Mosquitoes of Etiological Concern in Kenya and Possible Control Strategies
Abstract
1. Introduction
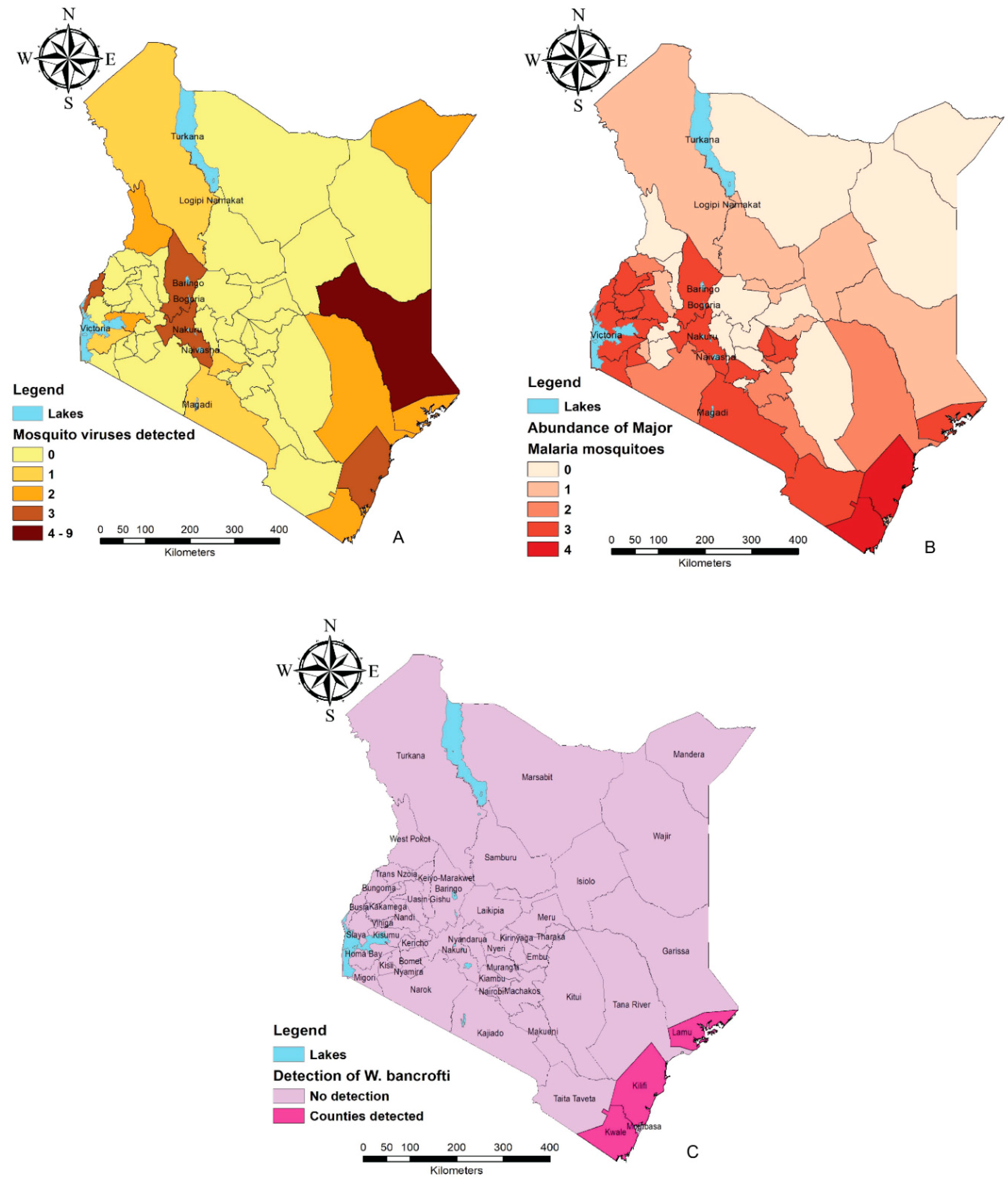
2. Mosquito-Borne Disease Endemic Regions in Kenya
3. Overview of the Main Mosquito Genera of Public Health Importance in Kenya and Their Geographical Distribution
3.1. Aedes
3.2. Anopheles
3.3. Culex
3.4. Mansonia
4. Mosquito Control Strategies in Kenya
4.1. What Has Been Done
4.1.1. Entomologic Surveillance
4.1.2. Use of Synthetic Pesticides
4.1.3. Insect Growth Regulators (IGRs)
4.2. What More Needs to Be Done?
4.2.1. Public Awareness on Environmental Management
4.2.2. Embracing Biological Controls
Use of Natural Predators
Large-Scale Applications of Entomopathogens
Incompatible Insect Technique (IIT)
4.2.3. Genetically Engineered Mosquitoes
The Sterile Insect Technique (SIT)
Release of Insects Carrying Dominant Lethality (RIDL)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Vector Control Response 2017–2030. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/259205/9789241512978-eng.pdf;jsessionid=9C11656832A16B1404639714E44BF08B?sequence=1 (accessed on 4 March 2019).
- Braack, L.; Gouveia De Almeida, A.P.; Cornel, A.J.; Swanepoel, R.; De Jager, C. Mosquito-borne arboviruses of African origin: Review of key viruses and vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Lymphatic Filariasis: Epidemiology and Risk Factors. 2019. Available online: https://www.cdc.gov/parasites/lymphaticfilariasis/epi.html (accessed on 4 March 2019).
- Ochieng, C.; Lutomiah, J.; Makio, A.; Koka, H.; Chepkorir, E.; Yalwala, S.; Mutisya, J.; Musila, L.; Khamadi, S.; Richardson, J.; et al. Mosquito-borne arbovirus surveillance at selected sites in diverse ecological zones of Kenya; 2007–2012. Virol. J. 2013, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Altizer, S.; Dobson, A.; Hosseini, P.; Hudson, P.; Pascual, M.; Rohani, P. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 2006, 9, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Patz, J.A.; Githeko, A.K.; McCarty, J.P.; Hussein, S.; Confalonieri, U.; DeWet, N. Climate Change and Infectious Diseases. World Health Organization, 2003. Available online: https://www.who.int/globalchange/publications/climatechangechap6.pdf (accessed on 4 March 2019).
- Shi, Z. Impact of Climate Change on the Global Environment and Associated Human Health. Sci. Res. 2018, 5, e4934. [Google Scholar] [CrossRef]
- Himeidan, Y.E.; Kweka, E.J. Malaria in East African highlands during the past 30 years: Impact of environmental changes. Front. Physiol. 2012, 3, 315. [Google Scholar] [CrossRef] [PubMed]
- Owino, E.A. Aedes spp. mosquitoes and emerging neglected diseases of Kenya. Int. J. Mosq. Res. 2018, 5, 1–11. [Google Scholar]
- Woods, C.W.; Karpati, A.M.; Grein, T.; McCarthy, N.; Gaturuku, P.; Muchiri, E.; Dunster, L.; Henderson, A.; Khan, A.S.; Al, R.S. An Outbreak of Rift Valley Fever in Northeastern Kenya, 1997–98. Emerg. Infect. Dis. 2002, 8, 138–144. [Google Scholar] [CrossRef]
- Sang, R.; Kioko, E.; Lutomiah, J.; Warigia, M.; Ochieng, C.; O’Guinn, M.; Lee, J.S.; Koka, H.; Godsey, M.; Hoel, D.; et al. Rift Valley fever virus epidemic in Kenya, 2006/2007: The entomologic investigations. Am. J. Trop. Med. Hyg. 2010, 83, 28–37. [Google Scholar] [CrossRef]
- Lutomiah, J.; Barrera, R.; Makio, A.; Mutisya, J.; Koka, H.; Owaka, S.; Koskei, E.; Nyunja, A.; Eyase, F.; Coldren, R.; et al. Dengue Outbreak in Mombasa City, Kenya, 2013–2014: Entomologic Investigations. PLoS Negl. Trop. Dis. 2016, 10, e0004981. [Google Scholar] [CrossRef]
- World Health Organization. Chikungunya—Mombasa, Kenya. 2018. Available online: https://www.who.int/csr/don/27-february-2018-chikungunya-kenya/en/ (accessed on 10 March 2019).
