Reduced Glutamine Synthetase Activity Alters the Fecundity of Female Bactrocera dorsalis (Hendel)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Gene Expression in Adults
2.3. RNAi Bioassay
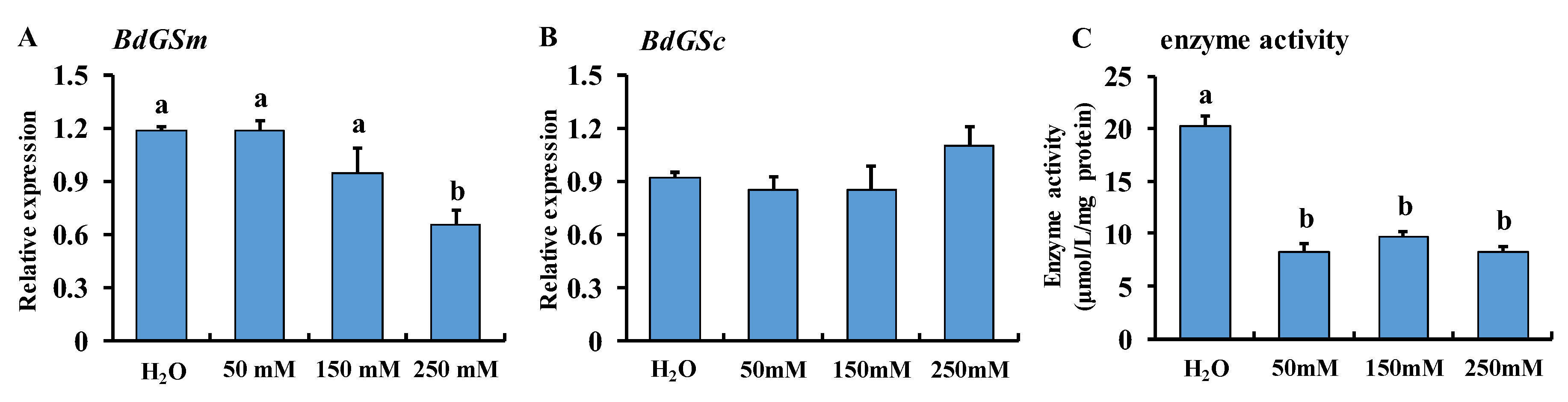
2.4. The MSX Bioassay
2.5. Statistical Analysis
3. Results
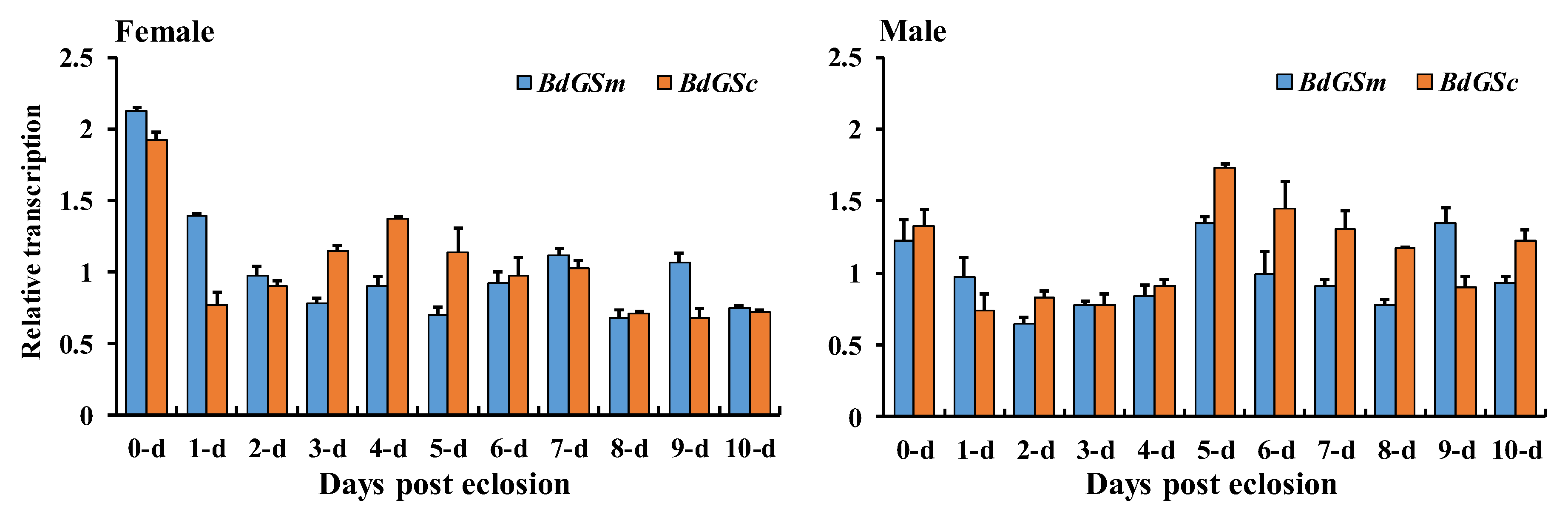
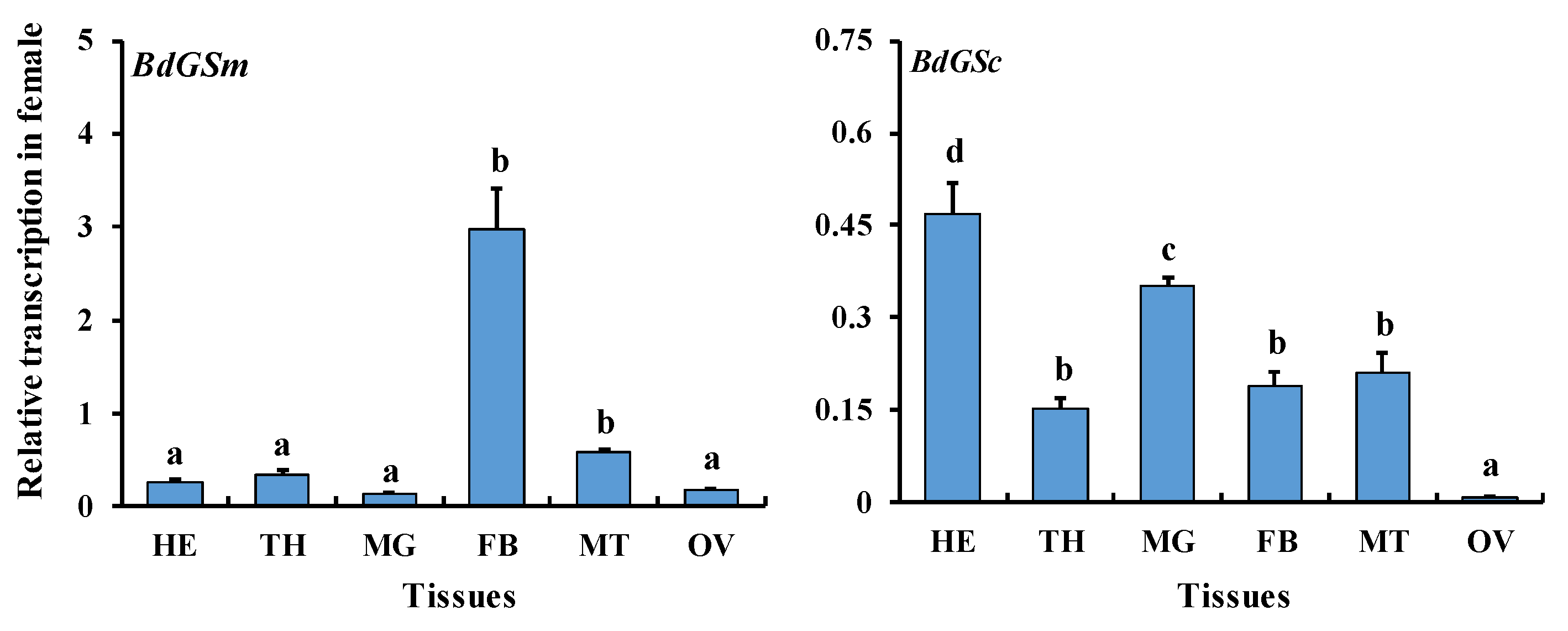
3.1. Gene Expression during Sex Maturation
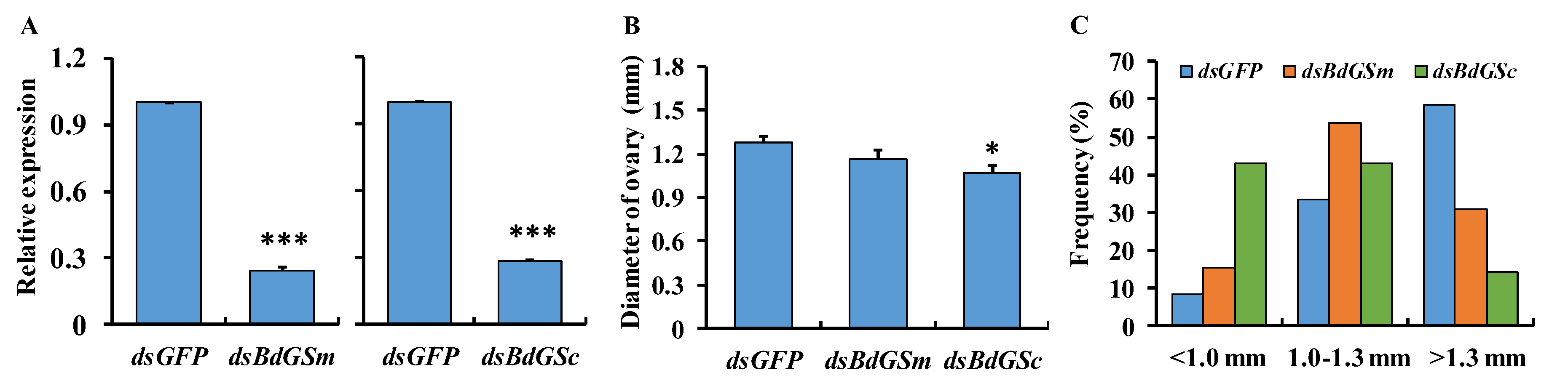
3.2. RNAi Bioassay
3.3. MSX Feeding
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lai, X.F.; Gao, H.; Kong, J.; Wang, Q.Y.; Wang, W.J.; Meng, X.H. Cloning and characterization of the glutamine synthetase gene from Chinese shrimp Fenneropenaeus chinensis. Aquacult. Int. 2011, 19, 873–889. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, Y.Z.; Yan, Y.; Xu, B.H.; Guo, X.Q. Identification and abiotic stress response of a glutamine synthetase gene (AccGS) from the Asiatic honeybee, Apis cerana cerana (Hymenoptera: Apidae). Eur. J. Entomol. 2014, 111, 1–9. [Google Scholar] [CrossRef]
- Eelen, G.; Dubois, C.; Cantelmo, A.R.; Goveia, J.; Bruning, U.; DeRan, M.; Jarugumilli, G.; van Rijssel, J.; Saladino, G.; Comitani, F.; et al. Role of glutamine synthetase in angiogenesis beyond glutamine synthesis. Nature 2018, 561, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Isu, S.; Kaneko, G.; Yamada, H.; Hara, T.; Itoh, Y.; Watabe, S. The