Functional Morphology and Defensive Behavior in a Social Aphid
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling and Observation
2.2. Morphometry
2.3. Statistical Analysis
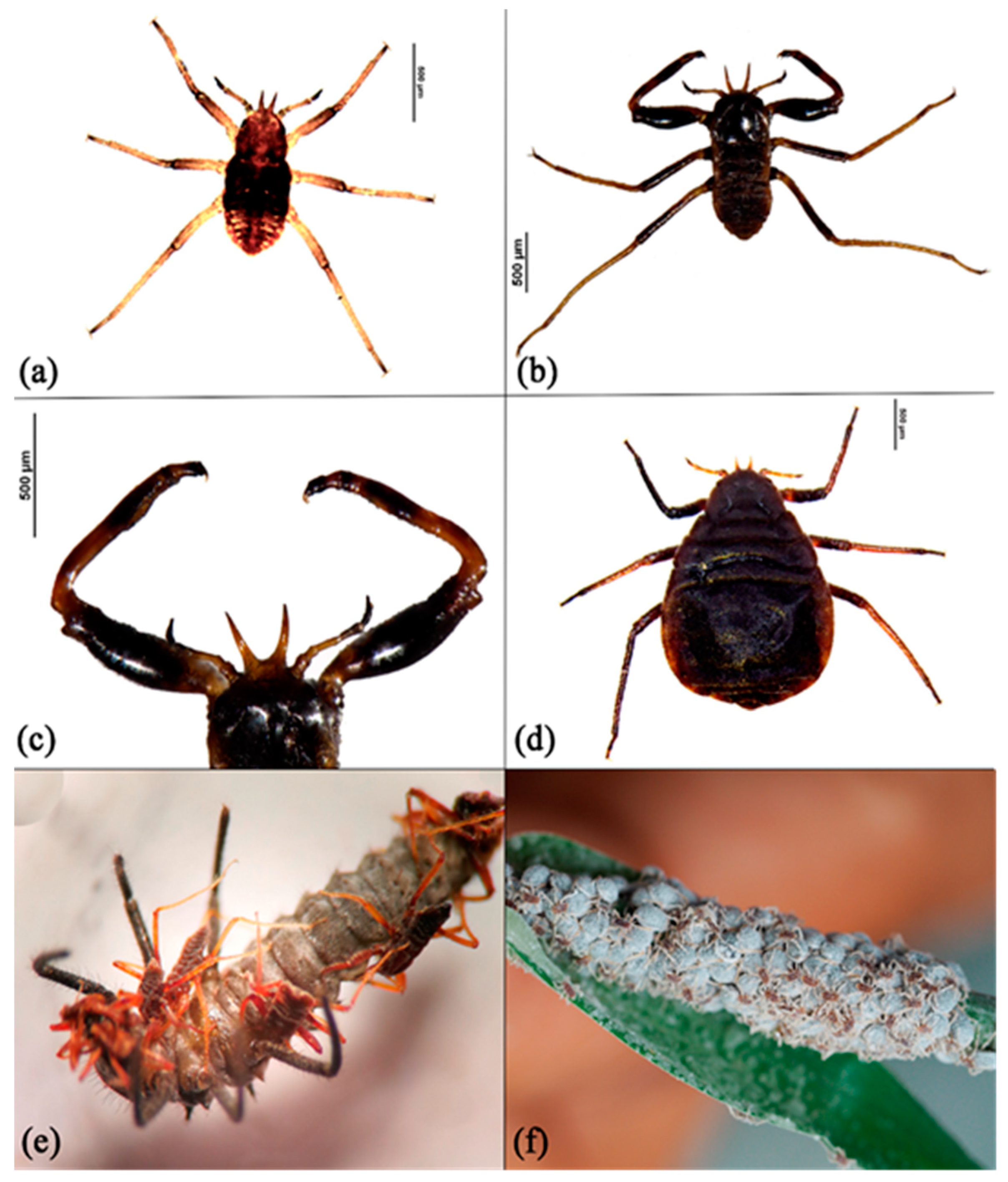
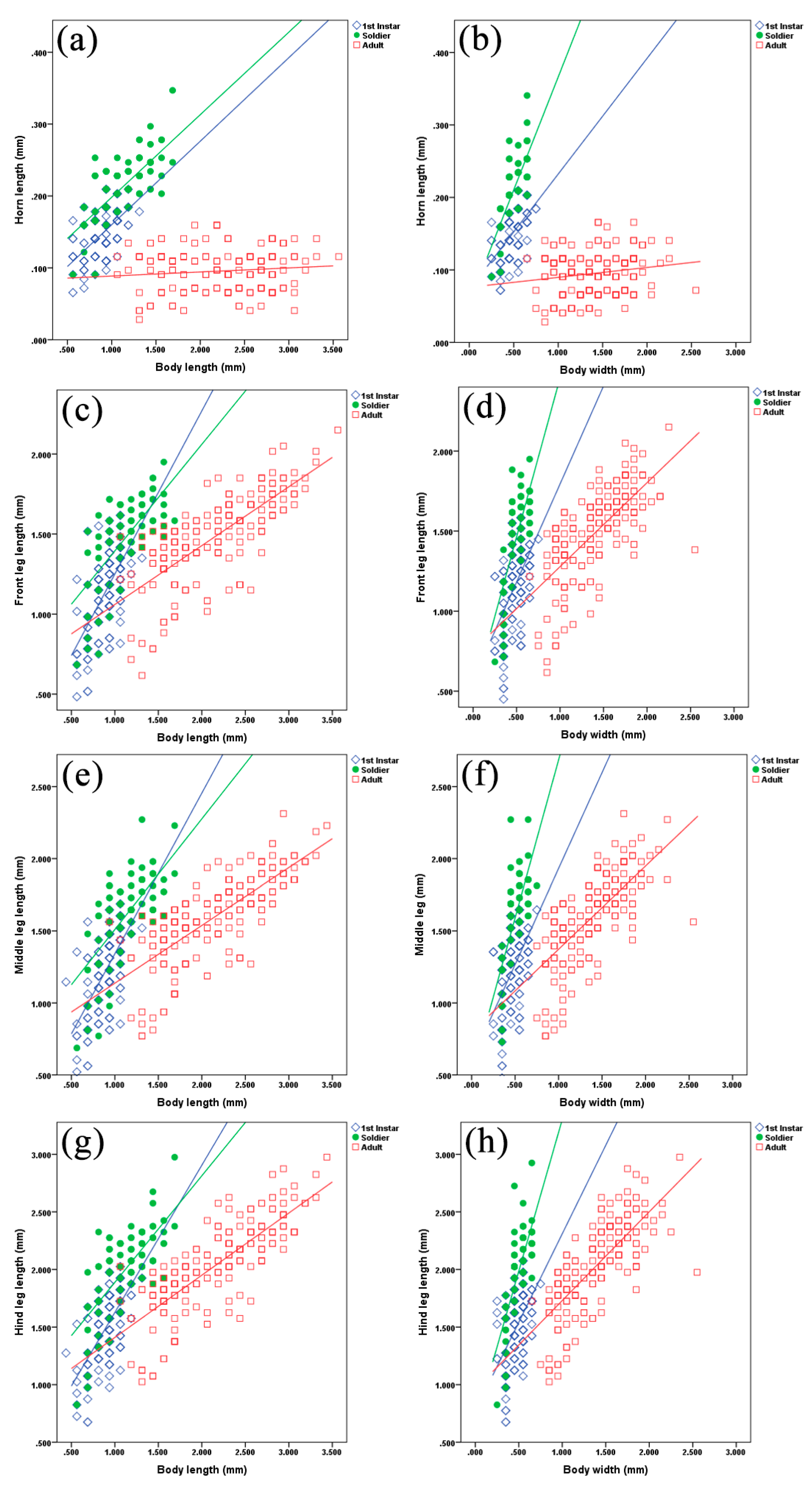
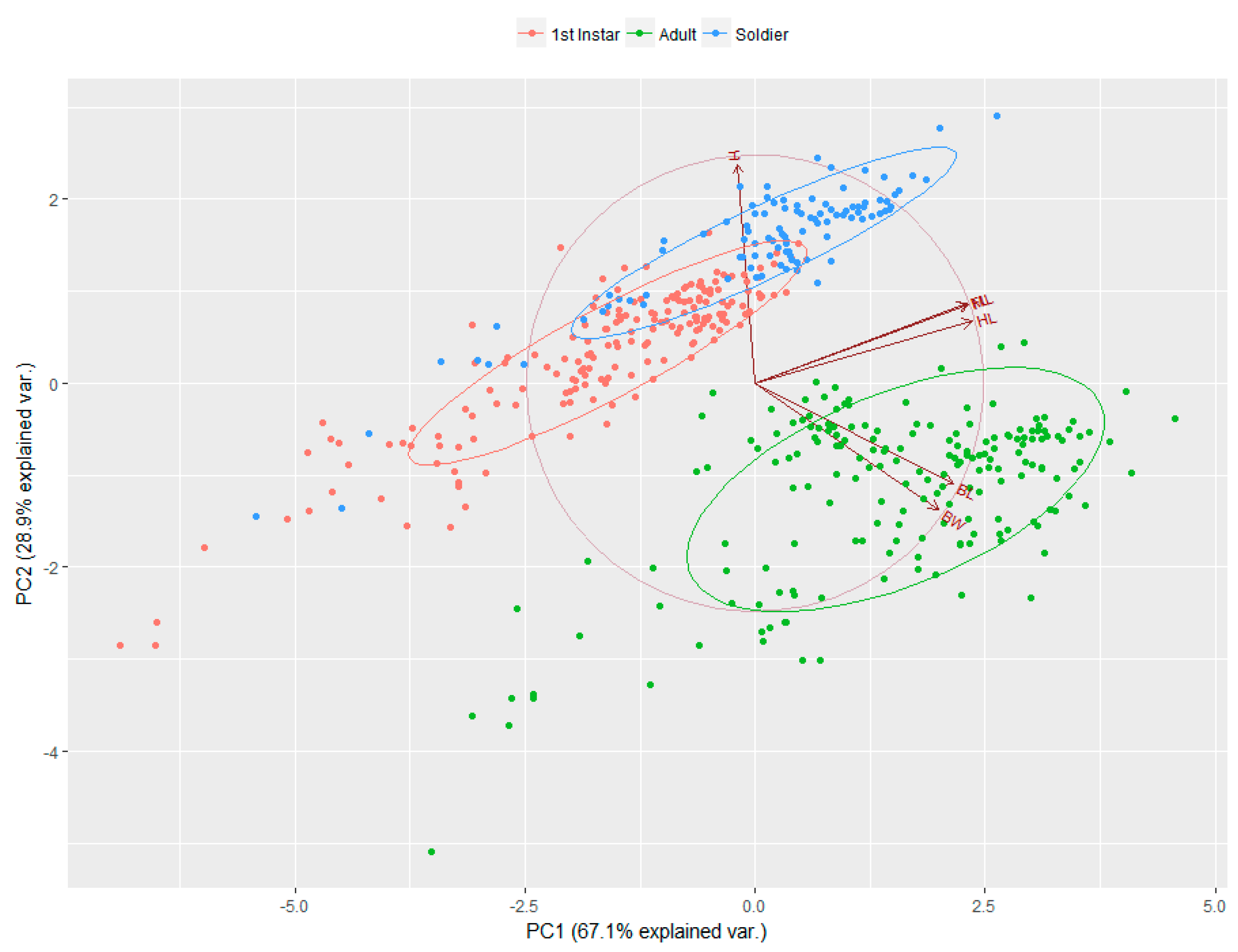
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coelho, P.; Kaliontzopoulou, A.; Rasko, M.; Meijden, A. Van Der A ‘striking’ relationship: Scorpion defensive behaviour and its relation to morphology and performance. Funct. Ecol. 2017, 31, 1390–1404. [Google Scholar] [CrossRef]
- Umbers, K.D.L.; Lehtonen, J.; Mappes, J. Deimatic displays. Curr. Biol. 2015, 25, R58–R59. [Google Scholar] [CrossRef] [PubMed]
- Umbers, K.D.L.; Mappes, J. Postattack deimatic display in the mountain katydid, Acripeza reticulata. Anim. Behav. 2015, 100, 68–73. [Google Scholar] [CrossRef]
- Vallin, A.; Jakobsson, S.; Wiklund, C. “An eye for an eye?”—On the generality of the intimidating quality of eyespots in a butterfly and a hawkmoth. Behav. Ecol. Sociobiol. 2007, 61, 1419–1424. [Google Scholar] [CrossRef]
- Kang, C.; Moon, H.; Sherratt, T.N.; Lee, S.I.; Jablonski, P.G. Multiple lines of anti-predator defence in the spotted lanternfly, Lycorma delicatula (Hemiptera: Fulgoridae). Biol. J. Linnean Soc. 2017, 120, 115–124. [Google Scholar] [CrossRef]
- Ijichi, N.; Shibao, H.; Miura, T.; Matsumoto, T.; Fukatsu, T. Soldier differentiation during embryogenesis of a social aphid, Pseudoregma bambucicola. Entomol. Sci. 2004, 7, 143–155. [Google Scholar] [CrossRef]
- Aoki, S.; Akimoto, S.; Yamane, S. Observations on Pseudoregma alexanderi (Homoptera, Pemphigidae), an aphid species producing pseudoscorpion-like soldiers on bamboos. Kontyu 1981, 49, 355–366. [Google Scholar]