- Mwangangi, J.M.; Midega, J.; Kahindi, S.; Njoroge, L.; Nzovu, J.; Githure, J.; Mbogo, C.M.; Beier, J.C. Mosquito species abundance and diversity in Malindi, Kenya and their potential implication in pathogen transmission. Parasitol. Res. 2012, 110, 61–71. [Google Scholar] [CrossRef]
- Ogola, E.O.; Odero, J.O.; Mwangangi, J.M.; Masiga, D.K.; Tchouassi, D.P. Population genetics of Anopheles funestus, the African malaria vector, Kenya. Parasites Vectors 2019, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Atoni, E.; Wang, Y.; Karungu, S.; Waruhiu, C.; Zohaib, A.; Obanda, V.; Agwanda, B.; Mutua, M.; Xia, H.; Yuan, Z. Metagenomic virome analysis of Culex mosquitoes from Kenya and China. Viruses 2018, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, N.; Dida, G.O.; Sonye, G.O.; Futami, K.; Njenga, S.M. Malaria vectors in Lake Victoria and adjacent habitats in western Kenya. PLoS ONE 2012, 7, e32725. [Google Scholar] [CrossRef] [PubMed]
- Ajamma, Y.K.; Villinger, J.; Omondi, D.; Salifu, D.; Onchuru, T.O.; Njoroge, L.; Muigai, A.W.T.; Masiga, D.K. Composition and genetic diversity of mosquitoes (Diptera: Culicidae) on Islands and mainland shores of Kenya’s lakes Victoria and baringo. J. Med. Entomol. 2016, 53, 1348–1363. [Google Scholar] [CrossRef] [PubMed]
- Ondiba, I.M.; Oyieke, F.A.; Nyamongo, I.K.; Estambale, B.B. Diversity, distribution and abundance of potential rift valley fever vectors in Baringo County, Kenya. Int. J. Mosq. Res. 2017, 4, 42–48. [Google Scholar]
- Ajamma, Y.U.; Onchuru, T.O.; Ouso, D.O.; Omondi, D.; Masiga, D.K.; Villinger, J. Vertical transmission of naturally occurring Bunyamwera and insect-specific flavivirus infections in mosquitoes from islands and mainland shores of Lakes Victoria and Baringo in Kenya. PLoS Negl. Trop. Dis. 2018, 12, e0006949. [Google Scholar] [CrossRef] [PubMed]
- Ofulla, A.V.O.; Karanja, D.; Omondi, R.; Okurut, T.; Matano, A.; Jembe, T.; Abila, R.; Boera, P.; Gichuki, J. Relative abundance of mosquitoes and snails associated with water hyacinth and hippo grass in the Nyanza gulf of Lake Victoria. Lakes Reserv. Res. Manag. 2010, 15, 225–271. [Google Scholar] [CrossRef]
- Lutomiah, J.; Bast, J.; Clark, J.; Richardson, J.; Yalwala, S.; Oullo, D.; Mutisya, J.; Mulwa, F.; Musila, L.; Khamadi, S.; et al. Abundance, diversity, and distribution of mosquito vectors in selected ecological regions of Kenya: Public health implications. J. Vector Ecol. 2013, 38, 134–142. [Google Scholar] [CrossRef]
- Turell, M.J.; Lee, J.S.; Richardson, J.H.; Sang, R.C.; Kioko, E.N.; Agawo, M.O.; Pecor, J.; O’guinn, M.L. Vector Competence of Kenyan Culex zombaensis And Culex quinquefasciatus mosquitoes for Rift Valley Fever Virus. J. Am. Mosq. Control Assoc. 2007, 23, 378–382. [Google Scholar] [CrossRef]
- Britch, S.C.; Binepal, Y.S.; Ruder, M.G.; Kariithi, H.M.; Linthicum, K.J.; Anyamba, A.; Small, J.L.; Tucker, C.J.; Ateya, L.O.; Oriko, A.A.; et al. Rift Valley Fever Risk Map Model and Seroprevalence in Selected Wild Ungulates and Camels from Kenya. PLoS ONE 2013, 8, e66626. [Google Scholar] [CrossRef]
- Tuno, N.; Okeka, W.; Minakawa, N.; Takagi, M.; Yan, G. Survivorship of Anopheles gambiae sensu stricto (Diptera: Culicidae) Larvae in Western Kenya Highland Forest. J. Med. Entomol. 2005, 42, 270–277. [Google Scholar] [CrossRef]
- Doucoure, S.; Drame, P.M. Salivary biomarkers in the control of mosquito-borne diseases. Insects 2015, 6, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Iwashita, H.; Higa, Y.; Futami, K.; Lutiali, P.A.; Njenga, S.M.; Nabeshima, T.; Minakawa, N. Mosquito arbovirus survey in selected areas of Kenya: Detection of insect-specific virus. Trop. Med. Health 2018, 46. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.; Roque, V.G.; Brett, J.; Dizon, R.; L’Azou, M. Epidemiology of dengue disease in the Philippines (2000–2011): A systematic literature review. PLoS Negl. Trop. Dis. 2014, 8, e3027. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Rodriguez-Morales, A.J.; Levi, J.E.; Cao-Lormeau, V.M.; Gubler, D.J. Unexpected outbreaks of arbovirus infections: Lessons learned from the Pacific and tropical America. Lancet Infect. Dis. 2018, 18, e355–e361. [Google Scholar] [CrossRef]
- Carrington, L.B.; Simmons, C.P. Human to mosquito transmission of dengue viruses. Front. Immunol. 2014, 5, 290. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.M.; Mackay, A.J.; Santiago, G.A.; Hunsperger, E.; Nilles, E.J.; Perez-Padilla, J.; Tikomaidraubuta, K.S.; Colon, C.; Amador, M.; Chen, T.; et al. Characteristics of a dengue outbreak in a remote Pacific Island chain—Republic of the Marshall Islands, 2011–2012. PLoS ONE 2014, 9, e108445. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.N.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Bortel, W.V.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. elife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Ngala, J.C.; Schmidt Chanasit, J. Entomological Coinfections of Arboviruses Dengue and Chikungunya Viruses along the Coastline of Kenya. J. Mosq. Res. 2018, 8, 1. [Google Scholar] [CrossRef]
