238-6. [Google Scholar]
- Thompson, H.A.; Dennis, D.T.; Dasch, G.A. Q fever. In Tick-borne Diseases of Humans; Goodman, J.L., Dennis, D.T., Sonenshine, D.E., Eds.; ASM Press: Washington, DC, USA, 2005; pp. 328–342. ISBN 978-1-55581-238-6. [Google Scholar]
- Wormser, G.P.; Liveris, D.; Nowakowski, J.; Nadelman, R.B.; Holmgren, D.; Bittker, S.; Cooper, D.; Wang, G.; Schwartz, I. Microbiologic evaluation of patients from Missouri with erythema migrans. Clin. Infect. Dis. 2005, 40, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Stromdahl, E.Y.; Nadolny, R.M.; Hickling, G.J.; Hamer, S.A.; Ogden, N.H.; Casal, C.; Heck, G.A.; Gibbons, J.A.; Cremeans, T.F.; Pilgard, M.A. Amblyomma americanum (Acari: Ixodidae) ticks are not vectors of the Lyme disease agent, Borrelia burgdorferi (Spirocheatales: Spirochaetaceae): A review of the evidence. J. Med. Entomol. 2018, 55, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Masters, E.J.; Grigery, C.N.; Masters, R.W. STARI, or Masters disease: Lone star tick–vectored Lyme-like illness. Infect. Dis. Clin. North Am. 2008, 22, 361–376. [Google Scholar] [CrossRef]
- Paddock, C.D.; Sumner, J.W.; Comer, J.A.; Zaki, S.R.; Goldsmith, C.S.; Goddard, J.; McLellan, S.L.F.; Tamminga, C.L.; Ohl, C.A. Rickettsia parkeri: A newly recognized cause of spotted dever rickettsiosis in the United States. Clin. Infect. Dis. 2004, 38, 805–811. [Google Scholar] [CrossRef]
- Sumner, J.W.; Durden, L.A.; Goddard, J.; Stromdahl, E.Y.; Clark, K.L.; Reeves, W.K.; Paddock, C.D. Gulf Coast ticks (Amblyomma maculatum) and Rickettsia parkeri, United States. Emerg. Infect. Dis. 2007, 13, 751–753. [Google Scholar] [CrossRef]
- Ewing, S.A.; Panciera, R.J. American canine hepatozoonosis. Clin. Microbiol. Rev. 2003, 16, 688–697. [Google Scholar] [CrossRef]
- Uilenberg, G. Experimental transmission of Cowdria ruminantium by the Gulf coast tick Amblyomma maculatum: danger of introducing heartwater and benign African theileriasis onto the American mainland. Am. J. Vet. Res. 1982, 43, 1279–1282. [Google Scholar] [PubMed]
- Mahan, S.M.; Peter, T.F.; Simbi, B.H.; Kocan, K.; Camus, E.; Barbet, A.F.; Burridge, M.J. Comparison of efficacy of American and African Amblyomma ticks as vectors of heartwater (Cowdria ruminantium) infection by molecular analyses and transmission trials. J. Parasitol. 2000, 86, 44–49. [Google Scholar] [CrossRef]
- Labruna, M.B. Ecology of Rickettsia in South America. Ann. N. Y. Acad. Sci. 2009, 1166, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Childs, J.E.; Paddock, C.D. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 2003, 48, 307–337. [Google Scholar] [CrossRef] [PubMed]