- Sakata, K.; Itô, Y.; Yukawa, J.; Yamane, S. Ratio of sterile soldiers in the bamboo aphid, Pseudoregma bambucicola (Homoptera: Aphididae), colonies in relation to social and habitat conditions. App. Entomol. Zool. 1991, 26, 463–468. [Google Scholar] [CrossRef][Green Version]
- Aoki, S. Evolution of sterile soldiers in aphids. In Animal Societies: Theories and Facts; Japan Scientific Societies Press: Tokyo, Japan, 1987; pp. 53–65. [Google Scholar]
- Sunose, T.; Yamane, S.; Tsuda, K.; Takasu, K. What do the Soldiers of Pseudoregma bambucicola (Homoptera, Aphidoidea) Defend? Jpn. J. Entomol. 1991, 59, 141–148. [Google Scholar]
- Ohara, K. Observations of the prey-predator relationship between Pseudoregma bambucicola (Homoptera, Pemphigidae) and Metasyrphus coivfra ter (Diptera, Syrphidae), with special reference to the behaviour of tjhe aphid soldiers. ESAKIA 1985, 23, 107–110. [Google Scholar]
- Stern, B.Y.D.L.; Foster, W.A.; Foster, W.A. The evolution of soldiers in aphids. Biol. Rev. 1996, 71, 27–79. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Ito, Y. Life history characteristics and behaviour of the bamboo aphid, Pseudoregma bambucicola (Hemiptera: Pemphigidae), having sterile soldiers. Insectes Sociaux 1991, 38, 317–326. [Google Scholar] [CrossRef]
- Aoki, S.; Kurosu, U. A review of the biology of Cerataphidini (Hemiptera, Aphididae, Hormaphidinae), focusing mainly on their life cycles, gall formation, and soldiers. Psyche 2010, 2010, 380351. [Google Scholar] [CrossRef]
- Braendle, C.; Foster, W.A. Defensive behavior in primary- and secondary-host generations of the soldier-producing aphid, Pemphigus bursarius (Hemiptera: Aphididae). J. Insect Behav. 2004, 17, 663–672. [Google Scholar] [CrossRef]