- Arum, S.O.; Weldon, C.W.; Orindi, B.; Landmann, T.; Tchouassi, D.P.; Affognon, H.D.; Sang, R. Distribution and diversity of the vectors of Rift Valley fever along the livestock movement routes in the northeastern and coastal regions of Kenya. Parasites Vectors 2015, 8, 294. [Google Scholar] [CrossRef]
- Tchouassi, D.P.; Bastos, A.D.S.; Sole, C.L.; Diallo, M.; Lutomiah, J.; Mutisya, J.; Mulwa, F.; Borgemeister, C.; Sang, R.; Torto, B.; et al. Population Genetics of Two Key Mosquito Vectors of Rift Valley Fever Virus Reveals New Insights into the Changing Disease Outbreak Patterns in Kenya. PLoS Negl. Trop. Dis. 2014, 8, e3364. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, M.; Sang, R.; Lutomiah, L.; Richardson, J.; Miller, B. Arbovirus surveillance of mosquitoes collected at sites of active Rift Valley fever virus transmission: Kenya, 2006–2007. J. Med. Entomol. 2009, 46, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Sigei, F.; Nindo, F.; Mukunzi, S.; Ng’ang’a, Z.; Sang, R. Evolutionary analyses of Sindbis virus strains isolated from mosquitoes in Kenya. Arch. Virol. 2018, 163, 2465–2469. [Google Scholar] [CrossRef] [PubMed]
- Lutomiah, J.; Ongus, J.; Linthicum, K.J.; Sang, R. Natural Vertical Transmission of Ndumu Virus in Culex pipiens (Diptera: Culicidae) Mosquitoes Collected as Larvae. J. Med. Entomol. 2014, 51, 1091–1095. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sang, R.; Lutomiah, J.; Said, M.; Makio, A.; Koka, H.; Koskei, E.; Nyunja, A.; Owaka, S.; Matoke-Muhia, D.; Bukachi, S.; et al. Effects of irrigation and rainfall on the population dynamics of rift valley fever and other arbovirus mosquito vectors in the epidemic-prone Tana-River County, Kenya. J. Med. Entomol. 2017, 54, 460–470. [Google Scholar] [CrossRef]
- Lutomiah, J.; Omondi, D.; Masiga, M.; Mutai, C.; Mireji, P.O.; Ongus, J.; Linthicum, K.J.; Sang, R. Blood Meal Analysis and Virus Detection in Blood-Fed Mosquitoes Collected During the 2006–2007 Rift Valley Fever Outbreak in Kenya. Vector-Borne Zoonotic Dis. 2014, 14, 656–664. [Google Scholar] [CrossRef]
- Sanders, E.J.; Marfin, A.A.; Tukei, P.M.; Kuria, G.; Ademba, G.; Agata, N.N.; Ouma, J.O.; Cropp, C.B.; Karabatsos, N.; Reiter, P.; et al. First recorded outbreak of yellow fever in Kenya, 1992–1993. II. Entomologic investigations. Am. J. Trop. Med. Hyg. 1998, 59, 644–649. [Google Scholar] [CrossRef]
- Ellis, B.R.; Sang, R.C.; Horne, K.M.; Higgs, S.; Wesson, D.M. Yellow fever virus susceptibility of two mosquito vectors from Kenya, East Africa. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 387–389. [Google Scholar] [CrossRef]
- Mulwa, F.; Lutomiah, J.; Chepkorir, E.; Okello, S.; Eyase, F.; Tigoi, C.; Kahato, M.; Sang, R. Vector competence of Aedes bromeliae and Aedes vitattus mosquito populations from Kenya for chikungunya virus. PLoS Negl. Trop. Dis. 2018, 12, e0006746. [Google Scholar] [CrossRef]
- Sang, R.; Arum, S.; Chepkorir, E.; Mosomtai, G.; Tigoi, C.; Sigei, F.; Lwande, O.W.; Landmann, T.; Affognon, H.; Ahlm, C.; et al. Distribution and abundance of key vectors of Rift Valley fever and other arboviruses in two ecologically distinct counties in Kenya. PLoS Negl. Trop. Dis. 2017, 11, e0005341. [Google Scholar] [CrossRef]
- Powell, J.; Tabachnick, W. History of domestication and spread of Aedes aegypti—A Review. Memórias do Instituto Oswaldo Cruz 2013, 108, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Yalwala, S.; Clark, J.; Oullo, D.; Ngonga, D.; Abuom, D.; Wanja, E.; Bast, J. Comparative efficacy of existing surveillance tools for Aedes aegypti in Western Kenya. J. Vector Ecol. 2015, 40, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ndenga, B.A.; Mutuku, F.M.; Ngugi, H.N.; Mbakaya, J.O.; Aswani, P.; Musunzaji, P.S.; Vulule, J.; Mukoko, D.; Kitron, U.; LaBeaud, A.D. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS ONE 2017, 12, e0189971. [Google Scholar] [CrossRef]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Monath, T. Dengue: The risk to developed and developing countries. Proc. Natl. Acad. Sci. USA 1994, 91, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Ngugi, H.N.; Mutuku, F.M.; Ndenga, B.A.; Musunzaji, P.S.; Mbakaya, J.O.; Aswani, P.; Irungu, L.W.; Mukoko, D.; Vulule, J.; Kitron, U.; et al. Characterization and productivity profiles of Aedes aegypti (L.) breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasites Vectors 2017, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Musoke, S.; Ocheng, D.; Gichogo, A.; Rees, P. Dengue-2 Virus in Kenya. Lancet 1982, 320, 208–209. [Google Scholar] [CrossRef]
- Obonyo, M.; Fidhow, A.; Ofula, V. Investigation of laboratory confirmed dengue outbreak in north-eastern Kenya, 2011. PLoS ONE 2018, 13, e0198556. [Google Scholar] [CrossRef]
- Voice of Africa. Kenya Health Officials Issue Alert Over Dengue Fever Outbreak. 2017. Available online: https://www.voanews.com/a/kenya-health-officials-alert-dengue-fever-outbreak/3843040.html (accessed on 25 March 2019).
- World Health Organization. Rift Valley fever—Kenya. 2018. Available online: https://www.who.int/csr/don/18-june-2018-rift-valley-fever-kenya/en/ (accessed on 25 March 2019).