- Teel, P.D.; Ketchum, H.R.; Mock, D.E.; Wright, R.E.; Strey, O.F. The Gulf Coast tick: a review of the life history, ecology, distribution, and emergence as an arthropod of medical and veterinary importance. J. Med. Entomol. 2010, 47, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Kugeler, K.J.; Eisen, L.; Beard, C.B.; Paddock, C.D. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017, 58, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Beati, L.; Nava, S.; Burkman, E.J.; Barros-Battesti, D.M.; Labruna, M.B.; Guglielmone, A.A.; Cáceres, A.G.; Guzmán-Cornejo, C.M.; León, R.; Durden, L.A.; et al. Amblyomma cajennense (Fabricius, 1787) (Acari: Ixodidae), the Cayenne tick: phylogeography and evidence for allopatric speciation. BMC Evol. Biol. 2013, 13, 267. [Google Scholar] [CrossRef]
- Martins, T.F.; Barbieri, A.R.M.; Costa, F.B.; Terassini, F.A.; Camargo, L.M.A.; Peterka, C.R.L.; Pacheco, R.D.C.; Dias, R.A.; Nunes, P.H.; Marcili, A.; et al. Geographical distribution of Amblyomma cajennense (sensu lato) ticks (Parasitiformes: Ixodidae) in Brazil, with description of the nymph of A. cajennense (sensu stricto). Parasit. Vectors 2016, 9, 186. [Google Scholar] [CrossRef]
- Nava, S.; Beati, L.; Labruna, M.B.; Cáceres, A.G.; Mangold, A.J.; Guglielmone, A.A. Reassessment of the taxonomic status of Amblyomma cajennense (Fabricius, 1787) with the description of three new species, Amblyomma tonelliae n. sp., Amblyomma interandinum n. sp. and Amblyomma patinoi n. sp., and reinstatement of Amblyomma mixtum Koch, 1844, and Amblyomma sculptum Berlese, 1888 (Ixodida: Ixodidae). Ticks Tick Borne Dis. 2014, 5, 252–276. [Google Scholar]
- Piña, F.T.B.; da Silva Rodrigues, V.; de Oliveira Souza Higa, L.; Garcia, M.V.; Barros, J.C.; de León, A.A.P.; Andreotti, R. Life cycle of Amblyomma mixtum (Acari: Ixodidae) parasitizing different hosts under laboratory conditions. Exp. Appl. Acarol. 2017, 73, 257–267. [Google Scholar] [CrossRef]
- Furman, D.P.; Loomis, E.C. The Ticks of California (Acari:Ixodida); University of California Press: Berkeley, CA, USA, 1984; ISBN 978-0-520-09685-1. [Google Scholar]
- Keirans, J.E.; Durden, L.A. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 1998, 35, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.N.; Lane, R.S. Lyme disease in California: A novel enzootic transmission cycle of Borrelia burgdorferi. Science 1992, 256, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Postic, D.; Ras, N.M.; Lane, R.S.; Hendson, M.; Baranton, G. Expanded diversity among Californian Borrelia isolates and description of Borrelia bissettii sp. nov. (formerly Borrelia group DN127). J. Clin. Microbiol. 1998, 36, 3497–3504. [Google Scholar] [PubMed]
- Fedorova, N.; Kleinjan, J.E.; James, D.; Hui, L.T.; Peeters, H.; Lane, R.S. Remarkable diversity of tick or mammalian-associated Borreliae in the metropolitan San Francisco Bay Area, California. Ticks Tick Borne Dis. 2014, 5, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.; Foley, J. Parallelisms and contrasts in the diverse ecologies of the Anaplasma phagocytophilum and Borrelia burgdorferi complexes of bacteria in the far western United States. Vet. Sci. 2016, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture USDA/NASS QuickStats Ad-hoc Query Tool. Available online: https://quickstats.nass.usda.gov/results/896F9A71-7600-3DF6-B0F3-F5DB4D0172CA (accessed on 14 March 2019).