- Shibao, H.; Kutsukake, M.; Matsuyama, S.; Fukatsu, T.; Shimada, M. Mechanisms regulating caste differentiation in an aphid social system. Commun. Integr. Biol. 2010, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.L.; Whitfield, J.A.; Foster, W.A. Behavior and morphology of monomorphic soldiers from the aphid genus Pseudoregma (Cerataphidini, Hormaphididae): Implications for the evolution of morphological castes in social aphids. Insectes Soc. 1997, 44, 379–392. [Google Scholar] [CrossRef]
- Sunose, T.; Tsuda, K.; Ohseko, S. Seasonal change in ratios of soldier in a population of the bamboo aphid, Psuedoregma bambucicola. Bull. Soc. Popul. Ecol. 1982, 35, 59–61. [Google Scholar]
- Aoki, S.; Kurosu, U.; Sirikajornjaru, W. A New Soldier-Producing Aphid Species, Pseudoregma baenzigeri, sp. nov., from Northern Thailand. J. Insect Sci. 2007, 7, 38. [Google Scholar] [CrossRef]
- Shibao, H. Social structure and the defensive role of soldiers in a eusocial bamboo aphid, Pseudoregma bambucicola (Homoptera: Aphididae): A test of the defence-optimization hypothesis. Popul. Ecol. 1998, 40, 325–333. [Google Scholar] [CrossRef]
- Ohara, K. Observations on the oviposition behaviour of Metasyrphus confrater (Diptera, Syrphidae) and the defensive behaviour of soldiers of Pseudoregma bambucicola (Homoptera, Pemphigidae). ESAKIA 1985, 23, 99–105. [Google Scholar]
- Oster, G.F.; Wilson, E.O. Caste and Ecology in the Social Insects; Princeton University Press: Princeton, NJ, USA, 1979; ISBN 0691023611. [Google Scholar]
- Prestwich, G.D. Defense mechanisms of termites. Annu. Rev. Entomol. 1984, 29, 201–232. [Google Scholar] [CrossRef]
