Abstract
Numerous species of the family Chrysopidae, commonly found in agroecosystems, whose larvae predate on several pests of economic importance, are regarded as biological control agents. Their abundance and diversity are influenced by vegetation cover, although little is known about the effects of semi-natural habitats on their populations. The objective of this study is to gain a better understanding of the relationship between the trees in semi-natural habitats adjacent to olive groves, juvenile stages of the family Chrysopidae and factors influencing their population decline, which is crucial for an effective habitat management program aimed at conserving these important predators. Using cardboard band traps (eight per tree), the juvenile stages were collected from 25 almond, oak, olive and pine trees over a one-year sampling period. The population decline was caused by parasitoids (26.5%), predators (5.1%) and unknown factors (13.2%). In addition, chrysopids established in olive trees showed the lowest rate of parasitism. We identified ten chrysopid species that emerged from the juveniles collected from almond, oak, olive and pine trees, with a predominance of Pseudomallada prasinus. The chrysopid–parasitoid complex was composed of five species; Baryscapus impeditus (Eulophidae), which was the most abundant, was preferentially associated with Chrysopa pallens, Chrysoperla lucasina and Chrysoperla mediterranea.
1. Introduction
Of the many families of the Order Neuroptera, Chrysopidae attracted the most attention as compared to Coniopterygidae and Hemerobiidae [1], as numerous species belonging to the Chrysopidae family are regarded as biological control agents given their potential impact on pest populations in crops [2,3,4,5,6]. Larvae are active polyphagous predators of soft-bodied arthropods, such as aphids, whiteflies, thrips and mites, in addition to being widely distributed in agroecosystems [2,3,4,5,6].
Chrysopidae is the second most important family in terms of the number and diversity of species with 1423 valid species belonging to 82 genera [7]. Chrysoperla carnea (Stephens, 1836) sensu lato, which has been reared and released in crops around the world [8,9,10,11], is the species most commonly used in agricultural biological control programs [12]. There is evidence that C. carnea is a complex of at least 21 cryptic species [1,13,14]. Although some species are well defined with respect to morphological characteristics, habitats, courtship songs and molecular techniques, their taxonomy has not been fully resolved [15,16,17,18,19]. A recent review of the green lacewing showed that seven species belong to the Chrysoperla Steinmann, 1964 genus in the Iberian Peninsula and Balearic Island [1].
In previous studies 33 species of the Chrysopidae family were identified in olive groves, with the Chrysoperla carnea complex (Stephens, 1836) and the genus Pseudomallada Tsukaguchi, 1995 being particularly noteworthy [20,21,22,23]. The larval stages of these chrysopids are key predators of the three main pests in olive groves: Prays oleae (Bernard, 1788), Saissetia oleae (Olivier, 1791) and Euphyllura olivina (Costa, 1839) [3,20,24,25]. The use of green lacewings to improve biological pest control in olive groves has been evaluated [26]. McEwen et al. [27] attempted to attract C. carnea by spraying artificial honeydew [27], and another study has shown that a relationship exists between non-crop vegetation and green lacewing oviposition in olive groves [28]. Porcel et al. [29] also found that resident vegetation cover has a positive effect on green lacewings abundance and diversity in olive groves. However, the role of semi-natural habitats adjacent (bordering and around) to olive groves is poorly understood.