- Ogden, N.H.; Maarouf, A.; Barker, I.K.; Bigras-Poulin, M.; Lindsay, L.R.; Morshed, M.G.; O’Callaghan, C.J.; Ramay, F.; Waltner-Toews, D.; Charron, D.F. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int. J. Parasitol. 2006, 36, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Bigras-Poulin, M.; O’Callaghan, C.J.; Barker, I.K.; Lindsay, L.R.; Maarouf, A.; Smoyer-Tomic, K.E.; Waltner-Toews, D.; Charron, D. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 2005, 35, 375–389. [Google Scholar] [CrossRef]
- Estrada-Peña, A. Increasing habitat suitability in the United States for the tick that transmits Lyme disease: A remote sensing approach. Environ. Health Perspect. 2002, 110, 635–640. [Google Scholar]
- Springer, Y.P.; Jarnevich, C.S.; Barnett, D.T.; Monaghan, A.J.; Eisen, R.J. Modeling the present and future geographic distribution of the lone star tick, Amblyomma americanum (Ixodida: Ixodidae), in the continental United States. Am. J. Trop. Med. Hyg. 2015, 93, 875–890. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; de la Fuente, J.; Cabezas-Cruz, A. A comparison of the performance of regression models of Amblyomma americanum (L.) (Ixodidae) using life cycle or landscape data from administrative divisions. Ticks Tick Borne Dis. 2016, 7, 624–630. [Google Scholar] [CrossRef]
- Kessler, W.H.; Blackburn, J.K.; Sayler, K.A.; Glass, G.E. Estimating the geographic distribution of host-seeking adult Amblyomma americanum (Acari: Ixodidae) in Florida. J. Med. Entomol. 2019, 56, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.J.; Foré, S.A.; Kim, H.-J. Ecological modeling over seven years to describe the number of host-seeking Amblyomma americanum in each life stage in northeast Missouri. J. Vector Ecol. 2018, 43, 271–284. [Google Scholar] [CrossRef] [PubMed]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project, Version 3.4. 2019. Available online: https://qgis.org/en/site/ (accessed on 1 December 2018).
- Homer, C.G.; Dewitz, J.A.; Yang, L.; Jin, S.; Danielson, P.; Xian, G.; Coulston, J.; Herold, N.D.; Wickham, J.D.; Megown, K. Completion of the 2011 National Land Cover Database for the Conterminous United States—Representing a Decade of Land Cover Change Information. Photogramm. Eng. Remote Sensing 2015, 81, 345–354. [Google Scholar]
- Jackson, L.K.; Gaydon, D.M.; Goddard, J. Seasonal activity and relative abundance of Amblyomma americanum in Mississippi. J. Med. Entomol. 1996, 33, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.E.; Yates, K.F.; Dietrich, G.; MacMillan, K.; Graham, C.B.; Reese, S.M.; Helterbrand, W.S.; Nicholson, W.L.; Blount, K.; Mead, P.S.; et al. An acarologic survey and Amblyomma americanum distribution map with implications for tularemia risk in Missouri. Am. J. Trop. Med. Hyg. 2011, 84, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Scifres, C.J.; Oldham, T.W.; Teel, P.D.; Drawe, D.L. Gulf coast tick (Amblyomma maculatum) populations and responses to burning of coastal prairie habitats. Southwest. Nat. 1988, 33, 55–64. [Google Scholar] [CrossRef]
- Google Maps Development Team Google Maps—pan America. 2019. Available online: https://www.google.com/maps/ (accessed on 15 November 2018).
- Stromdahl, E.Y.; Evans, S.R.; O’Brien, J.J.; Gutierrez, A.G. Prevalence of infection in ticks submitted to the human tick test kit program of the U.S. Army Center for Health Promotion and Preventive Medicine. J. Med. Entomol. 2001, 38, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Stromdahl, E.Y.; Williamson, P.C.; Kollars, T.M.; Evans, S.R.; Barry, R.K.; Vince, M.A.; Dobbs, N.A. Evidence of Borrelia lonestari DNA in Amblyomma americanum (Acari: Ixodidae) removed from humans. J. Clin. Microbiol. 2003, 41, 5557–5562. [Google Scholar] [CrossRef] [PubMed]
- Stromdahl, E.Y.; Jiang, J.; Vince, M.; Richards, A.L. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group Rickettsiae in the United States. Vector Borne Zoonotic Dis. 2011, 11, 969–977. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. dismo: Species Distribution Modeling. Release Date 2017. R Package Version 1.1-4. Available online: https://CRAN.R-project.org/package=dismo (accessed on 1 April 2019).