- Smith, C.R.; Anderson, K.E.; Tillberg, C.V.; Gadau, J.; Suarez, A.V. Caste determination in a polymorphic social insect: Nutritional, social, and genetic factors. Am. Nat. 2008, 172, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.E. The developmental basis of worker polymorphism in fire ants. J. Insect Physiol. 1990, 36, 315–322. [Google Scholar] [CrossRef]
- Dixon, A.F.G. The escape responses shown by certain aphids to the presence of the coccinellid Adalia decempunctata (L.). Trans. R. Entomol. Soc. Lond. 1958, 110, 319–334. [Google Scholar] [CrossRef]
- Leung, T.L.F.; Poulin, R. Small worms, big appetites: Ratios of different functional morphs in relation to interspecific competition in trematode parasites. Int. J. Parasitol. 2011, 41, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Shibao, H.; Kutsukake, M.; Fukatsu, T. Density-dependent induction and suppression of soldier differentiation in an aphid social system. J. Insect Physiol. 2004, 50, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O. The Insect Societies; Harvard University Press: Cambridge, MA, USA, 1971; 55p. [Google Scholar]
- Benton, T.G.; Foster, W.A. Altruistic housekeeping in a social aphid. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1992, 247, 199–202. [Google Scholar]
- Pike, N.; Foster, W. Fortress repair in the social aphid species Pemphigus spyrothecae. Anim. Behav. 2004, 67, 909–914. [Google Scholar] [CrossRef]
- Aoki, S.; Miyazaki, M. Notes on the pseudoscorpion-like larvae of Pseudoregma alexanderi (Homoptera, Aphidoidea). Kontyu 1978, 46, 433–438. [Google Scholar]
- Aoki, S. A new species of Colophina (Homoptera, Aphidoidea) with soldiers. Insect 1977, 45, 333–337. [Google Scholar]