- Sergon, K.; Njuguna, C.; Kalani, R.; Ofula, V.; Onyango, C.; Konongoi, L.S.; Bedno, S.; Burke, H.; Dumilla, A.M.; Konde, J.; et al. Seroprevalence of Chikungunya Virus (CHIKV) Infection on Lamu Island, Kenya, October 2004. Am. J. Trop. Med. Hyg. 2008, 78, 333–337. [Google Scholar] [CrossRef]
- Berry, I.M.; Eyase, F.; Pollett, S.; Konongoi, S.L.; Joyce, M.G.; Figueroa, K.; Ofula, V.; Koka, H.; Koskei, E.; Nyunja, A.; et al. Global Outbreaks and Origins of a Chikungunya Virus Variant Carrying Mutations Which May Increase Fitness for Aedes aegypti: Revelations from the 2016 Mandera, Kenya Outbreak. Am. J. Trop. Med. Hyg. 2019, 100, 1249–1257. [Google Scholar] [CrossRef]
- Konongoi, S.L.; Ofula, V.; Nyunja, A.; Owaka, S.; Koka, H.; Makio, A.; Koskei, E.; Eyase, F.; Langat, D.; Schoepp, R.J.; et al. Detection of dengue virus serotypes 1, 2 and 3 in selected regions of Kenya: 2011–2014. Virol. J. 2016, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- Konongoi, S.L.; Nyunja, A.; Ofula, V.; Owaka, S.; Koka, H.; Koskei, E.; Eyase, F.; Langat, D.; Mancuso, J.; Lutomiah, J.; et al. Human and entomologic investigations of chikungunya outbreak in Mandera, Northeastern Kenya, 2016. PLoS ONE 2018, 13, e0205058. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.K.; Gichogo, A.; Gitau, G.; Patel, N.; Ademba, G.; Kirui, R.; Highton, R.B.; Smith, D.H. Recovery of o’nyong-nyong virus from Anopheles funestus in Western Kenya. Trans. R. Soc. Trop. Med. Hyg. 1981, 75, 239–241. [Google Scholar] [CrossRef]
- LaBeaud, A.D.; Sutherland, L.J.; Muiruri, S.; Muchiri, E.M.; Gray, L.R.; Zimmerman, P.A.; Hise, A.G.; King, C.H. Arbovirus prevalence in mosquitoes, Kenya. Emerg. Infect. Dis. 2011, 17, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.R.; Nasci, R.S.; Godsey, M.S.; Savage, H.M.; Lutwama, J.J.; Lanciotti, R.S.; Peters, C.J. First field evidence for natural vertical transmission of West Nile virus in Culex univittatus complex mosquitoes from Rift Valley province, Kenya. Am. J. Trop. Med. Hyg. 2000, 62, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Logan, T.M.; Linthicum, K.J.; Ksiazek, T.G. Isolation of Rift Valley fever virus from mosquitoes collected during an outbreak in domestic animals in Kenya. J. Med. Entomol. 1992, 28, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Anopheles Mosquitoes. 2015. Available online: https://www.cdc.gov/malaria/about/biology/index.html#tabs-1-5 (accessed on 25 March 2019).
- Harbach, R.E. The Culicidae (Diptera): A review of taxonomy, classification and phylogeny. Zootaxa 2007, 1668, 591–638. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report at Glance. 2018. Available online: https://www.who.int/malaria/media/world-malaria-report-2018/en/ (accessed on 25 March 2019).
- Afrane, Y.A.; Bonizzoni, M.; Yan, G. Secondary Malaria Vectors of Sub-Saharan Africa: Threat to Malaria Elimination on the Continent? IntechOpen 2016, 20, 473–490. [Google Scholar] [CrossRef]
- Mouchet, J.; Carnevale, P.; Coosemans, M.; Julvez, J.; Manguin, S.; Richard-Lenoble, D.; Sircoulon, J. Biodiversity of Malaria Worldwide; John Libbey Eurotext: Montrouge, France, 2001. [Google Scholar]
- Coetzee, M.; Hunt, R.H.; Wilkerson, R.; Della Torre, A.; Coulibaly, M.B.; Besansky, N.J. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 2013, 3619, 246–274. [Google Scholar] [CrossRef]
- Kibret, S.; Wilson, G.G.; Tekie, H.; Petros, B. Increased malaria transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malar. J. 2014, 13, 360. [Google Scholar] [CrossRef]
- Lobo, N.F.; St Laurent, B.; Sikaala, C.H.; Hamainza, B.; Chanda, J.; Chinula, D.; Krishnankutty, S.M.; Mueller, J.D.; Deason, N.A.; Hoang, Q.T.; et al. Unexpected diversity of Anopheles species in Eastern Zambia: Implications for evaluating vector behavior and interventions using molecular tools. Sci. Rep. 2015, 5, 17952. [Google Scholar] [CrossRef] [PubMed]
- Okiro, E.A.; Alegana, V.A.; Noor, A.M.; Snow, R.W. Changing malaria intervention coverage, transmission and hospitalization in Kenya. Malar. J. 2010, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. CDC Activities in Kenya. 2018. Available online: https://www.cdc.gov/malaria/malaria_worldwide/cdc_activities/kenya.html (accessed on 25 March 2019).
- Macharia, P.M.; Giorgi, E.; Noor, A.M.; Waqo, E.; Kiptui4, R.; Okiro, E.A.; Snow, R.W. Spatio-temporal analysis of Plasmodium falciparum prevalence to understand the past and chart the future of malaria control in Kenya. Malar. J. 2018, 17, 340. [Google Scholar] [CrossRef] [PubMed]
- Okara, R.M.; Sinka, M.E.; Minakawa, N.; Mbogo, C.M.; Hay, S.I.; Snow, R.W. Distribution of the main malaria vectors in Kenya. Malar. J. 2010, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Olanga, E.A.; Okombo, L.; Irungu, L.W.; Mukabana, W.R. Parasites and vectors of malaria on Rusinga Island, Western Kenya. Parasites Vectors 2015, 8, 250. [Google Scholar] [CrossRef]
- Ototo, E.N.; Mbugi, J.P.; Wanjala, C.L.; Zhou, G.; Githeko, A.K.; Yan, G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar. J. 2015, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Ochomo, E.; Bayoh, N.M.; Kamau, L.; Atieli, F.; Vulule, J.; Ouma, C.; Ombok, M.; Njagi, K.; Soti, D.; Mathenge, E.; et al. Pyrethroid susceptibility of malaria vectors in four Districts of western Kenya. Parasites Vectors 2014, 7, 310. [Google Scholar] [CrossRef]
- Kipyab, P.C.; Khaemba, B.M.; Mwangangi, J.M.; Mbogo, C.M. The physicochemical and environmental factors affecting the distribution of Anopheles merus along the Kenyan coast. Parasites Vectors 2015, 8, 221. [Google Scholar] [CrossRef]
- Mukiama, T.K.; Mwangi, R.W. Seasonal population changes and malaria transmission potential of Anopheles pharoensis and the minor anophelines in Mwea Irrigation Scheme, Kenya. Acta Trop. 1989, 4, 181–189. [Google Scholar] [CrossRef]
- Degefa, T.; Yewhalaw, D.; Zhou, G.; Lee, M.; Atieli, H.; Githeko, A.K.; Yan, G. Indoor and outdoor malaria vector surveillance in western Kenya: Implications for better understanding of residual transmission. Malar. J. 2017, 16, 443. [Google Scholar] [CrossRef]
- Ministry of Health. The Epidemiology and Control Profile of Malaria in Kenya: Reviewing the Evidence to Guide the future Vector Control. National Malaria Control Programme, Ministry of Health Technical support provided by the LINK Project Nairobi, Kenya. , 2016. Available online: https://virtual.lshtm.ac.uk/wp-content/uploads/2016/11/Kenya-Epidemiological-Profile.pdf (accessed on 28 March 2019).
- Kweka, E.J.; Munga, S.; Himeidan, Y.; Githeko, A.K.; Yan, G. Assessment of mosquito larval productivity among different land use types for targeted malaria vector control in the western Kenya highlands. Parasites Vectors 2015, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- President’s Malaria Initiative. Kenya Malaria Operational Plan FY 2019. 2019. Available online: https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy19/fy-2019-kenya-malaria-operational-plan.pdf?sfvrsn=3 (accessed on 1 March 2019).