- Gilbert, L. Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases? Oecologia 2010, 162, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, X.; Ma, J.; Jiao, Z.; Xiao, J.; Wang, H. Influence of host and environmental factors on the distribution of the Japanese encephalitis vector Culex tritaeniorhynchus in China. Int. J. Environ. Res. Public Health 2018, 15, 1848. [Google Scholar] [CrossRef] [PubMed]
- Gorsevki, P.V.; Gessler, P.; Foltz, R.B. Spatial Prediction of Landslide Hazard Using Discriminant Analysis and GIS. In Proceedings of the Applications for the 21st Century, Denver, CO, USA, 25–27 September 2000. [Google Scholar]
- Bishopp, F.C.; Trembley, H.L. Distribution and hosts of certain North American ticks. J. Parasitol. 1945, 31, 1–54. [Google Scholar] [CrossRef]
- Sonenshine, D.E.; Levy, G.F. The ecology of the lone star tick, Amblyomma americanum (L.), in two contrasting habitats in Virginia (Acarina: Ixodidae). J. Med. Entomol. 1971, 8, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- PRISM Climate Group PRISM Gridded Climate Data: AN81d dataset. 2019. Available online: http://www.prism.oregonstate.edu/ (accessed on 16 May 2019).
- Beck, D.L.; Zavala, J.; Montalvo, E.O.; Quintana, F.G. Meteorological indicators for Amblyomma cajennense and population dynamics in the Tamaulipan Biotic Province in Texas. J. Vector Ecol. 2011, 36, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Neteler, M.; Bowman, M.H.; Landa, M.; Metz, M. GRASS GIS: A multi-purpose open source GIS. Environ. Model. Softw. 2012, 31, 124–130. [Google Scholar] [CrossRef]
- Hofierka, J.; Mitášová, H.; Neteler, M. Chapter 17 Geomorphometry in GRASS GIS. In Developments in Soil Science; Elsevier BV: Amsterdam, The Netherlands, 2009; Volume 33, pp. 387–410. ISBN 978-0-12-374345-9. [Google Scholar]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. Release Date 2016. R Package Version 2.6-7. Available online: https://CRAN.R-project.org/package=raster (accessed on 1 April 2019).
- Phillips, S.; Anderson, R.; Schapire, R. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum Entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Eisen, R.J.; Feirer, S.; Padgett, K.A.; Hahn, M.B.; Monaghan, A.J.; Kramer, V.L.; Lane, R.S.; Kelly, M. Modeling climate suitability of the western blacklegged tick in California. J. Med. Entomol. 2018, 55, 1133–1142. [Google Scholar] [CrossRef]
- Elith, J.H.; Graham, C.P.; Anderson, R.; Dudík, M.; Ferrier, S.; Guisan, A.; J. Hijmans, R.; Huettmann, F.; R. Leathwick, J.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Rodríguez, J.D.; Pérez, A.; Lozano, J.A. Sensitivity analysis of kappa-fold cross validation in prediction error estimation. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 32, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Scientific Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, S.F.; Paddock, C.D.; Springer, Y.P.; Eisen, R.J.; Behravesh, C.B. Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am. J. Trop. Med. Hyg. 2016, 94, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Burks, C.S.; Stewart, R.L.; Needham, G.R.; Lee, R.E. The role of direct chilling injury and inoculative freezing in cold tolerance of Amblyomma americanum, Dermacentor variabilis and Ixodes scapularis. Physiol. Entomol. 1996, 21, 44–50. [Google Scholar] [CrossRef]