- Aoki, S.; Kurosu, U. Soldiers of a European gall aphid, Pemphigus spyrotecae (Homoptera: Aphidoidea): Why do they molt? J. Ethol. 1986, 4, 97–104. [Google Scholar] [CrossRef]
- Umbers, K.D.L.; Fabricant, S.A.; Gawryszewski, F.M.; Seago, A.E.; Herberstein, M.E. Reversible colour change in A rthropoda. Biol. Rev. 2014, 89, 820–848. [Google Scholar] [CrossRef] [PubMed]
- Bilska, A.; Francikowski, J.; Wyglenda, A.; Masłowski, A.; Kaszyca, N.; Depa, L. Aphids Playing Possum—Defensive or Mutualistic Response? J. Insect Behav. 2018, 31, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Dunning, D.C. Warning sounds of moths. Zeitschrift für Tierpsychologie 1968, 25, 129–138. [Google Scholar] [PubMed]
- Johnson, J.A.; Brodie, E.D., Jr. The selective advantage of the defensive posture of the newt, Taricha granulosa. Am. Midl. Nat. 1975, 93, 139–148. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, J.A.; Zou, X.; Liu, Q.; Zhang, H.; Lin, X.; Huang, X. Functional Morphology and Defensive Behavior in a Social Aphid. Insects 2019, 10, 163. https://doi.org/10.3390/insects10060163
Siddiqui JA, Zou X, Liu Q, Zhang H, Lin X, Huang X. Functional Morphology and Defensive Behavior in a Social Aphid. Insects. 2019; 10(6):163. https://doi.org/10.3390/insects10060163
Chicago/Turabian StyleSiddiqui, Junaid Ali, Xuting Zou, Qian Liu, Hui Zhang, Xiaolan Lin, and Xiaolei Huang. 2019. "Functional Morphology and Defensive Behavior in a Social Aphid" Insects 10, no. 6: 163. https://doi.org/10.3390/insects10060163
APA StyleSiddiqui, J. A., Zou, X., Liu, Q., Zhang, H., Lin, X., & Huang, X. (2019). Functional Morphology and Defensive Behavior in a Social Aphid. Insects, 10(6), 163. https://doi.org/10.3390/insects10060163





