Toxicity of Kadsura coccinea (Lem.) A. C. Sm. Essential Oil to the Bed Bug, Cimex lectularius L. (Hemiptera: Cimicidae)
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Sample Preparation
2.3. GC/MS Analysis of K. Coccinea Essential Oil
2.4. Chemicals
2.5. Rearing of Bed Bugs
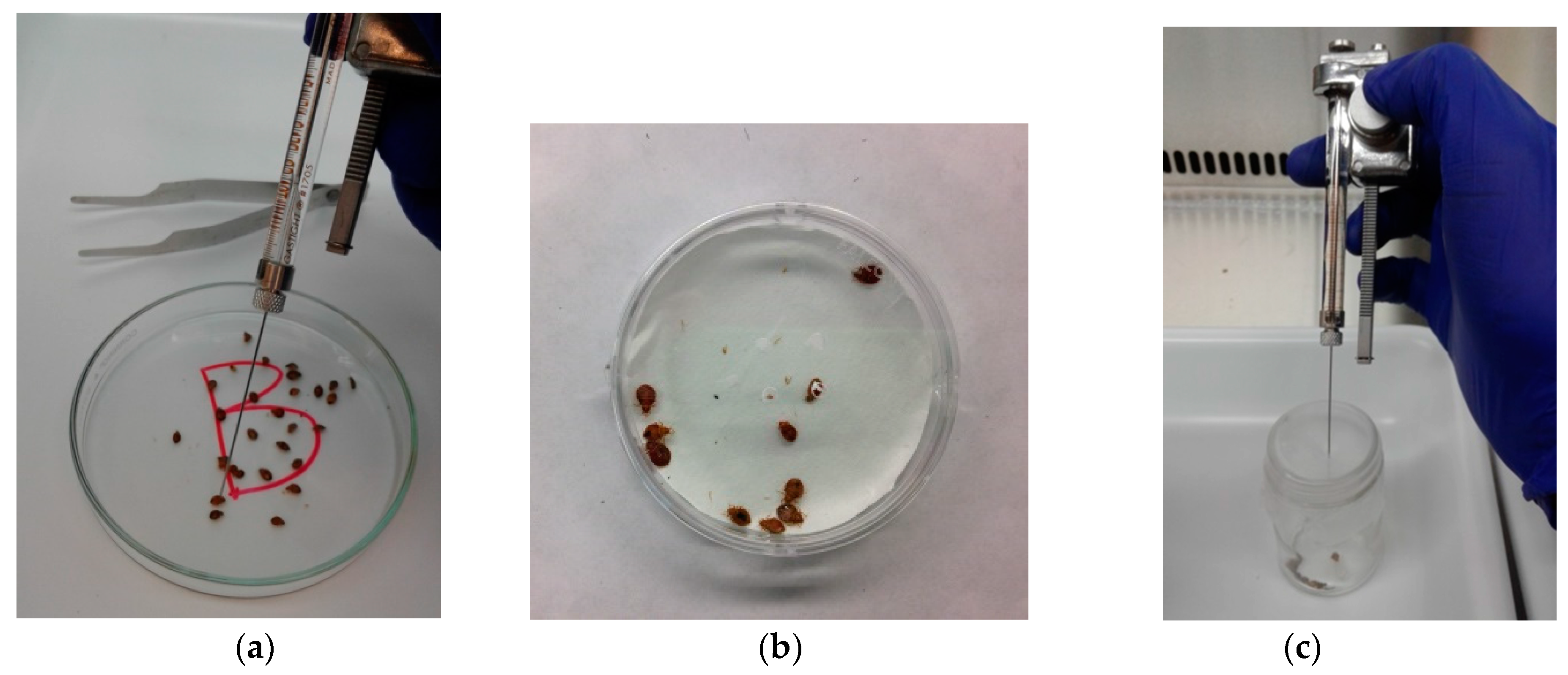
2.6. Topical Bioassay
2.7. Residual Bioassay
2.8. Fumigation Bioassay
3. Results and Discussion
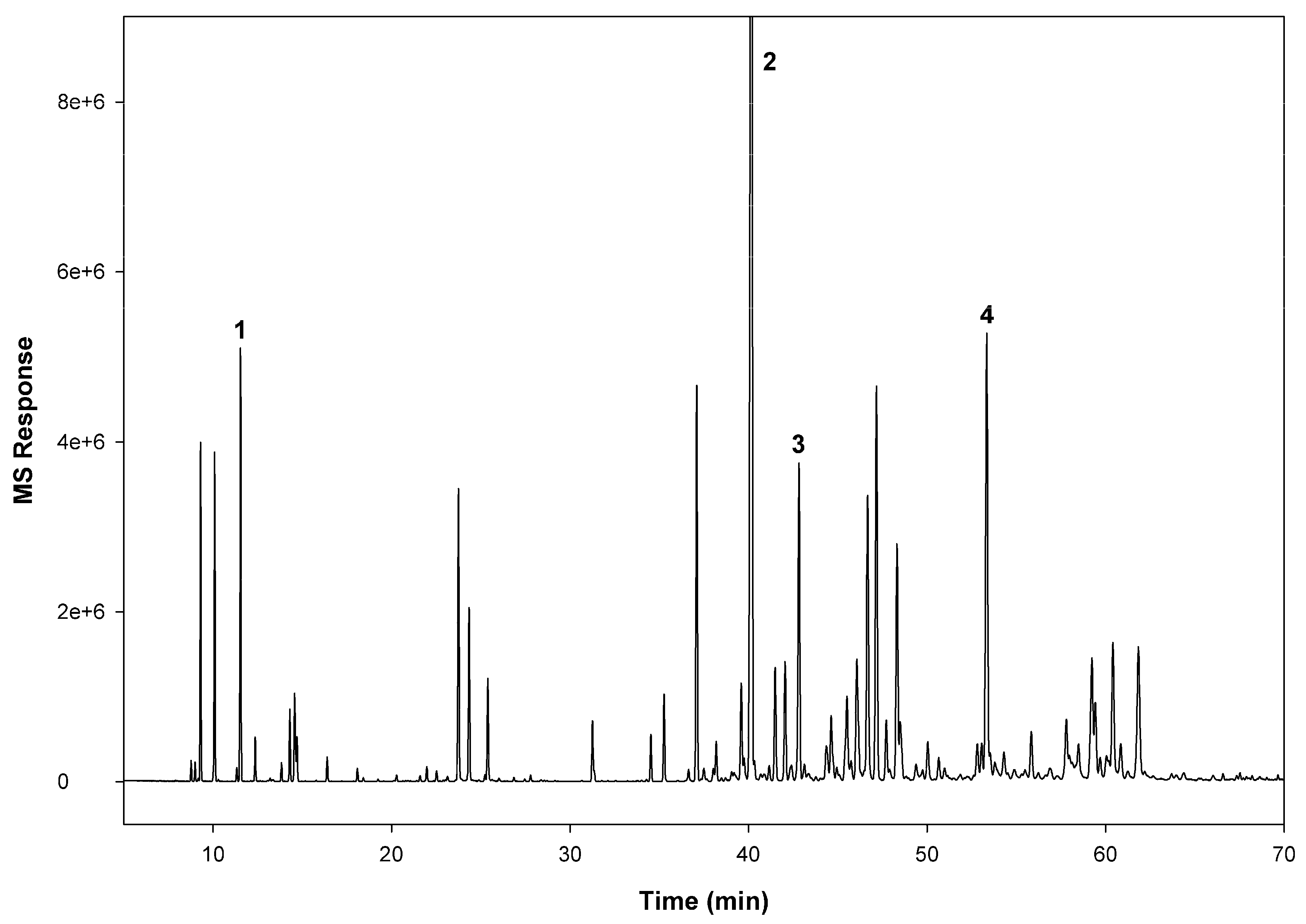
3.1. GC/MS Analysis
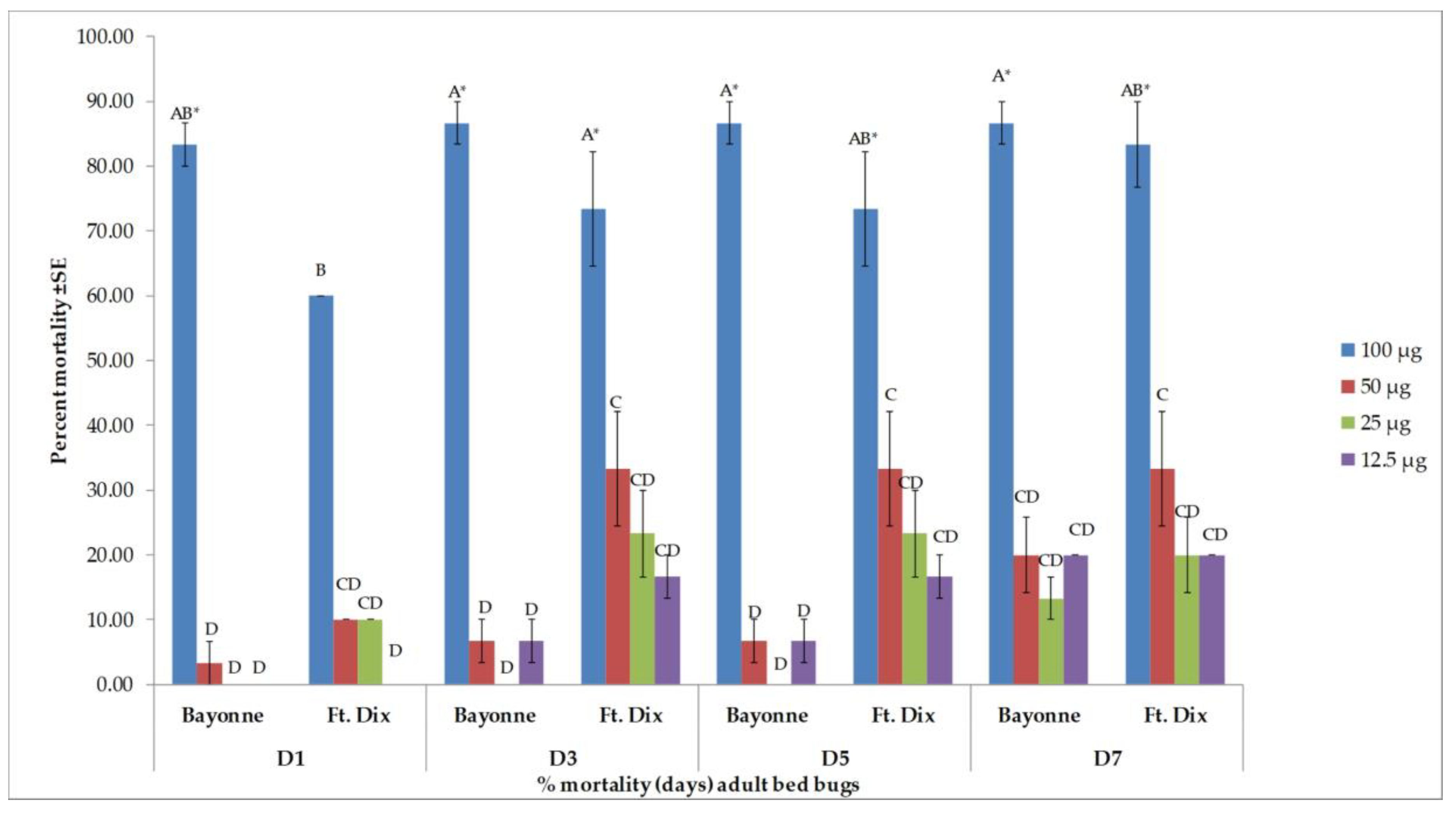
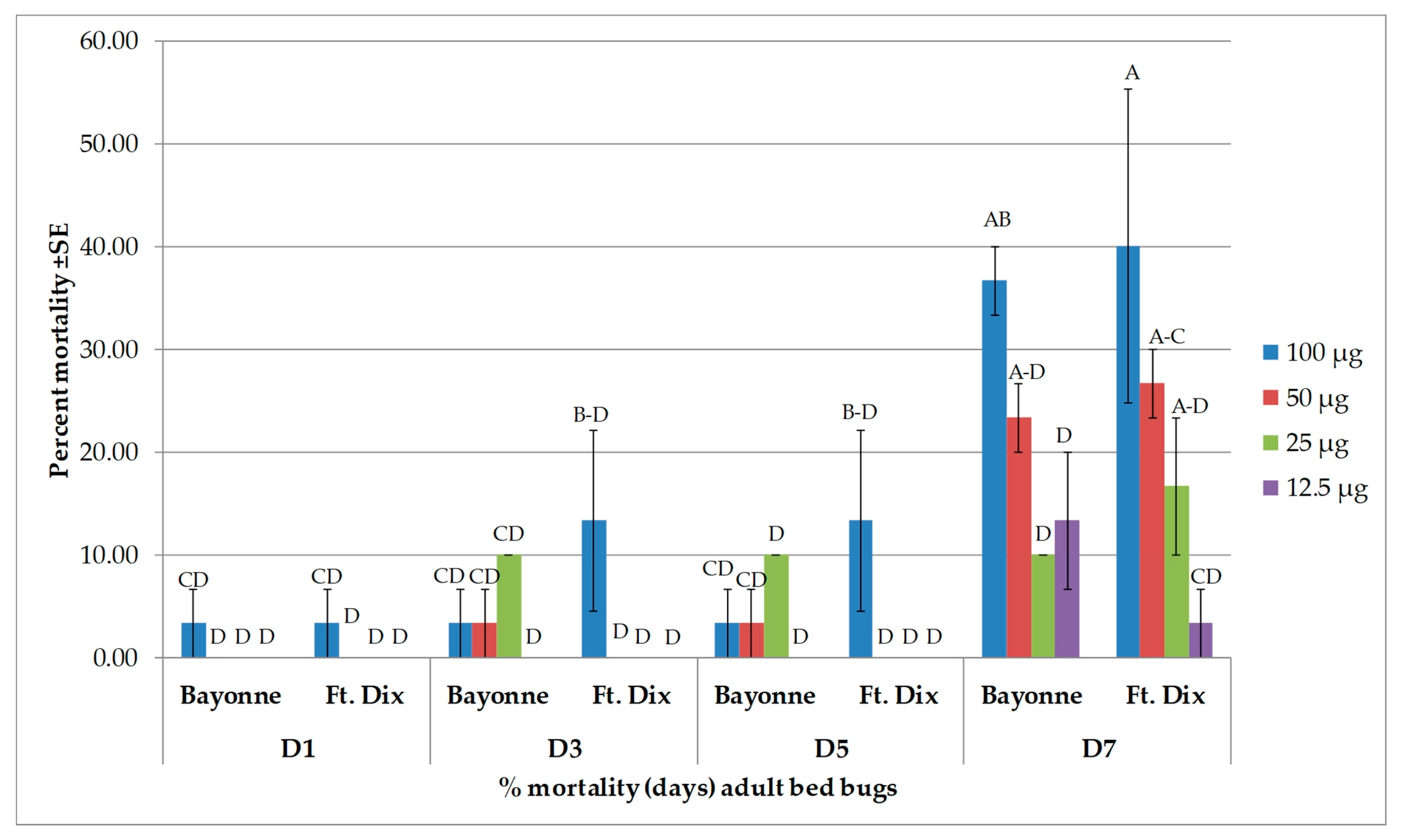
3.2. Toxicity Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghauri, M.S.K. Hemiptera (bugs). In Insects and Other Arthropods of Medical Importance; Smith, K.G.V., Ed.; British Museum: London, UK, 1973; pp. 373–393. [Google Scholar]
