Detection of Yellow Fever Virus in Sylvatic Mosquitoes during Disease Outbreaks of 2017–2018 in Minas Gerais State, Brazil
Abstract
1. Introduction
2. Materials and Methods
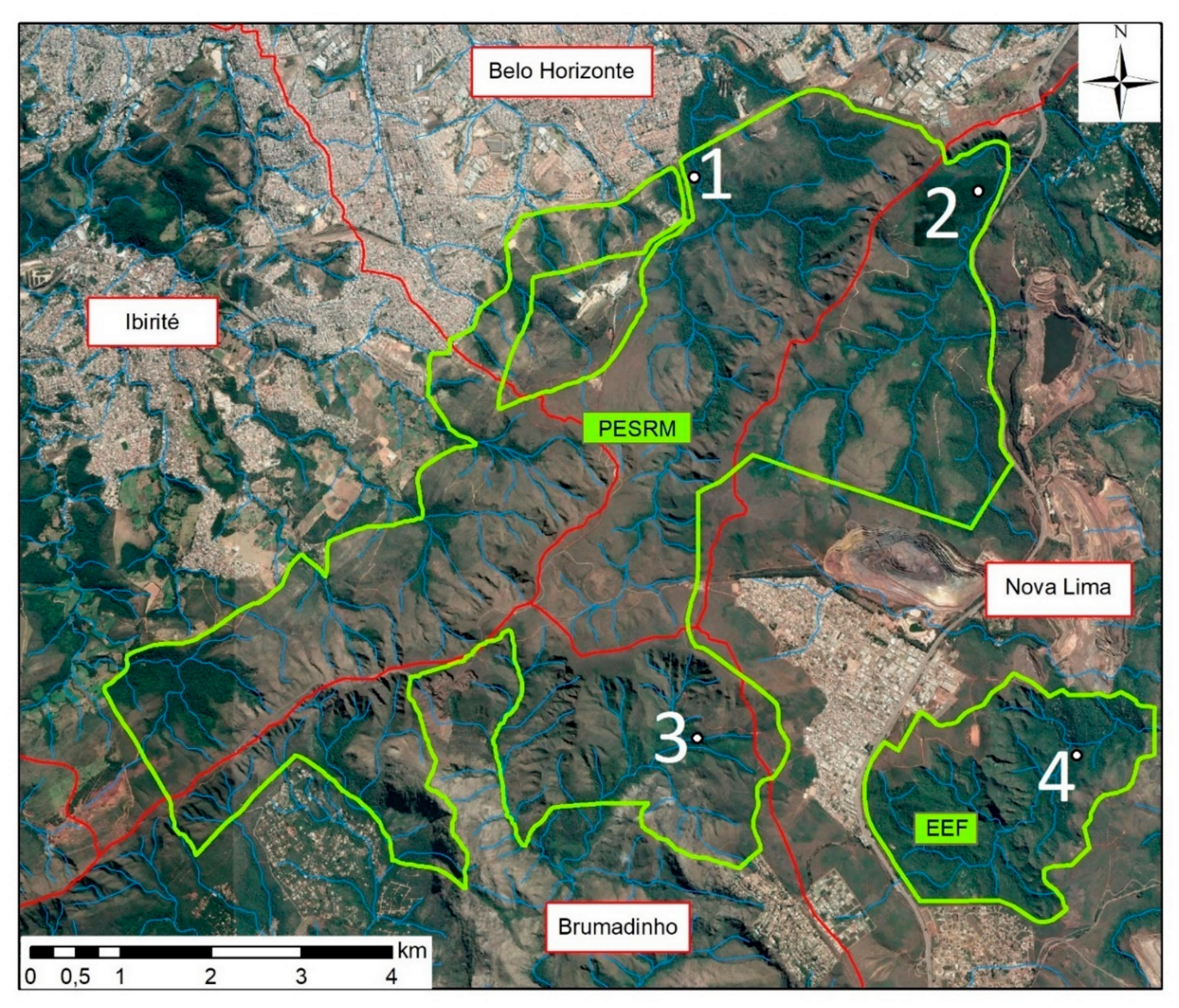
2.1. Study Area
2.2. Mosquito Collection and Identification
2.3. Virus Detection
2.4. Virus Isolation
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monath, T.P. Treatment of yellow fever. Antiviral Res. 2008, 78, 116–124. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow fever. J. Clin. Virol. 2015, 64, 160–173. [Google Scholar]
- Goldani, L.Z. Yellow fever outbreak in Brazil, 2017. Brazilian J. Infect. Dis. 2017, 21, 123–124. [Google Scholar] [CrossRef]
- Forattini, O.P. Principais mosquitos de importância sanitária no Brasil. Cad. Saude Publica 1995. [Google Scholar] [CrossRef]
- Consoli, R.; de Oliveira, R. Principais Mosquitos de Importância Sanitária no Brasil; Editora FIOCRUZ: Rio de Janeiro, Brazil, 1994; pp. 39–152. [Google Scholar]
- Franco, O. História da febre amarela no Brasil; Ministério da Saúde: Rio de Janeiro, Brazil, 1969; pp. 8–95. [Google Scholar]
- World Health Organization. A Global Strategy to Eliminate Yellow Fever Epidemics (EYE) 2017–2026. WHO: Geneva, Switzerland, 2017; ISBN 9789241513661. [Google Scholar]
- Monitoramento do Período Sazonal da Febre Amarela Brasil—2017/2018. Available online: http://portalarquivos2.saude.gov.br/images/pdf/2018/outubro/08/Informe-FA.pdf (accessed on 29 March 2019).