- Owino, E.A. Kenya needs cohesive policies and better strategies in its war against malaria in arid and semi arid areas. Int. J. Mosq. Res. 2018, 5, 124–126. [Google Scholar]
- Bowman, D.D.; Liu, Y.; McMahan, C.S.; Nordone, S.K.; Yabsley, M.J.; Lund, R.B. Forecasting United States heartworm Dirofilaria immitis prevalence in dogs. Parasites Vectors 2016, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Labarthe, N.; Guerrero, J. Epidemiology of heartworm: What is happening in South America and Mexico? Vet. Parasitol. 2005, 133, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Corbet, P.S.; Williams, M.C.; Gillett, J.D. O’nyong nyong fever: An epidemic virus disease in East Africa. IV. Vector studies at epidemic sites. Trans. R. Soc. Trop. Med. Hyg. 1961, 55, 463–480. [Google Scholar] [CrossRef]
- LaBeaud, A.D.; Banda, T.; Brichard, J.; Muchiri, E.M.; Mungai, P.L.; Mutuku, F.M.; Borland, E.; Gildengorin, G.; Pfeil, S.; Teng, C.Y.; et al. High Rates of O’Nyong Nyong and Chikungunya Virus Transmission in Coastal Kenya. PLoS Negl. Trop. Dis. 2015, 9, e0003436. [Google Scholar] [CrossRef] [PubMed]
- Njenga, S.M.; Mwandawiro, C.S.; Wamae, C.N.; Mukoko, D.A.; Omar, A.A.; Shimada, M.; Bockarie, M.J.; Molyneux, D.H. Sustained reduction in prevalence of lymphatic filariasis infection in spite of missed rounds of mass drug administration in an area under mosquito nets for malaria control. Parasites Vectors 2011, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Njenga, S.M.; Kanyi, H.M.; Mutungi, F.M.; Okoyo, C.; Matendechero, H.S.; Pullan, R.L.; Halliday, K.E.; Brooker, S.J.; Wamae, C.N.; Onsongo, J.K.; et al. Assessment of lymphatic filariasis prior to re-starting mass drug administration campaigns in coastal Kenya. Parasites Vectors 2017, 10, 99. [Google Scholar] [CrossRef]
- Mwandawiro, C.S.; Fujimaki, Y.; Mitsui, Y.; Katsivo, M. Mosquito vectors of bancroftian filariasis in Kwale District, Kenya. East Afr. Med. J. 1997, 74, 288–293. [Google Scholar]
- Moraga, P.; Cano, J.; Baggaley, R.F.; Gyapong, J.O.; Njenga, S.M.; Nikolay, B.; Davies, E.; Rebollo, M.P.; Pullan, R.L.; Bockarie, M.J.; et al. Modelling the distribution and transmission intensity of lymphatic filariasis in sub-Saharan Africa prior to scaling up interventions: Integrated use of geostatistical and mathematical modelling. Parasites Vectors 2015, 8, 560. [Google Scholar] [CrossRef]
- Ogola, E.O.; Fillinger, U.; Ondiba, I.M.; Villinger, J.; Masiga, D.K.; Torto, B.; Tchouassi, D.P. Insights into malaria transmission among Anopheles funestus mosquitoes, Kenya. Parasites Vectors 2018, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Ogola, E.O.; Fillinger, U.; Isabella, M.V.; Jandouwe, M.; Daniel, K.; Torto, B.; Tchouassi, D.P. Malaria in Kakuma refugee camp, Turkana, Kenya: Facilitation of Anopheles arabiensis vector populations by installed water distribution and catchment systems. Malar. J. 2011, 10, 149. [Google Scholar] [CrossRef]
- Njenga, S.M.; Muita, M.; Kirigi, G.; Mbugua, J.; Mitsui, Y.; Fujimaki, Y.; Aoki, Y. Bancroftian filariasis in Kwale District, Kenya. East Afr. Med. J. 2000, 77, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Kramer, L.D. Vector-virus interactions and transmission dynamics of West Nile virus. Viruses 2013, 5, 3021–3047. [Google Scholar] [CrossRef] [PubMed]
- Burkett-Cadena, N.D.; Bingham, A.M.; Porterfield, C.; Unnasch, T.R. Innate preference or opportunism: Mosquitoes feeding on birds of prey at the Southeastern Raptor Center. J. Vector Ecol. 2014, 39, 21–31. [Google Scholar] [CrossRef]
- Zittra, C.; Flechl, E.; Kothmayer, M.; Vitecek, S.; Rossiter, H.; Zechmeister, T.; Fuehrer, H.P. Ecological characterization and molecular differentiation of Culex pipiens complex taxa and Culex torrentium in eastern Austria. Parasites Vectors 2016, 9, 197. [Google Scholar] [CrossRef]
- Beji, M.; Rhim, A.; Roiz, D.; Bouattour, A. Ecophysiological characterization and molecular differentiation of Culex pipiens forms (Diptera: Culicidae) in Tunisia. Parasites Vectors 2017, 10, 3021–3047. [Google Scholar] [CrossRef][Green Version]
- Scott, J.G.; Yoshimizu, M.H.; Kasai, S. Pyrethroid resistance in Culex pipiens mosquitoes. Pestic. Biochem. Physiol. 2015, 120, 68–76. [Google Scholar] [CrossRef]
- Centers for Disease Control. Vectors of Lymphatic Filariasis. 2018. Available online: https://www.cdc.gov/parasites/lymphaticfilariasis/gen_info/vectors.html (accessed on 13 March 2019).
- World Health Oganisation. Lymphatic Filariasis. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis (accessed on 26 March 2019).
- Turell, M.J. Members of the Culex pipiens Complex as Vectors of Viruses. J. Am. Mosq. Control Assoc. 2012, 28, 123–126. [Google Scholar] [CrossRef]
- Rappole, J.H.; Hubalek, Z. Migratory birds and West Nile virus. J. Appl. Microbiol. 2003, 94, 47–58. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of west nile virus. Biomed. Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef] [PubMed]
- Kopp, A.; Gillespie, T.R.; Hobelsberger, D.; Estrada, A.; Harper, J.M.; Miller, R.A.; Eckerle, I.; Müller, M.A.; Podsiadlowski, L.; Leendertz, F.H.; et al. Provenance and geographic spread of St. Louis encephalitis virus. MBio 2013, 4, e00322-13. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, E.R.; Weaver, S.C. Vector competence of Culex (Melanoconion) taeniopus for equine-virulent subtype IE strains of Venezuelan equine encephalitis virus. Am. J. Trop. Med. Hyg. 2010, 82, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.M.; Elaagip, A.H.; Kenawy, M.A.; Ayres, C.F.J.; Peterson, A.T.; Soliman, D.E. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS ONE 2016, 11, e0163863. [Google Scholar] [CrossRef] [PubMed]
- Amraoui, F.; Atyame-Nten, C.; Vega-Rúa, A.; Lourenço-De-Oliveira, R.; Vazeille, M.; Failloux, A.B. Culex mosquitoes are experimentally unable to transmit zika virus. Euro. Surveill. 2016, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Ayers, V.B.; Lyons, A.C.; Unlu, I.; Alto, B.W.; Cohnstaedt, L.W.; Higgs, S.; Vanlandingham, D.L. Culex Species Mosquitoes and Zika Virus. Vector-Borne Zoonotic Dis. 2016, 16, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Phumee, A.; Chompoosri, J.; Intayot, P.; Boonserm, R.; Boonyasuppayakorn, S.; Buathong, R.; Thavara, U.; Tawatsin, A.; Joyjinda, Y.; Wacharapluesadee, S.; et al. Vertical transmission of Zika virus in Culex quinquefasciatus Say and Aedes aegypti (L.) mosquitoes. Sci. Rep. 2019, 9, 5257. [Google Scholar] [CrossRef]
- Grossi-Soyster, E.N.; Banda, T.; Teng, C.Y.; Muchiri, E.M.; Mungai, P.L.; Mutuku, F.M.; Gildengorin, G.; Kitron, U.; King, C.H.; Desiree, L.A. Rift valley fever seroprevalence in Coastal Kenya. Am. J. Trop. Med. Hyg. 2017, 97, 115–120. [Google Scholar] [CrossRef]
- Harbach, R. Genus Mansonia Blanchard, 1901. Mosquito Taxonomic Inventory, 2008. Available online: http://mosquito-taxonomic-inventory.info/genus-emmansoniaem-blanchard-1901 (accessed on 1 March 2019).