- Machado, C.D.; Raman, V.; Rehman, J.U.; Maia, B.H.L.N.S.; Meneghetti, E.K.; Almeida, V.P.; Silva, R.Z.; Farago, P.V.; Khan, I.A.; Budel, J.M. Schinus molle: Anatomy of leaves and stems, chemical composition and insecticidal activities of volatile oil against bed bug (Cimex lectularius). Revista Brasileira Farmacognosia 2018. [Google Scholar] [CrossRef]
- Usinger, R.L. Monograph of Cimicidae (Hemiptera, Heteroptera); Entomological Society of America: College Park, MD, USA, 1966; Volume 7. [Google Scholar]
- Walker, A.R. Chapter 6. Blood sucking bugs—Hemiptera. In Arthropods of Humans and Domestic Animals: A Guide to Preliminary Identification; Walker, A.R., Ed.; Chapman and Hall: London, UK, 1994; pp. 155–160. [Google Scholar]
- Goddard, J. Bed Bugs: Do They Transmit Diseases? In Infectious Diseases and Arthropods; Goddard, J., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 177–181. [Google Scholar]
- Koganemaru, R.; Miller, D.M. The bed bug problem: Past, present, and future control methods. Pestic. Biochem. Physiol. 2013, 106, 177–189. [Google Scholar] [CrossRef]
- Dang, K.; Doggett, S.L.; Veera Singham, G.; Lee, C.-Y.J.P. Insecticide resistance and resistance mechanisms in bed bugs, Cimex spp. (Hemiptera: Cimicidae). Parasit. Vectors 2017, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Toi, C.S.; Lilly, D.G.; Bu, W.; Doggett, S.L. Detection of knockdown resistance mutations in the common bed bug, Cimex lectularius (Hemiptera: Cimicidae), in Australia. Pest Manag. Sci. 2015, 71, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Lilly, D.G.; Dang, K.; Webb, C.E.; Doggett, S.L. Evidence for Metabolic Pyrethroid Resistance in the Common Bed Bug (Hemiptera: Cimicidae). J. Econ. Entomol. 2016, 109, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Koganemaru, R.; Miller, D.M.; Adelman, Z.N. Robust cuticular penetration resistance in the common bed bug (Cimex lectularius L.) correlates with increased steady-state transcript levels of CPR-type cuticle protein genes. Pestic. Biochem. Physiol. 2013, 106, 190–197. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- New Medical College of Jiangsu. Great Dictionary of Chinese Medicine; Shanghai Science and Technology Press: Shanghai, China, 1977; pp. 814–815. [Google Scholar]
- Pharmacopoeia of the People’s Republic of China; Guangdong Science and Technology Press: Beijing, China, 1977; Volume 1, p. 594.