occurrence of eukaryotic type III glutamine synthetase in the marine diatom Chaetoceros compressum. Marine Genomics 2009, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Caggese, C.; Barsanti, P.; Viggiano, L.; Bozzetti, M.P.; Caizzi, R. Genetic, molecular and developmental analysis of the glutamine synthetase isozymes of Drosophila melanogaster. Genetica 1994, 94, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Caggese, C.; Caizzi, R.; Barsanti, P.; Bozzetti, M.P. Mutations in the glutamine synthetase I (gsI) gene produce embryo-lethal female sterility in Drosophila melanogaster. Dev. Genet. 1992, 13, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Frenz, L.M.; Glover, D.M. A maternal requirement for glutamine synthetase I for the mitotic cycles of syncytial Drosophila embryos. J. Cell Sci. 1996, 109, 2649–2660. [Google Scholar]
- Smartt, C.T.; Kiley, L.M.; Hillyer, J.F.; Dasgupta, R.; Christensen, B.M. Aedes aegypti glutamine synthetase: Expression and gene structure. Gene 2001, 274, 35–45. [Google Scholar] [CrossRef]
- Smartt, C.T.; Chiles, J.; Lowenberger, C.; Christensen, B.M. Biochemical analysis of a blood meal-induced Aedes aegypti glutamine synthetase gene. Insect Biochem. Mol. Biol. 1998, 28, 935–945. [Google Scholar] [CrossRef]
- Hansen, A.K.; Moran, N.A. Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proc. Natl. Acad. Sci. USA 2011, 108, 2849–2854. [Google Scholar] [CrossRef]