- Yoder, J.A.; Tank, J.L. Similarity in critical transition temperature for ticks adapted for different environments: Studies on the water balance of unfed ixodid larvae. Int. J. Acarol. 2006, 32, 323–329. [Google Scholar] [CrossRef]
- Hoch, A.L.; Barker, R.W.; Hair, J.A. Measurement of physical parameters to determine the suitability of modified woodlots as lone star tick habitat. J. Med. Entomol. 1971, 8, 725–730. [Google Scholar] [CrossRef]
- Holden, K.; Boothby, J.T.; Anand, S.; Massung, R.F. Detection of Borrelia burgdorferi, Ehrlichia chaffeensis, and Anaplasma phagocytophilum in ticks (Acari: Ixodidae) from a coastal region of California. J. Med. Entomol. 2003, 40, 534–539. [Google Scholar] [CrossRef]
- California Department of Public Health. Vector-Borne Disease Section Annual Report 2016; California Department of Public Health: Sacramento, CA, USA, 2017; pp. 8–13. [Google Scholar]
- Li, Y.; Wang, C.; Allen, K.E.; Little, S.E.; Ahluwalia, S.K.; Gao, D.; Macintire, D.K.; Blagburn, B.L.; Kaltenboeck, B. Diagnosis of canine Hepatozoon spp. infection by quantitative PCR. Vet. Parasitol. 2008, 157, 50–58. [Google Scholar] [CrossRef]
- Lado, P.; Nava, S.; Mendoza-Uribe, L.; Caceres, A.G.; Delgado-de la Mora, J.; Licona-Enriquez, J.D.; Delgado-de la Mora, D.; Labruna, M.B.; Durden, L.A.; Allerdice, M.E.J.; et al. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae) group of ticks: Phenotypic plasticity or incipient speciation? Parasit. Vectors 2018, 11, 610. [Google Scholar] [CrossRef]
- Guglielmone, A.A.; Estrada-Peña, A.; Keirans, J.E.; Robbins, R.G. Ticks (Acari: Ixodida) of the neotropical zoogeographic region; Universiteit Utrecht, J. Bovy-Verbeek (public relations ICTTD): Universiteit Utrecht: Utrecht, The Netherlands, 2003; ISBN 978-987-43-6828-7. [Google Scholar]
- Estrada-Peña, A.; Venzal, J.M.; Mangold, A.J.; Cafrune, M.M.; Guglielmone, A.A. The Amblyomma maculatum Koch, 1844 (Acari: Ixodidae: Amblyomminae) tick group: Diagnostic characters, description of the larva of A. parvitarsum Neumann, 1901, 16S rDNA sequences, distribution and hosts. Syst. Parasitol. 2005, 60, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Tarragona, E.L.; Vesco, U.; de Meneghi, D.; Mastropaolo, M.; Mangold, A.J.; Guglielmone, A.A.; Nava, S. Divergent environmental preferences and areas of sympatry of tick species in the Amblyomma cajennense complex (Ixodidae). Int. J. Parasitol. 2014, 44, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.K.; da Fonseca, A.H. Distribution, diversity and seasonality of ticks in institutional environments with different human intervention degrees in the State of Rio de Janeiro, Brazil. Braz. J. Vet. Res. Anim. Sci. 2013, 35, 1–12. [Google Scholar]
- Álvarez-Hernández, G.; Roldán, J.F.G.; Milan, N.S.H.; Lash, R.R.; Behravesh, C.B.; Paddock, C.D. Rocky Mountain spotted fever in Mexico: past, present, and future. Lancet Infect. Dis. 2017, 17, e189–e196. [Google Scholar] [CrossRef]
- Tinoco-Gracia, L.; Lomelí, M.R.; Hori-Oshima, S.; Stephenson, N.; Foley, J. Molecular confirmation of Rocky Mountain spotted fever epidemic agent in Mexicali, Mexico. Emerg. Infect. Dis. 2018, 24, 1723–1725. [Google Scholar] [CrossRef] [PubMed]