- Islam, M.N.; ZulKifle, M.A.; Sherwani, M.K.; Ghosh, S.K.; Tiwari, S. Prevalence of malaria, dengue and chikungunya significantly associated with mosquito breeding sites. J. Islam. Med. Assoc. North Am. 2011, 43, 58–67. [Google Scholar] [CrossRef]
- Torres, R.; Samudio, R.; Carrera, J.P.; Young, J.; Maârquez, R.; Hurtado, L.; Weaver, S.; Chaves, L.F.; Tesh, R.; Caâceres, L. Enzootic mosquito vector species at equine encephalitis transmission foci in the República de Panama. PLoS ONE 2017, 12, e0185491. [Google Scholar] [CrossRef]
- Beranek, M.D.; Gallardo, R.; Almirón, W.R.; Contigiani, M.S. First detection of Mansonia titillans (Diptera: Culicidae) infected with St. Louis encephalitis virus (Flaviviridae: Flavivirus) and Bunyamwera serogroup (Peribunyaviridae: Orthobunyavirus) in Argentina. J. Vector Ecol. 2018, 43, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Rueanghiran, C.; Lertwatcharasarakul, P.; Ruangsittichai, J. Species-specific primers for the detection of lymphatic filariasis vectors: Mansonia bonneae and Mansonia dives. Trop. Biomed. 2017, 34, 615–621. [Google Scholar]
- Ughasi, J.; Bekard, H.E.; Coulibaly, M.; Adabie-Gomez, D.; Gyapong, J.; Appawu, M.; Wilson, M.D.; David, B.; Daniel, A. Mansonia africana and Mansonia uniformis are Vectors in the transmission of Wuchereria bancrofti lymphatic filariasis in Ghana. Parasites Vectors 2012, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Onapa, A.W.; Pedersen, E.M.; Reimert, C.M.; Simonsen, P.E. A role for Mansonia uniformis mosquitoes in the transmission of lymphatic filariasis in Uganda? Acta Trop. 2007, 101, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Epelboin, Y.; Talaga, S.; Epelboin, L.; Dusfour, I. Zika virus: An updated review of competent or naturally infected mosquitoes. PLoS Negl. Trop. Dis. 2017, 11, e0005933. [Google Scholar] [CrossRef] [PubMed]
- Kinyatta, N.M. Vectorial Potential of Mansonia Species in the Transmission of Wuchereria Bancrofti and Evaluation of Mosquito Collection Methods in Tana-Delta, Coastal Kenya. Master’s Thesis, Kenyatta University of Agriculture and Technology, Nairobi, Kenya, 2010. [Google Scholar]
- Ondeto, B.M.; Nyundo, C.; Kamau, L.; Muriu, S.M.; Mwangangi, J.M.; Njagi, K.; Mathenge, E.M.; Ochanda, H.; Mbogo, C.M. Current status of insecticide resistance among malaria vectors in Kenya. Parasites Vectors 2017, 10, 429. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommended Insecticides for Indoor Residual Spraying against Malaria Vectors. 2015. Available online: Https://www.who.int/neglected_diseases/vector_ecology/vector-control/Insecticides_IRS_2_March_2015.pdf (accessed on 11 March 2019).
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef]
- Wanjala, C.L.; Kweka, E.J. Malaria Vectors Insecticides Resistance in Different Agroecosystems in Western Kenya. Front. Public Health 2018, 6, 55. [Google Scholar] [CrossRef]
- Kiuru, C.W.; Oyieke, F.A.; Mukabana, W.R.; Mwangangi, J.; Kamau, L.; Muhia-Matoke, D. Status of insecticide resistance in malaria vectors in Kwale County, Coastal Kenya. Malar. J. 2018, 17, 3. [Google Scholar] [CrossRef]
- Schmidt, M.; Hrabcova, V.; Jun, D.; Kuca, K.; Musilek, K. Vector Control and Insecticidal Resistance in the African Malaria Mosquito Anopheles gambiae. Chem. Res. Toxicol. 2018, 31, 534–547. [Google Scholar] [CrossRef]
- Karunamoorthi, K. Vector control: A cornerstone in the malaria elimination campaign History of Malaria Control: Past Experience. Clin Microbiol. Infect. 2011, 17, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Dida, G.O.; Ohashi, K.; Kawashima, E.; Sonye, G.; Njenga, S.M.; Mwandawiro, C.; Minakawa, N. A small-scale field trial of pyriproxyfen-impregnated bed nets against pyrethroid-resistant Anopheles gambiae s.s. in Western Kenya. PLoS ONE 2014, 9, e111195. [Google Scholar] [CrossRef] [PubMed]
- Muema, J.M.; Bargul, J.L.; Nyanjom, S.G.; Mutunga, J.M.; Njeru, S.N. Potential of Camellia sinensis proanthocyanidins-rich fraction for controlling malaria mosquito populations through disruption of larval development. Parasites Vectors 2016, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.J.; Stanich, M.A.; McAbee, R.D.; Mulligan, F.S. High level methoprene resistance in the mosquito Ochlerotatus nigromaculis (Ludlow) in Central California. Pest Manag. Sci. 2002, 58, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.H.; Mulligan, F.S. Potential for resistance to pyriproxyfen: A promising new mosquito larvicide. J. Am. Mosq. Control Assoc. 1991, 7, 409–411. [Google Scholar] [PubMed]
- Mutero, C.M.; Mbogo, C.; Mwangangi, J.; Imbahale, S.; Kibe, L.; Orindi, B.; Girma, M.; Njui, A.; Lwande, W.; Affognon, H.; et al. An Assessment of Participatory Integrated Vector Management for Malaria Control in Kenya. Environ. Health Perspect. 2015, 123, 1145–1151. [Google Scholar] [CrossRef]
- Benelli, G.; Jeffries, C.L.; Walker, T. Biological control of mosquito vectors: Past, present, and future. Insects 2016, 7. [Google Scholar] [CrossRef]
- Vu, S.N.; Nguyen, T.Y.; Tran, V.P.; Truong, U.N.; Le, Q.M.; Le, V.L.; Le, T.N.; Bektas, A.; Briscombe, A.; Aaskov, J.G.; et al. Elimination of dengue by community programs using Mesocyclops (Copepoda) against Aedes aegypti in central Vietnam. Am. J. Trop. Med. Hyg. 2005, 72, 67–73. [Google Scholar]
- Howard, A.F.V.; Zhou, G.; Omlin, F.X. Malaria mosquito control using edible fish in western Kenya: Preliminary findings of a controlled study. BMC Public Health 2007, 7, 199. [Google Scholar] [CrossRef]
- Berry, C. The bacterium, Lysinibacillus sphaericus, as an insect pathogen. J. Invertebr. Pathol. 2012, 109. [Google Scholar] [CrossRef]