- Litvoc, M.N.; Novaes, C.T.G.; Lopes, M.I.B.F. Yellow fever. Rev. Assoc. Med. Bras. 2018, 64, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.M.; de Abreu, F.V.S.; Dos Santos, A.A.C.; de Mello, I.S.; Santos, M.P.; Ribeiro, I.P.; Ferreira-de-Brito, A.; de Miranda, R.M.; de Castro, M.G.; Ribeiro, M.S.; et al. Genomic and structural features of the yellow fever virus from the 2016-2017 Brazilian outbreak. J. Gen. Virol. 2018, 99, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Monitoramento dos casos de dengue, febre de chikungunya e doença aguda pelo vírus Zika até a Semana Epidemiológica 44 de 2018. Available online: http://portalarquivos2.saude.gov.br/images/pdf/2018/novembro/23/Publicacao-2018-57-SE44.pdf (accessed on 29 March 2019).
- Zuchi, N.; da Silva Heinen, L.B.; dos Santos, M.A.M.; Pereira, F.C.; Slhessarenko, R.D. Molecular detection of Mayaro virus during a dengue outbreak in the state of Mato Grosso, Central-West Brazil. Mem. Inst. Oswaldo Cruz 2014, 109, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Instituto Estadual de Florstas. Plano de Manejo do Parque Estadual da Serra do Rola-moça, incluindo Estação Ecológica de Fechos; IEF: Belo Horizonte, Brazil, 2007; pp. 11–35.
- Pugedo, H.; Barata, R.A.; França-Silva, J.C.; Silva, J.C.; Dias, E.S. HP: um modelo aprimorado de armadilha luminosa de sucção para a captura de pequenos insetos TT—HP: An improved model of sucction light trap for the capture of small insects. Rev. Soc. Bras. Med. Trop. 2005, 38, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Harbach, R.E.; Kitching, I.J. Phylogeny and classification of the Culicidae (Diptera). Syst. Entomol. 1998, 23. [Google Scholar] [CrossRef]
- Dutra, H.L.C.; Rodrigues, S.L.; Mansur, S.B.; de Oliveira, S.P.; Caragata, E.P.; Moreira, L.A. Development and physiological effects of an artificial diet for Wolbachia-infected Aedes aegypti. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Méndez, J.A.; Nakouné, E.R.; Niedrig, M. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.N.; Duarte, M.M.; Mansur, S.B.; Daoud, B.; Pereira, T.N.; Émile, T.; Adelino, R.; Giovanetti, M.; Carlos, L.; Alcantara, J.; et al. Pluripotency of Wolbachia against Arbovirus: The case of yellow fever. [version 1; peer review: 1 approved, 1 approved with reservations]. Gates Open Res. 2019, 1–17. [Google Scholar]
- Pastorino, B.; Bessaud, M.; Grandadam, M.; Murri, S.; Tolou, H.J.; Peyrefitte, C.N. Development of a TaqMan® RT-PCR assay without RNA extraction step for the detection and quantification of African Chikungunya viruses. J. Virol. Methods 2005, 124, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Pacidônio, E.C.; Caragata, E.P.; Alves, D.M.; Marques, J.T.; Moreira, L.A. The impact of Wolbachia infection on the rate of vertical transmission of dengue virus in Brazilian Aedes aegypti. Parasites Vectors 2017, 10, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Long, K.C.; Ziegler, S.A.; Thangamani, S.; Hausser, N.L.; Kochel, T.J.; Higgs, S.; Tesh, R.B. Experimental transmission of Mayaro virus by Aedes aegypti. Am. J. Trop. Med. Hyg. 2011, 85, 750–757. [Google Scholar] [CrossRef]
- Figueiredo, L.B.; Cecílio, A.B.; Ferreira, G.P.; Drumond, B.P.; de Oliveira, J.G.; Bonjardim, C.A.; Ferreira, P.C.P.; Kroon, E.G. Dengue virus 3 genotype 1 associated with dengue fever and dengue hemorrhagic fever, Brazil. Emerg. Infect. Dis. 2008, 14, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.P.; da Silva, J.E. Aspectos da biologia dos culicinae do Parque das Mangabeiras, Belo Horizonte. I. Espécies locais e variação estacional. Arq. da Esc. Veterinária da UFMG 1973, 25. [Google Scholar]