Chrysopid populations are regulated by predation (intraguild and cannibalism) and parasitism which are particularly harmful [30,31], and their development is also affected by abiotic conditions such as temperature, humidity and day length [32,33,34,35,36]. In fact, the eggs and larvae of C. carnea s.l. are attacked and killed by coccinellids, reduvids, carabids, spiders and ants [37,38,39,40,41], as well as by cannibalistic individuals from its own species [42]. The chrysopid–parasitoid complex is composed of species from the Orders Hymenoptera and Diptera, in addition to mites, fungae and certain viruses, which can affect all stages of chrysopid development, ranging from the egg and larva stages to adulthood [31,43,44,45,46,47]; some genera of the Order Hymenoptera, such as Isodromus Howard, 1887, Baryscapus Förster, 1856, Helorus Latreille, 1802 and Gelis Thunberg, 1827 are primary parasitoids of Chrysopidae, while others, such as Perilampus Latreille, 1809, Dichrogaster Doumerc, 1855, Pteromalus Swederus, 1795 and Eupelmus Dalman, 1820, are primary parasitoids of Chrysopidae and hyperparasitoids [47,48,49,50,51,52,53,54,55,56,57,58]. Other factors affecting larval mortality include abiotic conditions and the food resource availability. The impact of all these factors can vary according to the species of chrysopid and its habitat, which need to be accurately characterized when biological control is planned both for conservation purposes and through mass release of chrysopids [59].
Faced with natural enemies, chrysopids have developed defensive strategies and behaviours, such as nocturnal and twilight activity, cryptic, aposematic and disruptive coloration [60,61], stalked eggs [62,63,64], thanatosis [6], as well as segregation of foul-smelling substances produced by adults and toxic, crippling and disruptive substances secreted by larvae [6,61,65,66,67,68,69]. It has also been suggested that exogenous material on the backs of larvae of certain chrysopid genera (Pseudomallada and Rexa Navás, 1920) could act as a physical barrier against predators and parasitoids [62,69,70,71,72,73].
Given the generalist predatory behaviour and dispersive capacity of chrysopids, their populations in olive groves are influenced by the vegetation and natural habitats adjacent to the crop, where they can find alternative prey, pollen, nectar, as well as reproduction and refuge sites. Thus, spontaneous vegetation cover between the rows of olive trees has been reported to increase chrysopid abundance and diversity in the crop [29]. Additionally, tree species such as Quercus rotundifolia Lam., Pinus halepensis Mill. and Prunus dulcis (Mill.) D.A. Webb, which are an integral part of the olive grove landscape in Spain, are visited by chrysopids [3,21,74] and used as oviposition sites by different species [75]. Studies of their population dynamics in olive groves should therefore include the effect of adjacent vegetation, as research on chrysopid parasitism has, up to now, focused on different arboreal species and crops while neglecting activity in the surrounding landscape [45,47,49,51,58,76,77,78,79].
This study aims to assess the relationship between trees in semi-natural habitats adjacent to olive groves, the juvenile stages of the family Chrysopidae and population decline factors (parasitism, predation and unknown factors).
We expected (a) to collect chrysopid juveniles from all the tree species studied, from which adult chrysopids had previously been sampled [21], and (b) to record a medium to high chrysopid parasitism rate in olive trees which was predicted to be similar in all three trees species (almond, oak and pine) [50,78,80]. Finally, as we expected the chrysopids to be parasitized, we studied the relationship between parasitoid and chrysopid assemblages while taking into account the season and tree species (almond, oak, olive and pine) in which the interaction occurred.
The knowledge acquired is a crucial prerequisite for an effective habitat management program aimed at conserving the populations of these important predators.
2. Materials and Methods
2.1. Area of Study
The study was carried out in the Montes Orientales region, 20 km to the north of the Andalusian province of Granada, which is the fourth largest area devoted to olive grove crops, covering 198,331 hectares (ha) [81]. The landscape in this region is dominated by olive plantations, with patches of semi-natural vegetation mostly composed of P. halepensis, Q. rotundifolia and P. dulcis, in addition to less abundant species, such as Quercus coccifera L. (Fagales: Fagaceae), Juniperus oxycedrus L. (Pinales: Cupressaceae), Cistus albidus L. (Malvales: Cistaceae), Cistus clusii Dunal (Malvales: Cistaceae), Genista cinerea (Vill.) DC. (Fabales: Fabaceae), Lavandula latifolia Medik. (Lamiales: Lamiaceae), Pistacia terebinthus L. (Sapindales: Anacardiaceae), Rosmarinus officinalis L. (Lamiales: Lamiaceae), Thymus mastichina (L.) L. subsp. mastichina (Lamiales: Lamiaceae), Thymus zygis L. subsp. gracilis (Boiss) R. Morales (Lamiales: Lamiaceae) and Ulex parviflorus Pourr. (Fabales: Fabaceae).