- Liu, Y.; Yang, Y.; Tasneem, S.; Hussain, N.; Daniyal, M.; Yuan, H.; Xie, Q.; Liu, B.; Sun, J.; Jian, Y.; et al. Lignans from Tujia Ethnomedicine Heilaohu: Chemical Characterization and Evaluation of Their Cytotoxicity and Antioxidant Activities. Molecules 2018, 23, 2147. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Bai, C.Q.; Chu, S.S.; Zhou, L.; Liu, S.S.D.Z.L.; Liu, Q.Z. Chemical composition and toxicities of the essential oil derived from Kadsura heteroclita stems against Sitophilus zeamais and Meloidogyne incognita. J. Med. Plants Res. 2011, 5, 4943–4948. [Google Scholar]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Rajeswary, M.; Benelli, G. delta-Cadinene, Calarene and delta-4-Carene from Kadsura heteroclita Essential Oil as Novel Larvicides Against Malaria, Dengue and Filariasis Mosquitoes. Comb. Chem. High Throughput Screen. 2016, 19, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides: For richer, for poorer. Pest Manag. Sci. 2008, 64, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B.; Machial, C.M. Chapter 2 Pesticides based on plant essential oils: From traditional practice to commercialization. Adv. Phytomed. 2006, 3, 29–44. [Google Scholar] [CrossRef]