- Dennison, P.E.; Brewer, S.C.; Arnold, J.D.; Moritz, M.A. Large wildfire trends in the western United States, 1984–2011. Geophys. Res. Lett. 2014, 41, 2928–2933. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Kravitz, B.; Rasch, P.J.; Simon Wang, S.-Y.; Gillies, R.R.; Hipps, L. Extreme fire season in California: A glimpse into the future? Bull. Am. Meteorol. Soc. 2015, 96, S5–S9. [Google Scholar] [CrossRef]
- Beck, D.L.; Orozco, J.P. Diurnal questing behavior of Amblyomma mixtum (Acari: Ixodidae). Exp. Appl. Acarol. 2015, 66, 613–621. [Google Scholar] [CrossRef]
- Su, W.; Yu, D.; Sun, Z.; Zhan, J.; Liu, X.; Luo, Q. Vegetation changes in the agricultural-pastoral areas of northern China from 2001 to 2013. J. Integr. Agric. 2016, 15, 1145–1156. [Google Scholar] [CrossRef]
- Bonthoux, S.; Lefèvre, S.; Herrault, P.-A.; Sheeren, D. Spatial and temporal dependency of NDVI satellite imagery in predicting bird diversity over France. Remote Sensing 2018, 10, 1136. [Google Scholar] [CrossRef]
- Boehnke, D.; Gebhardt, R.; Petney, T.; Norra, S. On the complexity of measuring forests microclimate and interpreting its relevance in habitat ecology: the example of Ixodes ricinus ticks. Parasit. Vectors 2017, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A.; Hung, R.W. Effects of microscale habitat physiognomy on the focal distribution of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. Environ. Entomol. 2002, 31, 1085–1090. [Google Scholar] [CrossRef]
- Shender, L.A.; Botzler, R.G.; George, T.L. Analysis of serum and whole blood values in relation to helminth and ectoparasite infections of feral pigs in Texas. J. Wildl. Dis. 2002, 38, 385–394. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanders, D.M.; Schuster, A.L.; McCardle, P.W.; Strey, O.F.; Blankenship, T.L.; Teel, P.D. Ixodid ticks associated with feral swine in Texas. J. Vector Ecol. 2013, 38, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.B.; Auckland, L.D.; Marra, P.P.; Hamer, S.A. Avian migrants facilitate invasions of neotropical ticks and tick-borne pathogens into the United States. Appl. Environ. Microbiol. 2015, 81, 8366–8378. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.A.; Rausch, B.A.; Hedges, B.Z.; Stueber, S.E.; Jajack, A.J.; Benoit, J.B. The effects of water exposure, soil conditions, and fungus exposure on hatching of the larval lone star tick, Amblyomma americanum (Acari: Ixodidae). Int. J. Acarol. 2012, 38, 344–352. [Google Scholar] [CrossRef]
- Civitello, D.J.; Flory, S.L.; Clay, K. Exotic grass invasion reduces survival of Amblyomma americanum and Dermacentor variabilis ticks (Acari: Ixodidae). J. Med. Entomol. 2008, 45, 867–872. [Google Scholar] [CrossRef]
- Allerdice, M.E.J.; Beati, L.; Yaglom, H.; Lash, R.R.; Delgado-de la Mora, J.; Licona-Enriquez, J.D.; Delgado-de la Mora, D.; Paddock, C.D. Rickettsia parkeri (Rickettsiales: Rickettsiaceae) detected in ticks of the Amblyomma maculatum (Acari: Ixodidae) group collected from multiple locations in southern Arizona. J. Med. Entomol. 2017, 54, 1743–1749. [Google Scholar] [CrossRef]
| Environmental Predictor Variable | A. americanum | A. maculatum | A. cajennense | A. mixtum | ||||
|---|---|---|---|---|---|---|---|---|