- Dégallier, N.; Travassos da Rosa, A.P.A.; Vasconcelos, P.F.C.; Travassos da Rosa, E.S.; Rodrigues, S.G.; Sa, F.G.C.; Travassos da Rosa, J.F.S. New entomological and virological data on the vectors of sylvatic yellow fever in Brazil. Arthropod-Borne Virus Inf. Exch. 1993, 21–22. [Google Scholar]
- Goenaga, S.; Fabbri, C.; Dueñas, J.C.R.; Gardenal, C.N.; Rossi, G.C.; Calderon, G.; Morales, M.A.; Garcia, J.B.; Enria, D.A.; Levis, S. Isolation of Yellow Fever Virus from Mosquitoes in Misiones Province, Argentina. Vector-Borne Zoonotic Dis. 2012, 12, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Hervé, J.-P.; Dégallier, N.; Rosa, A.P.A.T.; da Filho, G.C.S. A febre amarela silvestre no Brasil e os riscos de propagação urbana. Hiléia Médica 1985, 7, 31–40. [Google Scholar]
- Cardoso, J.d.C.; de Almeida, M.A.B.; dos Santos, E.; da Fonseca, D.F.; Sallum, M.A.M.; Noll, C.A.; Monteiro, H.A.d.O.; Cruz, A.C.R.; Carvalho, V.L.; Pinto, E.V.; et al. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus mosquitoes, Southern Brazil, 2008. Emerg. Infect. Dis. 2010, 16, 1918–1924. [Google Scholar]
- de Souza, R.P.; Petrella, S.; Coimbra, T.L.M.; Maeda, A.Y.; Rocco, I.M.; Bisordi, I.; Silveira, V.R.; Pereira, L.E.; Suzuki, A.; dos Santos Silva, S.J.; et al. Isolation of yellow fever virus (YFV) from naturally infected Haemagogus (Conopostegus) leucocelaenus (Diptera, Culicidae) in São Paulo state, Brazil, 2009. Rev. Inst. Med. Trop. Sao Paulo 2011, 53, 133–139. [Google Scholar] [CrossRef]
- Marcondes, C.B.; Alencar, J. Revisão de mosquitos Haemagogus Williston (Diptera: Culicidae) do Brasil. Rev. Biomed. 2010, 21, 221–238. [Google Scholar]
- Prefeitura de Belo Horizonte—PBH A experiência de Belo Horizonte no enfrentamento às arboviroses: Dengue, zika e chikungunya. Secr. Munic. Saúde 2016, 28.
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139. [Google Scholar] [CrossRef] [PubMed]
- Terzian, A.C.B.; Zini, N.; Sacchetto, L.; Rocha, R.F.; Parra, M.C.P.; Del Sarto, J.L.; Dias, A.C.F.; Coutinho, F.; Rayra, J.; da Silva, R.A.; et al. Evidence of natural Zika virus infection in neotropical non-human primates in Brazil. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Pereira Serra, O.; Fernandes Cardoso, B.; Maria Ribeiro, A.L.; dos Santos, F.A.L.; Dezengrini Slhessarenko, R. Mayaro virus and dengue virus 1 and 4 natural infection in culicids from Cuiabá, state of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Mondet, B.; Vasconcelos, P.F.C.; Travassos da Rosa, A.P.A.; Travassos da Rosa, E.S.; Rodrigues, S.G.; Travassos da Rosa, J.F.S.; Bicout, D.J. Isolation of Yellow Fever Virus from Nulliparous Haemagogus (Haemagogus) janthinomys in Eastern Amazonia. Vector-Borne Zoonotic Dis. 2002, 2, 47–50. [Google Scholar] [CrossRef]
- Martins, R.M.; Maia, M.D.L.S.; Farias, R.H.G.; Camacho, L.A.B.; Freire, M.S.; Galler, R.; Yamamura, A.M.Y.; Almeida, L.F.C.; Lima, S.M.B.; Nogueira, R.M.R.; et al. 17DD yellow fever vaccine: A double blind, randomized clinical trial of immunogenicity and safety on a dose-response study. Hum. Vaccines Immunother. 2013, 9, 879–888. [Google Scholar] [CrossRef]
- Secretaria Estadual de Saúde/MG. Cobertura Vacinal em Minas Gerais. Available online: http://www.saude.mg.gov.br/febreamarela (accessed on 9 April 2019).