- Campbell, B.E.; Miller, D.M. Insecticide Resistance in Eggs and First Instars of the Bed Bug, Cimex lectularius (Hemiptera: Cimicidae). Insects 2015, 6, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.; Cuadrillero, C.; Vilella, D. Maintenance of a laboratory colony of Cimex lectularius (Hemiptera: Cimicidae) using an artificial feeding technique. J. Med. Entomol. 2002, 39, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Potter, M.F.; Haynes, K.F. Evaluation of piperonyl butoxide as a deltamethrin synergist for pyrethroid-resistant bed bugs. J. Econ. Entomol. 2009, 102, 2310–2315. [Google Scholar] [CrossRef]
- SAS Institut. Version 6; SAS Institute: Cary, NC, USA, 2005. [Google Scholar]
- Govindarajan, M.; Benelli, G. Artemisia absinthium-borne compounds as novel larvicides: Effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol. Res. 2016, 115, 4649–4661. [Google Scholar] [CrossRef]
- Choi, W.-S.; Park, B.-S.; Lee, Y.-H.; Jang, D.Y.; Yoon, H.Y.; Lee, S.-E. Fumigant toxicities of essential oils and monoterpenes against Lycoriella mali adults. Crop Prot. 2006, 25, 398–401. [Google Scholar] [CrossRef]
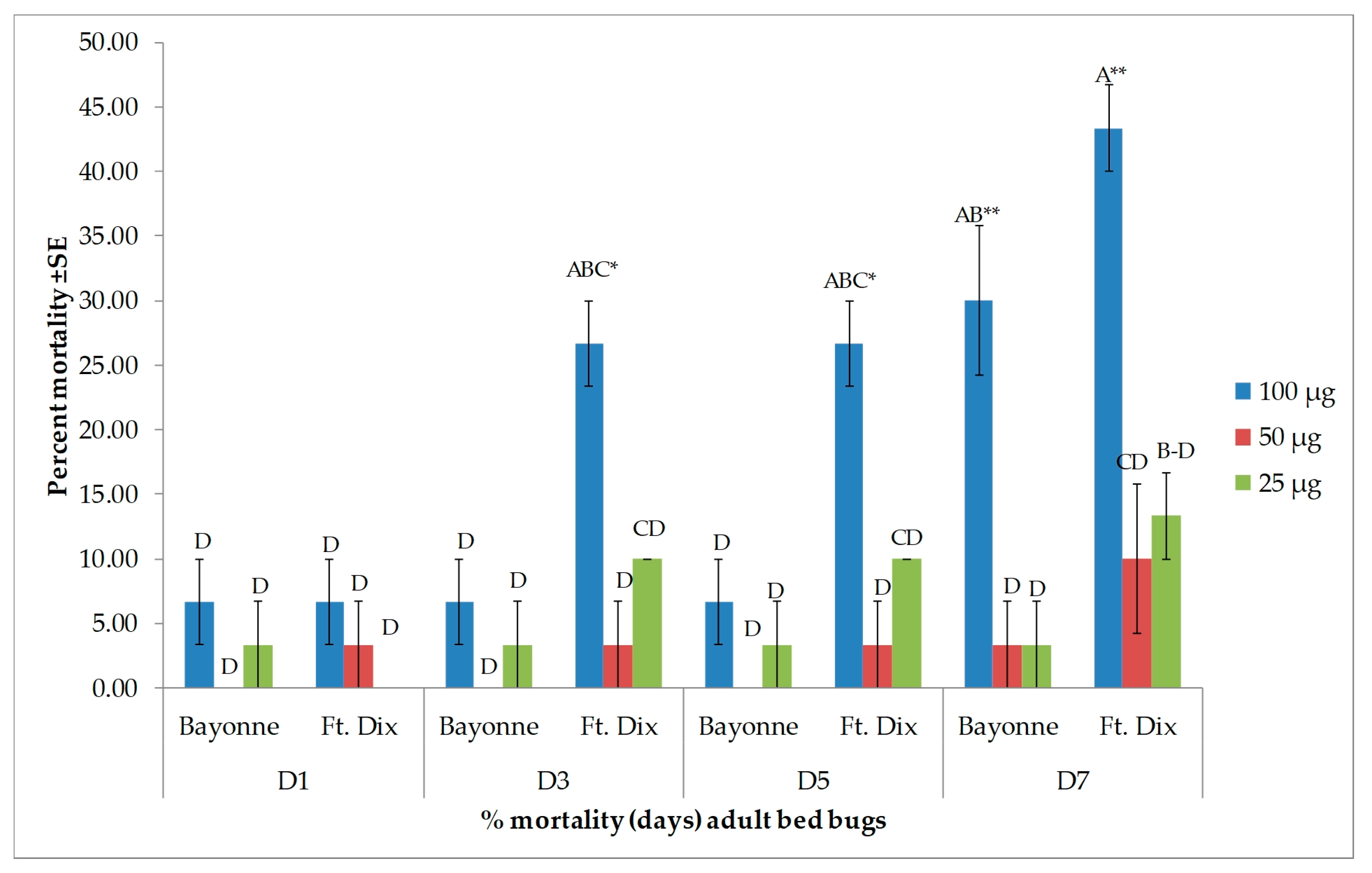
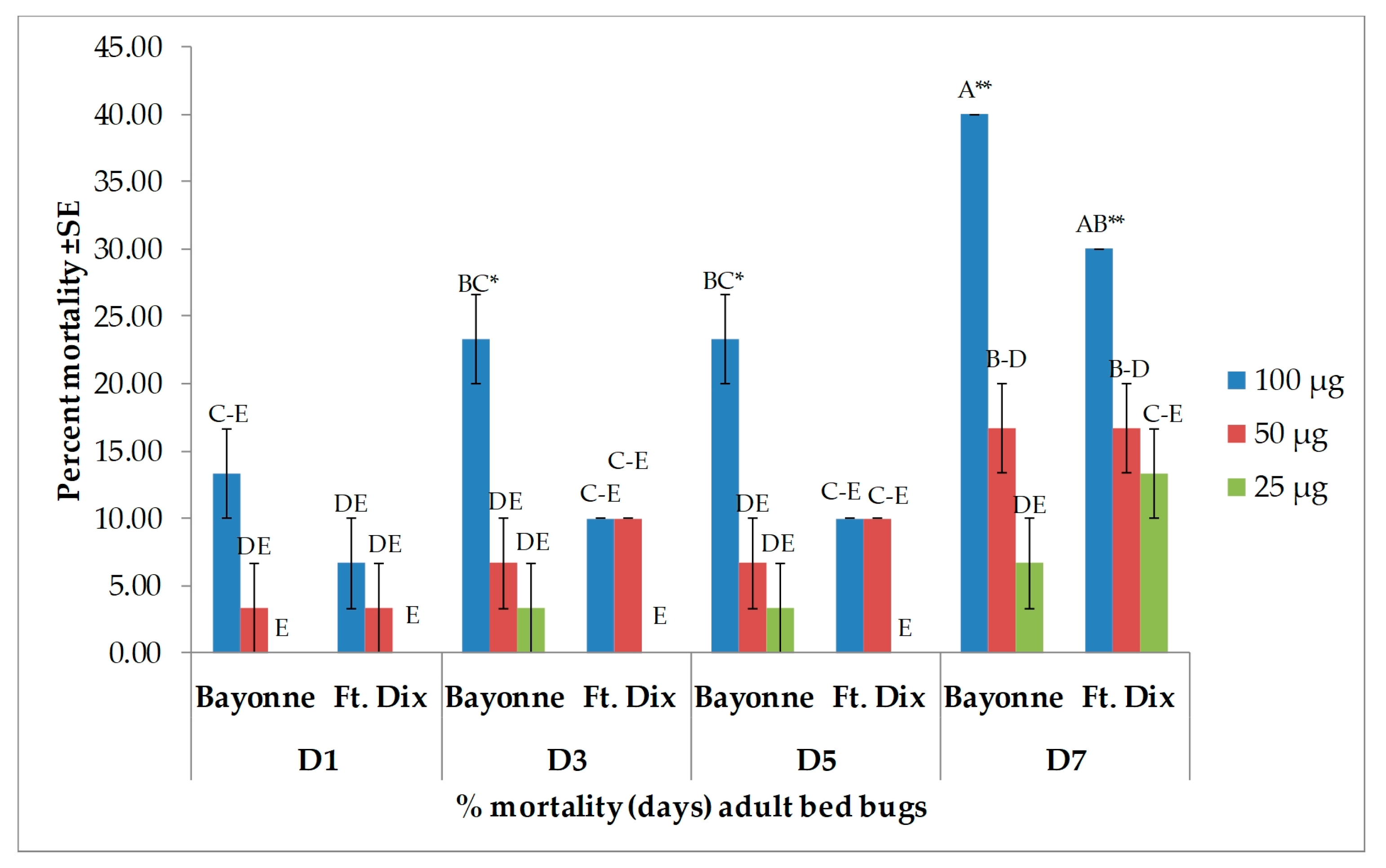
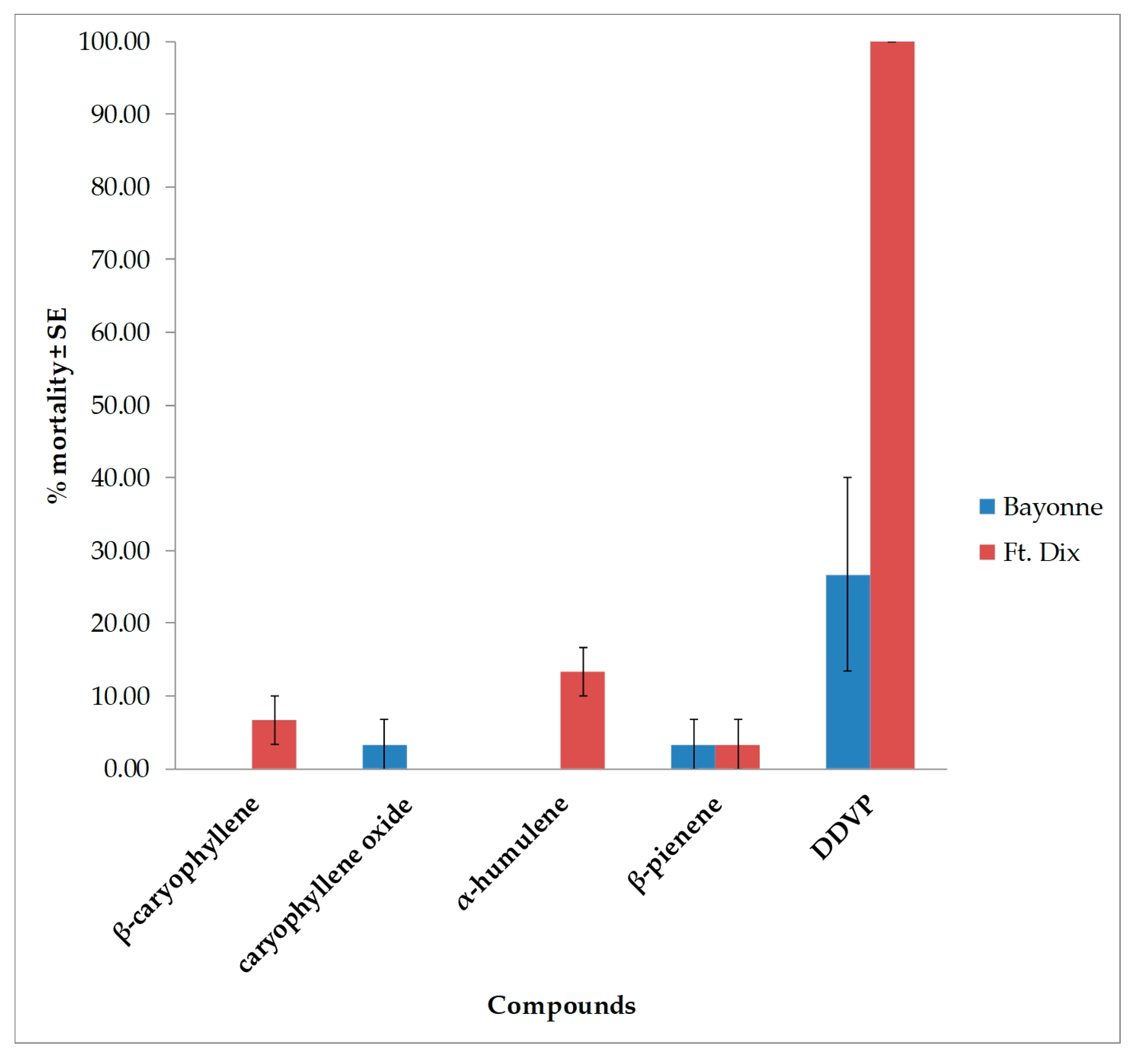
| Days after Treatment | Strain | Dose (µg/bed bug) | ||
|---|---|---|---|---|
| 100 | 50 | 25 | ||
| 1 | Bayonne | 61.9 ± 2.38 B | 7.1 ± 0.00 EF | 0.0 ± 0.00 F |