Sampling was carried out in five organic olive farms (Table 1) in conformity with EU legislation [82,83]. All these farms are located at a similar altitude of 800 to 1100 m above sea level, the variety of Olea europaea L. is “Picual” and the plantation schemes are very similar (8 × 8 and 12 × 12 m), with areas ranging from 0.9 to 215 ha. Soil management practices on these farms include the maintenance of spontaneous vegetation cover, which is eliminated by mechanical mowing and/or grazing between April and May. In addition, during the post-harvest period, the soil is fertilized with organic matter, and crushed pruning waste is placed in the rows between crops to create inert cover. The incidence of disease (such as Fusicladium oleagineum) and pests (such as P. oleae and Bactrocera oleae (Gmelin, 1790)) was remedied by timely and targeted treatment (two aimed at diseases and one for pests) using products listed in Annex II of Commission Regulation (EC) no. 889/2008.

Table 1.
Characteristics and availability of each tree species and number of tree species sampled in each site per month sampled.
2.2. Collection of Samples
To collect the juvenile stages of chrysopids (larvae and prepupae/pupae), eight corrugated cardboard band traps (10 × 17.5 cm) were placed in a total of 100 trees (25 trees per species): O. europaea (olive), Q. rotundifolia (oak), P. dulcis (almond) and P. halepensis (pine), whose distribution in the sampling sites depended on their availability in the study area (Table 1). The band traps were installed on different branches located 160–170 cm from the ground taking into account the four cardinal directions (two band traps per direction). The 800 band traps were changed each month between June 2016 and May 2017 (a total of 12 sampling events) on the same 100 trees (identified by number).
In the laboratory, the juvenile stages—larvae, “open cocoons”, with one or more apertures caused by the emergence of chrysopid or parasitoid adults and predators feeding on juveniles, as well as “closed cocoons”, with no apertures and containing a chrysopid larva—were individually labelled and kept in Petri dishes (55 mm in diameter) for observation and monitoring. The trash-bearing juveniles (with exogenous material on their backs) and naked juveniles (with no exogenous material) were also quantified. The larval instars and “closed cocoons” were kept in an incubation chamber (Fitoclima S600 PLH; Aralab, Rio de Mouro, Portugal) in order to monitor their development at a temperature of 25 ± 1 °C, a humidity of 50%–60% and a photoperiod of 16:8 (Light:Dark) hours.
The individual larvae were fed ad libitum with Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs (EphestiaTop; Biotop; Livron-sur-Drôme; France) to facilitate the completion of their biological cycle up to the adult stage and taxonomic identification.
The juveniles that failed to reach the adult stage were inspected under a stereomicroscope (Nikon SMZ 800; Nikon, Tokyo, Japan) in order to ascertain whether death was due to parasitoids or unknown factors. Additionally, we determined whether the aperture in the “open cocoons” was caused by the emergence of an adult chrysopid, a parasitoid or by the feeding of predators. In parasitized cocoons, the number of emerged adult parasitoids, as well as the number and average diameter of exit apertures were quantified (Figure 1).
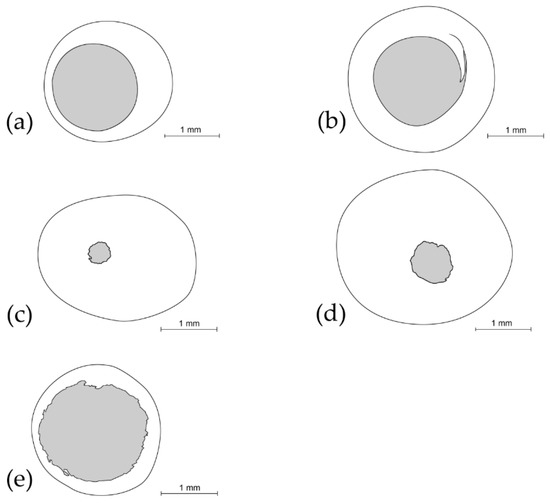
Figure 1.