- Secretaria Estadual de Saúde/MG. Boletim epidemiológico—21/06/2018. Available online: http://www.saude.mg.gov.br/component/gmg/story/10625-informe-epidemiologico-da-febre-amarela-21-06 (accessed on 9 April 2019).
- Possas, C.; Lourenço-de-Oliveira, R.; Tauil, P.L.; Pinheiro, F.d.P.; Pissinatti, A.; Cunha, R.V.d.C.; Freire, M.; Martins, R.M.; Homma, A. Yellow fever outbreak in Brazil: The puzzle of rapid viral spread and challenges for immunisation. Mem. Inst. Oswaldo Cruz 2018, 113, e180278. [Google Scholar] [CrossRef]
- Secretaria Estadual de Saúde/SP. Boletim Epidemiológico Febre Amarela—04/02/2019. Available online: http://www.saude.sp.gov.br/resources/cve-centro-de-vigilancia-epidemiologica/areas-de-vigilancia/doencas-de-transmissao-por-vetores-e-zoonoses/doc/famarela/2019/fa19_boletim_epid_0402.pdf (accessed on 9 April 2019).
- Bicca-marques, J.C.; Freitas, D.S. De Conservation letter The role of monkeys, mosquitoes, and humans in the occurrence of a yellow fever outbreak in a fragmented landscape in south Brazil: Protecting howler monkeys is a matter of public health. Access 2010, 3, 78–89. [Google Scholar]
- Almeida, M.A.B.; da Cardoso, J.C.; dos Santos, E.; da Fonseca, D.F.; Cruz, L.L.; Faraco, F.J.C.; Bercini, M.A.; Vettorello, K.C.; Porto, M.A.; Mohrdieck, R.; et al. Surveillance for Yellow Fever Virus in Non-Human Primates in Southern Brazil, 2001–2011: A Tool for Prioritizing Human Populations for Vaccination. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Causey, O.R.; Kumm, H.W.; Laemmert, H.W. Dispersion of forest mosquitoes in Brazil; further studies. Am. J. Trop. Med. Hyg. 1950, s1-30. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Travassos da Rosa, A.P.A.; Moraes, M.A.P. An epidemic of yellow fever in central Brazil, 1972-1973. II. Ecological studies. Am. J. Trop. Med. Hyg. 1981, 30. [Google Scholar] [CrossRef]

| Virus | Sequence | Reference |
|---|---|---|
| Yellow fever virus (YFV) | F-5’/GCT AAT TGA GGT GYA TTG GTC TGC/3’ | [17] |
| R-5’/CTG CTA ATC GCT CAA MGA ACG/3’ | ||
| P-5’/FAM/ATC GAG TTG/ZEN/CTA GGC AAT AAA CAC/3lAbRQSp/3’ | ||
| Chikungunya virus (CHIKV) | F-5’/AAG CTY CGC GTC CTT TAC CAA G/3’ | [19] |
| R-5’/CCA AAT TGT CCY GGT CTT CCT/3’ | ||
| P-5´/5HEX/CCA ATG TCY/ZEN/TCM GCC TGG ACA CCT TT/3IABkFQ/3´ | ||
| Dengue virus (DENV) | F-5´/GGA AGC TGT ACC TTG GTG GTA AGG A/3’ | [20] with modifications |
| R-5’/CGT TCT GTG CCT GGA ATG ATG/3’ | ||
| P-5’/TEX615/AAC AGC ATA TTG ACG CTG GGA GAG ACC AGA/3IAbRQSp/3’ | ||
| Zika virus (ZIKV) | F-5’/TTG GTC ATG ATA CTG CTG ATT GC/3’ | [21] |
| R-5’/CCT TCC ACA AAG TCC CTA TTG C/3’ | ||
| P-5’/FAM/CGG CAT ACA/ZEN/GCA TCA GGT GCA TAG GAG/3IABKFQ/3’ | ||
| Mayaro virus (MAYV) | F-5′/GTG GTC GCA CAG TGA ATC TTT C/3′ | [22] |
| R-5′/CAA ATG TCC ACC AGG CGA AG/3′ | ||
| P-5′/HEX/ATG GTG GTA/ZEN/GGC TAT CCG ACA GGT C/3IABKFQ/3’ |