- Poopathi, S.; Abidha, S. Mosquitocidal bacterial toxins (Bacillus sphaericus and Bacillus thuringiensis serovar israelensis): Mode of action, cytopathological effects and mechanism of resistance. J. Physiol. Pathophysiol. 2010, 1, 22–38. [Google Scholar]
- Nareshkumar, A.; Murugan, K.; Baruah, I.; Madhiyazhagan, P.; Nataraj, T. Larvicidal Potentiality, Longevity and Fecundity Inhibitory Activities of Bacillus Sphaericus (Bs G3-IV) on Vector Mosquitoes, Aedes aegypti and Culex quinquefasciatus. J. Entomol. Acarol. Res. 2012, 44, 3. [Google Scholar] [CrossRef]
- Mwangangi, J.M.; Kahindi, S.C.; Lydiah, W.K.; Nzovu, J.G.; Luethy, P.; Githure, J.I.; Mbogo, C.M. Wide-scale application of Bti/Bs biolarvicide in different aquatic habitat types in urban and peri-urban. Parasitol. Res. 2011, 108, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Derua, Y.A.; Kahindi, S.C.; Mosha, F.W.; Kweka, E.J.; Atieli, H.E.; Wang, X.; Zhou, G.; Lee, M.; Githeko, A.K.; Yan, G. Microbial larvicides for mosquito control: Impact of long lasting formulations of Bacillus thuringiensis var. israelensis and Bacillus sphaericus on non-target organisms in western Kenya highlands. Ecol. Evol. 2018, 8, 7563–7573. [Google Scholar] [CrossRef] [PubMed]
- Karungu, S.; Huang, D.; Atoni, E.; Waruhiu, C.; Agwanda, B.; Hu, X.; Yuan, Z. Isolation, identification and evaluation of mosquito entomopathogenic Bacillus species and related genera from randomly selected sites in Kenya. Afr. J. Microbiol. Res. 2018, 12, 290–299. [Google Scholar] [CrossRef]
- Aw, K.M.S.; Hue, S.M. Mode of Infection of Metarhizium spp. Fungus and Their Potential as Biological Control Agents. J. Fungi 2017, 3, 30. [Google Scholar] [CrossRef]
- Aliana, Z.D.; Anggriani, N.; Supriatna, A.K. The Use of Metarhizium anisopliae and Beauveria bassiana as Fungal Pathogen to Control the Growth of Aedes aegypti Population: A Preliminary Result. In Proceedings of the 3rd International Conference on Chemical, Agricultural and Medical Sciences (CAMS-2015), Singapore, 10–11 December 2015; pp. 7–8. [Google Scholar]
- Lee, S.J.; Kim, S.; Yu, J.S.; Kim, J.C.; Nai, Y.S.; Kim, J.S. Biological control of Asian tiger mosquito, Aedes albopictus (Diptera: Culicidae) using Metarhizium anisopliae JEF-003 millet grain. J. Asia. Pac. Entomol. 2015, 18, 217–221. [Google Scholar] [CrossRef]
- Bukhari, T.; Takken, W.; Koenraadt, C.J.M. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasites Vectors 2011, 4, 23. [Google Scholar] [CrossRef]
- Niassy, S.; Subramanian, S.; Ekesi, S.; Bargul, J.L.; Villinger, J.; Maniania, N.K. Use of Metarhizium anisopliae chitinase genes for genotyping and virulence characterization. Biomed. Res. Int. 2013, 2013, 465213. [Google Scholar] [CrossRef]
- Murigu, M.M.; Nana, P.; Waruiru, R.M.; Nga’nga’, C.J.; Ekesi, S.; Maniania, N.K. Laboratory and field evaluation of entomopathogenic fungi for the control of amitraz-resistant and susceptible strains of Rhipicephalus decoloratus. Vet. Parasitol. 2016, 225, 12–18. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-NG, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl. Trop. Dis. 2019, 13, e0006822. [Google Scholar] [CrossRef]
- Fraser, J.E.; De Bruyne, J.T.; Iturbe-Ormaetxe, I.; Stepnell, J.; Burns, R.L.; Flores, H.A.; O’Neill, S.L. Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog. 2017, 13, e1006751. [Google Scholar] [CrossRef] [PubMed]
- Mains, J.; Brelsfoard, C.; Rose, R.; Dobson, S. Female Adult Aedes albopictus Suppression by Wolbachia- Infected Male Mosquitoes. Sci Rep. 2016, 6, 33846. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Plichart, C.; Sang, A.; Brelsfoard, C.; Bossin, H.; Dobson, S. Open Release of Male Mosquitoes Infected with a Wolbachia Biopesticide: Field Performance and Infection Containment. PLoS Negl. Trop. Dis. 2012, 6, e1797. [Google Scholar] [CrossRef] [PubMed]
- Proverbs, M. Induced Sterilization and Control of Insects. Annu. Rev. Entomol. 1969, 14, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Caputo, B.; Ienco, A.; Cianci, D.; Pombi, M.; Petrarca, V.; Baseggio, A.; Devine, G.J.; della Torre, A. The ‘auto-dissemination’ approach: A novel concept to fight Aedes albopictus in urban areas. PLoS Negl. Trop. Dis. 2012, 12, e1793. [Google Scholar] [CrossRef]
- Thomas, D.D.; Donnelly, C.A.; Wood, R.J.; Alphey, L.S. Insect population control using a dominant, repressible, lethal genetic system. Science 2000, 287, 2474–2476. [Google Scholar] [CrossRef]
- Harris, A.F.; McKemey, A.R.; Nimmo, D.; Curtis, Z.; Black, I.; Morgan, S.A.; Oviedo, M.N.; Lacroix, R.; Naish, N.; Morrison, N.I.; et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 2012, 30, 828–830. [Google Scholar] [CrossRef]
- Carvalho, D.O.; McKemey, A.R.; Garziera, L.; Lacroix, R.; Donnelly, C.A.; Alphey, L.; Malavasi, A.; Capurro, M.L. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 2015, 9, e0003864. [Google Scholar] [CrossRef]
| Genera | Species | Virus Isolated/Detected 1 | County of Virus Detection | Reference |
|---|---|---|---|---|
| Aedes | A. aegypti | DENV, CHKV | Mombasa, Mandera, Kilifi, Lamu, Busia | [12,13,33,55,57,58] |
| A. africanus | YFV | Baringo, | [59] | |
| A. albicosta | DENV, CHKV | Mombasa, Kilifi, Lamu, Kwale | [33] | |
| A. circumluteolus | RVFV, BBKV, NDUV, SMFV | Garissa | [4,36] | |
| A. fryeri | DENV | Mombasa, Kilifi, Lamu, Kwale | [33] | |
| A. fulgens | DENV, CHKV | Mombasa, Kilifi, Lamu, Kwale | [33] | |
| A. keniensis | YFV | Baringo | [59] | |
| A. Luridus | NDUV | Tana River | [4] | |
| A. mcintoshi | RVFV, NDUV, PGAV, BUNV, BBKV, PGAV, SMFV, NRIV | Garissa | [4,11,36,40] | |
| DENV, CHKV | Mombasa, Kilifi, Lamu, Kwale | [33] | ||
| A. ochraceus | RVFV, NDUV, BUNV, BBKV, SNBV, SMFV | Garissa | [4,11,36,37,38] | |
| DENV, CHKV | Mombasa, Kilifi, Lamu, Kwale | [33] | ||