Examples of apertures in cocoons made by (a) the Chrysopidae family, by the most abundant parasitoid species: (b) Helorus ruficornis, (c) Baryscapus impeditus and (d) Isodromus puncticeps and by (e) predators.
The adult chrysopids that emerged in the laboratory were identified taxonomically up to species level according to the Monserrat key [1]. The emerged adult parasitoids in the laboratory were identified up to species level with the aid of taxonomists with specialist knowledge of the different families (see acknowledgements), the Plant Protection Group collection at the Estación Experimental del Zaidín (EEZ) and the Goulet and Huber key [84].
2.3. Statistical Analysis
All analyses were carried out using R software version 3.5.0 [85]. Statistical analysis began with data exploration [86]. We explored the total abundance of the juvenile stages collected in four categories (adult, parasitized, and predated chrysopids; unknown factors) in the tree species sampled throughout the study period. For data presentation purposes, the study period was simplified by grouping the sampling dates by season: Summer (June, July and August), autumn (September, October and November), winter (December, January and February) and spring (March, April and May). Juveniles (from larvae and “open or closed cocoons”), which produced an adult chrysopid and emerged either in the laboratory or in the field, were categorized under the heading “adult chrysopids”. A similar system was used for parasitoids from juveniles, which were grouped under the heading “parasitized chrysopids”. Death of juveniles caused by other population decline factors were classified as “unknown factors”. Finally, “open cocoons” with apertures due to attacks by predators, were defined as “predated chrysopids”.
We then analysed the total abundance of juvenile stages collected from each tree species sampled using a generalized linear mixed model (GLMM) with a negative binomial distribution (Equations (1)–(3)) and a log link function (Equation (4)) in relation to tree species, site and month sampled as fixed factors and the identification of the individual tree as the random factor (Equations (4) and (5)) using the “lme4” software package [87]:
Abundance of juvenile stages ~ NB(μij, k)
E(Abundance of juvenile stagesij) = μij
Log(μij) = tree speciesij+ siteij+ month sampledij + aj
aj ~ N(0, σ2individual tree)
We then calculated the rate of parasitism per tree (%) expressed as the number of juvenile stages affected by parasitism in each tree divided by the total number of juvenile stages collected from each tree multiplied by 100. The rate of parasitism was analysed with the aid of the GLMM with a binomial distribution (Equation (6)) and a logit link function (Equation (7)) using tree species, site and month sampled as fixed factors and the identification of the individual tree as the random factor (Equations (7) and (8)). The “lme4” software package was used for this analysis [87]:
Parasitism rateij ~ Bin(1, pij)
Logit(pij) = α + β1 x Tree speciesij+ β2 x siteij + β3 x month sampledij + aj
aj ~ N(0, σ2individual tree)
The models were constructed and selected according to Akaike Information Criteria (AIC) [88]. We also analysed the model residuals and checked for uniformity using the “DHARMa” software package [89]. The multiple comparisons in each model (chrysopid abundance and parasitism rate) for the tree species, site and month sampled variables were checked with the aid of the post-hoc Tukey test using the “multcomp” software package [90].
The data for juveniles categorized as “unknown factors”, “predated chrysopids” and “adult chrysopids” were analysed by applying the Kruskal–Wallis test with a Bonferroni adjustment with the aid of the “agricolae” software package [91].
In addition, we calculated the parasitism rate according to the trash-bearing and naked juveniles collected. The rate of parasitism was analysed by applying the Kruskal–Wallis test with a Bonferroni adjustment with the aid of the “agricolae” software package [91].