| Ft. Dix | 66.7 ± 2.38 B | 11.9 ± 6.30 DEF | 0.0 ± 0.00 F | |
| 3 | Bayonne | 61.9 ± 2.38 B | 14.3 ± 4.12 DEF | 0.0 ± 0.00 F |
| Ft. Dix | 66.7 ± 2.38 B | 28.6 ± 7.14 CDE | 4.8 ± 4.76 F | |
| 5 | Bayonne | 61.9 ± 2.38 B | 16.7 ± 2.38 DEF | 7.1 ± 7.14 EF |
| Ft. Dix | 90.5 ± 2.38 A | 28.6 ± 7.14 CDE | 4.8 ± 4.76 F | |
| 7 | Bayonne | 61.9 ± 2.38 B | 33.3 ± 6.30 CD | 16.7 ± 8.58 DEF |
| Ft. Dix | 90.5 ± 2.38 A | 47.6 ± 2.38 BC | 9.5 ± 2.38 EF | |
| Strain | DAY | β-caryophyllene | caryophyllene oxide | α-humulene | β-pinene | ||||
|---|---|---|---|---|---|---|---|---|---|
| 100 * | 300 * | 100 * | 300 * | 100 * | 300 * | 100 * | 300 * | ||
| Bayonne | 1 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0. 00 |
| 3 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| 5 | 3.3 ± 3.33 | 30.0 ± 15.27 | 3.3 ± 3.33 | 26.7 ± 21.85 | 10.0 ± 0.00 | 20.0 ± 5.77 | 0.0 ± 0.00 | 3.3 ± 3.33 | |
| 7 | 6.7 ± 6.67 | 33.3 ± 12.01 | 3.3 ± 3.33 | 30.0 ± 20.81 | 13.3 ± 3.33 | 26.7 ± 8.81 | 0.0 ± 0.00 | 10.0 ± 3.33 | |
| Ft. Dix | 1 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 |
| 3 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | 0.0 ± 0.00 | |
| 5 | 13.3 ± 3.33 | 40.0 ± 5.77 | 3.3 ± 3.33 | 13.3 ± 8.81 | 13.3 ± 3.33 | 30.0 ± 5.77 | 3.3 ± 3.33 | 13.3 ± 6.67 | |