| A. pembaensis | RVFV | Kilifi | [4] | |
| DENV, CHKV | Mombasa, Kilifi, Lamu, Kwale | [33] | ||
| A. sudanensis | BBKV, SNBV, WNV | Garissa | [36] | |
| NDUV | Tana River | [39] | ||
| Anopheles | An. funestus | ONNV | Kisumu | [59] |
| BUNV | Kajiado | [4] | ||
| NRIV | Tana River | [4] | ||
| An. gambiae | BUNV | Homabay | [20] | |
| An. squamosus | RVFV | Garissa | [11] | |
| Culex | Cx. bitaeniorhynchus | RVFV | Kilifi | [11] |
| NDUV | Tana River | [39] | ||
| Cx. cinereus | NDUV | Busia | [4] | |
| Cx. pipiens | USUV | Kisumu | [4] | |
| NDUV | Garissa, Tana River | [38,39] | ||
| Cx. poicilipes | RVFV | Kilifi | [11] | |
| Cx. quinquefasciatus | RVFV | Baringo, Garissa | [11,60] | |
| WNV, SNBV | Garissa | [36,60] | ||
| Cx. rubinotus | NDUV | Baringo | [4] | |
| Cx. univittatus | RVFV | Baringo | [11] | |
| BUNV | Homa Bay | [20] | ||
| SNBV | West Pokot, Nakuru, Busia | [4,37,61] | ||
| WNV | Garissa, Turkana, West Pokot | [4,61] | ||
| Cx. vansomereni | NDUV | Tana River | [39] | |
| BBKV, SNBV | Nakuru | [4,37] | ||
| Cx. zombaensis | RVFV | Nakuru | [62] | |
| BBKV | Kiambu | [4] | ||
| Mansonia | Mn. africana | RVFV | Nakuru, Baringo, Garissa | [11,60,62] |
| NDUV | Baringo | [4] | ||
| Mn. uniformis | RVFV | Baringo, Garissa | [40,60] | |
| NDUV | Baringo | [36] |
| Parasite | Associated Human Ailment | Dominant Mosquito Species | Counties of Vector Distribution | Reference |
|---|---|---|---|---|
| Plasmodium falciparum | Malaria | An. gambiae s.s., An. arabiensis, An. funestus, An. merus | Kwale, Kilifi | [71,75,81,93,94] |
| An. gambiae ss, An. arabiensis, An. funestus | Taita-Taveta, Lamu, Kajiado, Embu, Nakuru, Baringo, Bungoma, Kirinyaga, Kiambu, Busia, Siaya, Kakamega, Vihiga, Homabay, Migori, Kisii, Kisumu, Nandi | |||
| An. gambiae ss, An. arabiensis | Narok | |||
| An. arabiensis, An. funestus | Tana-River, Makueni, Machakos, Trans-Nzoia | |||
| An. funestus | Samburu, Isiolo, Garissa, Mombasa, Uasin-Gishu, Nyamira | |||
| An. arabiensis | Turkana | |||
| An. gambiae ss | Tharaka-Nithi | |||
| Wuchereria bancrofti | Bancroftian filariasis | An. gambiae sl, An. funestus, Cx. quinquefasciatus | Kwale, Kilifi, Lamu | [50,91,95] |
| Common Current Strategies | |||||
| Strategy | Description/products | Life stage target 1 | Development of resistance | Advantage | Major challenges |
| Surveillance | Use mosquito collection tools; (CO2-baited CDC (Centers for Disease Control) light traps and 350 mL larval dippers) | L, P, A | None | Identify mosquito distribution status (guide on control measures) | Time consuming |
| Synthetic pesticides | Involve chemicals (pyrethroids, carbamates B, organophosphates B, and organochlorines B) integrated in IRS and LLIN | L, P, A | High | Fast mode of action, broad spectrum | Environmental unfriendly, toxic to non-target biota and easy to develop resistance |
| Insect growth regulators (IGRs) | Involve chemicals or plant extracts to inhibit metamorphosis. (methoprene and pyriproxyfen) | E, L, P, A | Low | Safe to non-target organisms, target specific | Slow mode of action |
| Alternative Strategies | |||||
| Public awareness | Involving the public in clearing nearby breeding zones (open water containers, tires, tins, bottles, etc.) | E, L, P, A | None | Broad spectrum, minimal use of pesticides | Lack of funding for mass education countrywide |
| Biological control | Natural predators that feed on mosquito (Larvivorous fish, Omnivorous copepods, amphibians, etc.) | E, L, P, A | None | Broad spectrum, environmentally friendly | Limited area of applicability, threat to few non-target organisms |
| Entomopathogenic bacteria (Bacillus thuringiensis vr israelensis and Lysinibacillus sphaericus) | L | Low | Species specific, harmless to non-target organisms | Infective in cryptic breeding sites, limited to larvae control, active only after ingestion | |
| Entomopathogenic fungi (Beauveria bassiana and Metarhizium anisopliae) | L, A | Low | Broad spectrum, environmentally friendly | Cost ineffective, limited duration of efficacy | |
| Infecting male mosquito with Wolbachia (wmel Wolbachia strain) | A | Low | Species specific, environmentally friendly | Mating competitiveness, high cost of male mosquito rearing | |
| Genetic engineering | Sterile insect technique (SIT)—Genetic suppression of the male mosquito | A | None | Species specific, environmentally friendly | Mating competitiveness, high cost of male mosquito rearing and molecular tools |
| Release of insects carrying dominant lethality (RIDL)—transgenic prevention of adult development | A | None | Species specific, environmentally friendly | Mating competitiveness, high cost of male mosquito rearing and molecular tools | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karungu, S.; Atoni, E.; Ogalo, J.; Mwaliko, C.; Agwanda, B.; Yuan, Z.; Hu, X. Mosquitoes of Etiological Concern in Kenya and Possible Control Strategies. Insects 2019, 10, 173. https://doi.org/10.3390/insects10060173
Karungu S, Atoni E, Ogalo J, Mwaliko C, Agwanda B, Yuan Z, Hu X. Mosquitoes of Etiological Concern in Kenya and Possible Control Strategies. Insects. 2019; 10(6):173. https://doi.org/10.3390/insects10060173
Chicago/Turabian StyleKarungu, Samuel, Evans Atoni, Joseph Ogalo, Caroline Mwaliko, Bernard Agwanda, Zhiming Yuan, and Xiaomin Hu. 2019. "Mosquitoes of Etiological Concern in Kenya and Possible Control Strategies" Insects 10, no. 6: 173. https://doi.org/10.3390/insects10060173
APA StyleKarungu, S., Atoni, E., Ogalo, J., Mwaliko, C., Agwanda, B., Yuan, Z., & Hu, X. (2019). Mosquitoes of Etiological Concern in Kenya and Possible Control Strategies. Insects, 10(6), 173. https://doi.org/10.3390/insects10060173