- Fu, X.; Li, T.; Chen, J.; Dong, Y.; Qiu, J.; Kang, K.; Zhang, W. Functional screen for microRNAs of Nilaparvata lugens reveals that targeting of glutamine synthase by miR-4868b regulates fecundity. J. Insect Physiol. 2015, 83, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Sun, Z.; Zhang, J.; Kang, K.; Chen, J.; Zhang, W. Activation of the TOR signalling pathway by glutamine regulates insect fecundity. Sci. Rep. 2015, 5, 10694. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Armstrong, K.F.; Carmichael, A.E.; Milne, J.R.; Raghu, S.; Roderick, G.K.; Yeates, D.K. Invasive phytophagous pests arising through a recent tropical evolutionary radiation: The Bactrocera dorsalis complex of fruit flies. Annu. Rev. Entomol. 2005, 50, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Feng, Y.C.; Wei, D.D.; Yuan, G.R.; Dou, W.; Wang, J.J. Female remating inhibition and fitness of Bactrocera dorsalis (Diptera: Tephritidae) associated with male accessory glands. Fla. Entomol. 2015, 98, 52–58. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Wei, D.; Li, R.; Jia, H.T.; Liu, Y.W.; Taning, C.N.T.; Wang, J.J.; Smagghe, G. Cytoplasmic glutamine synthetase gene expression regulates larval development in Bactrocera dorsalis (Hendel). Arch. Insect Biochem. Physiol. 