| Taxonomic Category | Sep | Dec | Feb/Mar | May | Total |
|---|---|---|---|---|---|
| Psorophora ferox | 0 | 25 | 243 | 3 | 271 (31.3%) |
| Limatus durhamii | 7 | 36 | 118 | 5 | 166 (19.1%) |
| Haemagogus janthinomys | 0 | 77 | 81 | 0 | 158 (18.2%) |
| Aedes serratus | 0 | 53 | 43 | 1 | 97 (11.2%) |
| Haemagogus leucocelaenus | 0 | 24 | 18 | 0 | 42 (4.8%) |
| Culex (Anoedioporpa) sp | 30 | 0 | 0 | 0 | 30 (3.5%) |
| Sabethes purpureus | 6 | 4 | 12 | 2 | 24 (2.8%) |
| Aedes albopictus | 0 | 8 | 8 | 0 | 16 (1.8%) |
| Sabethes petrocchiae | 0 | 2 | 7 | 5 | 14 (1.6%) |
| Aedes terrens | 0 | 10 | 0 | 0 | 10 (1.2%) |
| Aedeomyia squamipennis | 2 | 2 | 0 | 1 | 5 (0.6%) |
| Culex sp | 0 | 4 | 1 | 0 | 5 (0.6%) |
| Culex (Culex) sp | 0 | 1 | 1 | 2 | 4 (0.5%) |
| Culex (Melanoconion) sp | 1 | 0 | 1 | 2 | 4 (0.5%) |
| Sabethes albiprivus | 2 | 0 | 2 | 0 | 4 (0.5%) |
| Aedes fluviatilis | 0 | 0 | 2 | 0 | 2 (0.2%) |
| Culex (Microculex) sp | 0 | 0 | 2 | 0 | 2 (0.2%) |
| Psorophora albigenu | 0 | 2 | 0 | 0 | 2 (0.2%) |
| Runchomyia cerqueirai | 2 | 0 | 0 | 0 | 2 (0.2%) |
| Wyeomyia fuscipes | 2 | 0 | 0 | 0 | 2 (0.2%) |
| Wyeomyia moerbista | 0 | 0 | 2 | 0 | 2 (0.2%) |
| Anopheles eiseni | 1 | 0 | 0 | 0 | 1 (0.1%) |
| Culex declarator | 0 | 0 | 1 | 0 | 1 (0.1%) |
| Runchomyia reversa | 0 | 1 | 0 | 0 | 1 (0.1%) |
| Wyeomyia alani | 0 | 0 | 1 | 0 | 1 (0.1%) |
| Wyeomyia sp | 0 | 0 | 1 | 0 | 1 (0.1%) |
| Total | 53 (6.1%) | 249 (28.7%) | 544 (62.7%) | 21 (2.4%) | 867 (100%) |
| Sampled Areas | Infected/Sampled | Infection Rate |
|---|---|---|
| Area 1 | 0/13 | 0% |
| Area 2 | 1/11 | 9.9% |
| Area 3 | 1/109 | 0.9% |
| Area 4 | 11/25 | 44% |
| Total | 13/158 | 8.2% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, G.G.; Rocha, M.N.; de Oliveira, M.A.; Moreira, L.A.; Andrade Filho, J.D. Detection of Yellow Fever Virus in Sylvatic Mosquitoes during Disease Outbreaks of 2017–2018 in Minas Gerais State, Brazil. Insects 2019, 10, 136. https://doi.org/10.3390/insects10050136
Pinheiro GG, Rocha MN, de Oliveira MA, Moreira LA, Andrade Filho JD. Detection of Yellow Fever Virus in Sylvatic Mosquitoes during Disease Outbreaks of 2017–2018 in Minas Gerais State, Brazil. Insects. 2019; 10(5):136. https://doi.org/10.3390/insects10050136
Chicago/Turabian StylePinheiro, Guilherme Garcia, Marcele Neves Rocha, Maria Angélica de Oliveira, Luciano Andrade Moreira, and José Dilermando Andrade Filho. 2019. "Detection of Yellow Fever Virus in Sylvatic Mosquitoes during Disease Outbreaks of 2017–2018 in Minas Gerais State, Brazil" Insects 10, no. 5: 136. https://doi.org/10.3390/insects10050136
APA StylePinheiro, G. G., Rocha, M. N., de Oliveira, M. A., Moreira, L. A., & Andrade Filho, J. D. (2019). Detection of Yellow Fever Virus in Sylvatic Mosquitoes during Disease Outbreaks of 2017–2018 in Minas Gerais State, Brazil. Insects, 10(5), 136. https://doi.org/10.3390/insects10050136