We employed redundancy analysis (RDA) to determine whether a relationship exists between the composition of chrysopid and parasitoid species and environmental variables (tree species and season). The results were presented using a tri-plot correlation with the aid of the “vegan” software package [92].
3. Results
3.1. Analysis of Collected Cocoons
We separated the “open cocoons” from “closed cocoons”. “Open cocoons” were classified as “adult chrysopids” (Figure 1a) which emerged from a single circular orifice with a regular border and an average diameter of 1.65 ± 0.01 mm (n = 5 cocoon apertures). Parasitized juveniles were classified as “parasitized chrysopids” (Figure 1b–d) which emerged through one, two or three regular or irregular circular apertures with a diameter ranging from 0.4 to 1.7 mm (n = 15 cocoon apertures), with the remains of the juvenile host still inside the cocoon. “Open cocoons” were also classified as “predated chrysopids”, with one or two even or uneven circular apertures with an average diameter of 1.7 ± 0.07 mm (n = 5 cocoon apertures) (Figure 1e) to feed on juvenile stages, without remains of the juvenile host inside the cocoon. “Closed cocoons” contained prepupa or pupa which could emerge as “adult chrysopids”, could have become “parasitized chrysopids” or may not have emerged at all and died due to “unknown factors”.
A total of 1345 juvenile stages of chrysopids were collected between June 2016 and May 2017, over half of which (741 juveniles; n = 1200 trees sampled) completed their development to adulthood in the laboratory or in the field. The other juveniles (604 juveniles; n = 1200 trees sampled) failed to reach adulthood due to the action of parasitoids (357 juveniles; n = 1200 trees sampled), predators (69 juveniles; n = 1200 trees sampled) and unknown factors (178 juveniles; n = 1200 trees sampled) (Table 2).

Table 2.
Abundance (%) and categories of juvenile stages in almond, oak, olive and pine trees by season.
3.2. Abundance and Identification of Chrysopids
The abundance of chrysopids fluctuated during all four seasons. According to the results of the GLMM (Table 3, Table S1), the summer months showed by far the greatest abundance of chrysopids per tree (2.58 ± 0.28; 774 juveniles; n = 300 trees sampled), while the winter months recorded the lowest abundance (0.23 ± 0.04; 68 juveniles; n = 300 trees sampled) (Table 2). The months of autumn (1.24 ± 0.14; 373 juveniles; n = 300 trees sampled) and spring (0.43 ± 0.06; 130 juveniles; n = 300 trees sampled) registered intermediate values. In the spring period, the abundance of juveniles in May (0.82 ± 0.16; n = 100 trees sampled) was higher than that in all the winter months: December (0.18 ± 0.05; n = 100 trees sampled), January (0.16 ± 0.05; n = 100 trees sampled) and February (0.33 ± 0.08; n = 100 trees sampled) (Table 3, Table S1).

Table 3.
ANOVA (type II Wald Chi-square test) results of generalized linear mixed models (GLMMs) (chrysopid abundance and parasitism rate). Significance codes: *** p < 0.001, ** p < 0.01, * p < 0.05.
Chrysopid abundance varied significantly between sites according to the GLMM (Table 3, Table S1); the Norberto farm presented the highest abundance (2.04 ± 0.25; n = 336 trees sampled) as compared to the other sites, while the Píñar farm (left) had the lowest abundance (0.47 ± 0.09; n = 216 trees sampled); the other sites (Los Almendros, Píñar farm (right) and La Pedriza) reported intermediate values and significant inter-site differences (Table 3, Table S1).
Tree species was also a variable factor in the abundance of juvenile stages of chrysopids (Table 3, Table S1). Pine trees exhibited significantly lower abundance of juveniles per tree (0.75 ± 0.13; 225 juveniles; n = 300 trees sampled) as compared to the other tree species: Almond (1.76 ± 0.27; 529 juveniles; n = 300 trees sampled), olive (1.19 ± 0.13; 356 juveniles; n = 300 trees sampled) and oak (0.78 ± 0.08; 235 juveniles; n = 300 trees sampled), with no significant differences being observed between the latter three species (Table 3, Table S1).