2018, 97, e21447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Wei, D.; Dou, W.; Hu, F.; Liu, W.F.; Wang, J.J. Toxicities and synergistic effects of several insecticides against the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, H.M.; Yang, W.J.; Wei, D.D.; Dou, W.; Huang, Y.; Wang, J.J. Transcriptome profiling of the testis reveals genes involved in spermatogenesis and marker discovery in the oriental fruit fly, Bactrocera dorsalis. Insect Mol. Biol. 2015, 24, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Jia, H.T.; Zhang, M.Y.; Li, R.; Smagghe, G.; Wang, J.J. Comparative analysis of differential gene expression profiling of sex-bias fat body of Bactrocera dorsalis (Diptera: Tephritidae) identifying a new vitellogenin gene. Ann. Entomol. Soc. Am. 2018, 111, 43–54. [Google Scholar] [CrossRef]
- Shen, G.M.; Jiang, H.B.; Wang, X.N.; Wang, J.J. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol. Biol. 2010, 11, 76. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△Ct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Yang, W.J.; Jiang, X.Z.; Niu, J.Z.; Shen, G.M.; Ran, C.; Wang, J.J. The essential role of vitellogenin receptor in ovary development and vitellogenin uptake in Bactrocera dorsalis (Hendel). Int. J. Mol. Sci. 2015, 16, 18368–18383. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Li, R.; Zhang, M.Y.; Liu, Y.W.; Zhang, Z.; Smagghe, G.; Wang, J.J. Comparative proteomic profiling reveals molecular characteristics associated with oogenesis and oocyte maturation during ovarian development of Bactrocera dorsalis (Hendel). Int. J. Mol. Sci. 2017, 18, 1379. [Google Scholar] [CrossRef] [PubMed]
- Scaraffia, P.Y.; Zhang, Q.; Thorson, K.; Wysocki, V.H.; Miesfeld, R.L. Differential ammonia metabolism in Aedes aegypti fat body and midgut tissues. J. Insect Physiol. 2010, 56, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Youji, H.; Hakvoort, T.B.M.; Vermeulen, J.L.M.; Labruyère, W.T.; D Rudi, D.W.; W Saskia, V.D.H.; Ruijter, J.M.; Uylings, H.B.M.; Lamers, W.H. Glutamine synthetase deficiency in murine astrocytes results in neonatal death. Glia 2010, 58, 741–754. [Google Scholar]
- Avisar, N.; Shiftan, L.; Ben-Dror, I.; Havazelet, N.; Vardimon, L. A silencer element in the regulatory region of glutamine synthetase controls cell type-specific repression of gene induction by glucocorticoids. J. Biol. Chem. 1999, 274, 11399–11407. [Google Scholar] [CrossRef]
- Boyan, G.; Williams, L. Embryonic development of the insect central complex: insights from lineages in the grasshopper and Drosophila. Arthropod Struct. Dev. 2011, 40, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Lin, C.P.; Lu, K.H. cDNA isolation, expression, and hormonal regulation of yolk protein genes in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). J. Insect Physiol. 