| Percent Contribution | Permutation Importance | Percent Contribution | Permutation Importance | Percent Contribution | Permutation Importance | Percent Contribution | Permutation Importance | |
| BIO1 = Annual mean temperature | na | na | na | na | 68.2 | 25.8 | na | na |
| BIO2 = Mean diurnal range (mean of monthly (max temp - min temp)) | 12.8 | 33.3 | na | na | na | na | na | na |
| BIO3 = Isothermality (BIO2/BIO7) (* 100) | na | na | na | na | na | na | na | na |
| BIO4 = Temperature seasonality (standard deviation *100) | na | na | na | na | na | na | na | na |
| BIO5 = Max temperature of warmest month | na | na | na | na | na | na | na | na |
| BIO6 = Min temperature of coldest month | 27.3 | 38.4 | 38.4 | 40.5 | 8.8 | 25.8 | 79.6 | 67.9 |
| BIO7 = Temperature annual range (BIO5 - BIO6) | na | na | na | na | na | na | na | na |
| BIO8 = Mean temperature of wettest quarter | na | na | na | na | na | na | 14.3 | 12.3 |
| BIO9 = Mean temperature of driest quarter | na | na | na | na | na | na | na | na |
| BIO10 = Mean temperature of warmest quarter | na | na | na | na | na | na | na | na |
| BIO11 = Mean temperature of coldest quarter | na | na | na | na | na | na | na | na |
| BIO12 = Annual precipitation | na | na | na | na | na | na | na | na |
| BIO13 = Precipitation of wettest month | na | na | 18.9 | 15.2 | na | na | na | na |
| BIO14 = Precipitation of driest month | 59.9 | 28.3 | na | na | na | na | na | na |
| BIO15 = Precipitation seasonality (coefficient of variation) | na | na | na | na | 12.4 | 21.9 | na | na |
| BIO16 = Precipitation of wettest quarter | na | na | na | na | na | na | na | na |
| BIO17 = Precipitation of driest quarter | na | na | na | na | na | na | na | na |
| BIO18 = Precipitation of warmest quarter | na | na | na | na | na | na | na | na |
| BIO19 = Precipitation of coldest quarter | na | na | na | na | na | na | na | na |
| Elevation | na | na | 30.6 | 29.8 | na | na | 0.7 | 1.0 |
| Slope | na | na | na | na | na | na | 5.3 | 18.8 |
| NDVI average (1985-2000) | na | na | 12.2 | 14.5 | 3.3 | 12.8 | na | na |
| NDVI standard deviation (1985-2000) | na | na | na | na | 7.4 | 13.7 | na | na |
| Average test AUC from 10 iterations (range) | 0.78 (0.69–0.84) | 0.74 (0.66–0.84) | 0.64 (0.55–0.75) | 0.73 (0.56–0.94) | ||||
| Maximum sensitivity at maximum specificity | 0.24 | 0.30 | 0.61 | 0.61 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascoe, E.L.; Marcantonio, M.; Caminade, C.; Foley, J.E. Modeling Potential Habitat for Amblyomma Tick Species in California. Insects 2019, 10, 201. https://doi.org/10.3390/insects10070201
Pascoe EL, Marcantonio M, Caminade C, Foley JE. Modeling Potential Habitat for Amblyomma Tick Species in California. Insects. 2019; 10(7):201. https://doi.org/10.3390/insects10070201
Chicago/Turabian StylePascoe, Emily L., Matteo Marcantonio, Cyril Caminade, and Janet E. Foley. 2019. "Modeling Potential Habitat for Amblyomma Tick Species in California" Insects 10, no. 7: 201. https://doi.org/10.3390/insects10070201
APA StylePascoe, E. L., Marcantonio, M., Caminade, C., & Foley, J. E. (2019). Modeling Potential Habitat for Amblyomma Tick Species in California. Insects, 10(7), 201. https://doi.org/10.3390/insects10070201