| 7 | 16.7 ± 3.33 | 43.3 ± 8.81 | 3.3 ± 3.33 | 16.7 ± 8.81 | 13.3 ± 3.33 | 36.7 ± 3.33 | 3.3 ± 3.33 | 16.7 ± 3.33 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, J.U.; Wang, M.; Yang, Y.; Liu, Y.; Li, B.; Qin, Y.; Wang, W.; Chittiboyina, A.G.; Khan, I.A. Toxicity of Kadsura coccinea (Lem.) A. C. Sm. Essential Oil to the Bed Bug, Cimex lectularius L. (Hemiptera: Cimicidae). Insects 2019, 10, 162. https://doi.org/10.3390/insects10060162
Rehman JU, Wang M, Yang Y, Liu Y, Li B, Qin Y, Wang W, Chittiboyina AG, Khan IA. Toxicity of Kadsura coccinea (Lem.) A. C. Sm. Essential Oil to the Bed Bug, Cimex lectularius L. (Hemiptera: Cimicidae). Insects. 2019; 10(6):162. https://doi.org/10.3390/insects10060162
Chicago/Turabian StyleRehman, Junaid U., Mei Wang, Yupei Yang, Yongbei Liu, Bin Li, Yan Qin, Wei Wang, Amar G. Chittiboyina, and Ikhlas A. Khan. 2019. "Toxicity of Kadsura coccinea (Lem.) A. C. Sm. Essential Oil to the Bed Bug, Cimex lectularius L. (Hemiptera: Cimicidae)" Insects 10, no. 6: 162. https://doi.org/10.3390/insects10060162
APA StyleRehman, J. U., Wang, M., Yang, Y., Liu, Y., Li, B., Qin, Y., Wang, W., Chittiboyina, A. G., & Khan, I. A. (2019). Toxicity of Kadsura coccinea (Lem.) A. C. Sm. Essential Oil to the Bed Bug, Cimex lectularius L. (Hemiptera: Cimicidae). Insects, 10(6), 162. https://doi.org/10.3390/insects10060162