2012, 58, 763–770. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, J.; Sun, Z.; Dong, X.; He, Y.; Kang, K.; Liu, Z.; Zhang, W. Proteomic and transcriptomic analyses of fecundity in the brown planthopper Nilaparvata lugens (Stål). J. Proteome Res. 2013, 12, 5199–5212. [Google Scholar] [CrossRef]
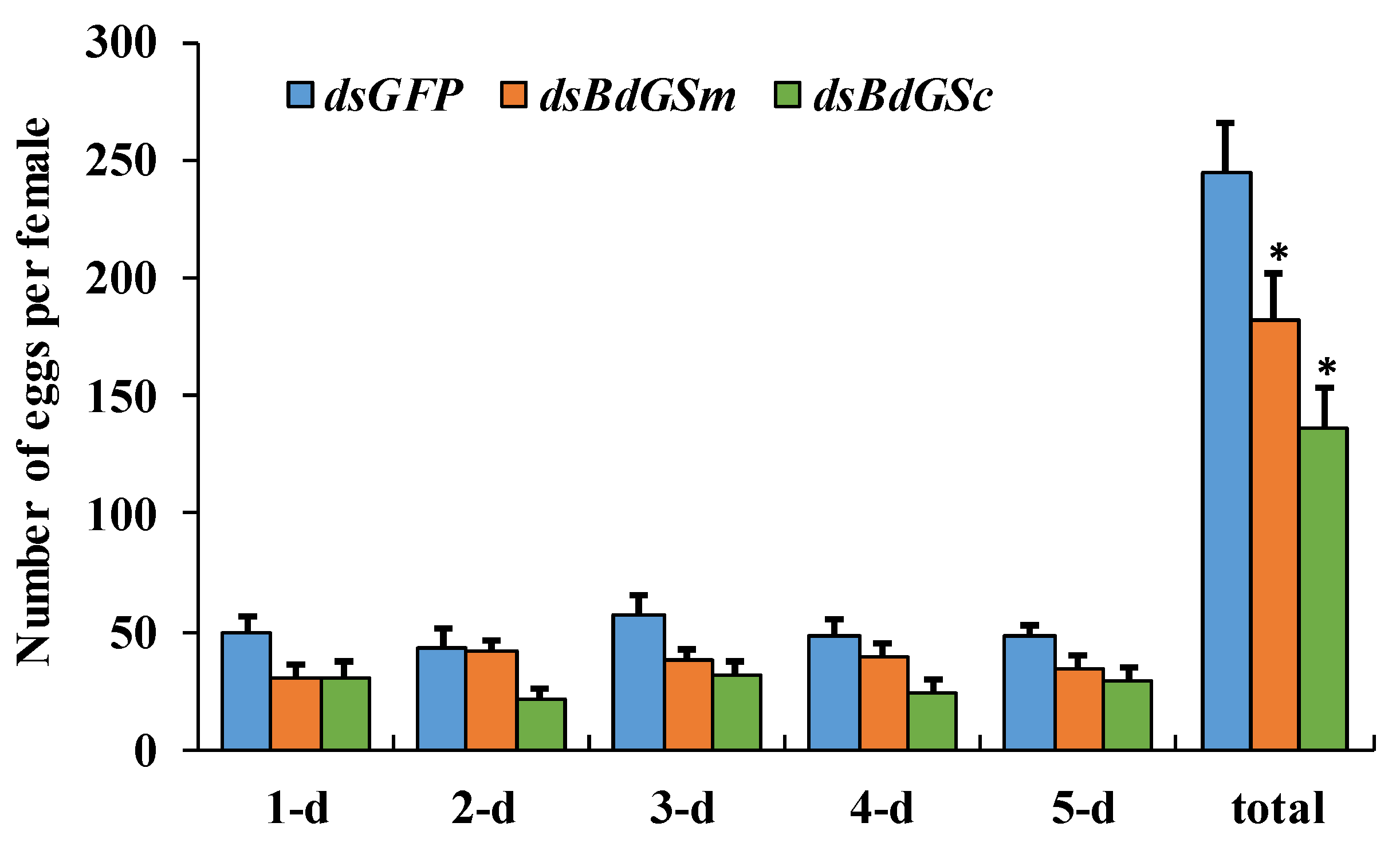

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Zhang, M.-Y.; Zhang, Y.-X.; Zhang, S.-Y.; Smagghe, G.; Wang, J.-J. Reduced Glutamine Synthetase Activity Alters the Fecundity of Female Bactrocera dorsalis (Hendel). Insects 2019, 10, 186. https://doi.org/10.3390/insects10070186
Wei D, Zhang M-Y, Zhang Y-X, Zhang S-Y, Smagghe G, Wang J-J. Reduced Glutamine Synthetase Activity Alters the Fecundity of Female Bactrocera dorsalis (Hendel). Insects. 2019; 10(7):186. https://doi.org/10.3390/insects10070186
Chicago/Turabian StyleWei, Dong, Meng-Yi Zhang, Ying-Xin Zhang, Su-Yun Zhang, Guy Smagghe, and Jin-Jun Wang. 2019. "Reduced Glutamine Synthetase Activity Alters the Fecundity of Female Bactrocera dorsalis (Hendel)" Insects 10, no. 7: 186. https://doi.org/10.3390/insects10070186
APA StyleWei, D., Zhang, M.-Y., Zhang, Y.-X., Zhang, S.-Y., Smagghe, G., & Wang, J.-J. (2019). Reduced Glutamine Synthetase Activity Alters the Fecundity of Female Bactrocera dorsalis (Hendel). Insects, 10(7), 186. https://doi.org/10.3390/insects10070186