The number of juveniles that completed their development to adulthood was by far the highest for those sampled from olive trees (0.87 ± 0.1; 225 juveniles; n = 300 trees sampled) (Kruskal–Wallis χ² = 28.57, d.f. = 3, p < 0.001) and lowest in oak trees (0.4 ± 0.05; 121 juveniles; n = 300 trees sampled), with almond and pine trees recording intermediate values and with no significant differences between almond, oak and pine trees (Table 2). The number of juveniles killed by “unknown factors” was significantly higher in almond trees (0.22 ± 0.03; 66 juveniles; n = 300 trees sampled) than in oak (0.09 ± 0.02; 28 juveniles; n = 300 trees sampled) and pine trees (0.09 ± 0.03; 29 juveniles; n = 300 trees sampled) (Kruskal–Wallis χ² = 22.79, d.f. = 3, p < 0.001), while no significant differences were observed between almond, oak and pine trees, on the one hand, and olive trees (0.18 ± 0.04; 55 juveniles; n = 300 trees sampled), on the other (Table 2). Moreover, the number of “predated chrysopids” in all tree species studied did not differ significantly (Kruskal–Wallis χ² = 5.33, d.f. = 3, p = 0.15).
With regard to temporal distribution, the number of juveniles killed by “unknown factors” collected in summer (0.3 ± 0.05; 90 juveniles; n = 300 trees sampled) and autumn (0.17 ± 0.03; 51 juveniles; n = 300 trees sampled) was significantly higher than in spring (0.07 ± 0.03; 21 juveniles; n = 300 trees sampled) and winter (0.05 ± 0.02; 16 juveniles; n = 300 trees sampled), although no significant inter-seasonal differences were observed (Kruskal–Wallis χ² = 49.72,d.f. = 3, p < 0.001). The number of juveniles reaching adulthood was significantly higher in summer (1.37 ± 0.14; 410 juveniles; n = 300 trees sampled), followed by autumn (0.68 ± 0.08; 205 juveniles; n = 300 trees sampled), spring (0.29 ± 0.04; 86 juveniles; n = 300 trees sampled) and winter (0.13 ± 0.03; 40 juveniles; n = 300 trees sampled), with significant differences being observed between these last three seasons (Kruskal–Wallis χ² = 126.1, d.f. = 3, p < 0.001) (Table 2).
A total of 440 adult chrysopids belonging to ten species from five different genera of the family Chrysopidae emerged in the laboratory: Chrysopa Leach, 1815 (1), Chrysoperla (4), Cunctochrysa Hölzel, 1972 (1), Pseudomallada (3) and Rexa (1) (Table 4).

Table 4.
Abundance (mean ± SE) of chrysopid species that emerged in laboratory from chrysopid juveniles collected from almond, oak, olive and pine trees by season.
Pseudomallada prasinus (Burmeister, 1839) was the most abundant species (242 individuals) followed by Chrysoperla pallida Henry, Brooks, Duelli and Johnson, 2002 (74 individuals) and Chrysoperla mediterranea (Hölzel, 1972) (63 individuals). The other species were much less numerous: Chrysoperla lucasina (Lacroix, 1912) (16), Chrysoperla mutata (McLachlan, 1898) (15), Rexa almerai (Navás, 1919) (10), Pseudomallada picteti (McLachlan, 1880) (7), Pseudomallada flavifrons (Brauer, 1851) (5), Chrysopa pallens (Rambur, 1838) (4) and Cunctochrysa baetica (Hölzel, 1972) (4).
3.3. Parasitism Rate and Juvenile Chrysopid Parasitoid Complex
The rate of parasitism differed significantly in the arboreal stratum (Table 3, Table S2); the rate for olive trees (4.2 ± 1%; 28 parasitized juveniles; n = 300 trees sampled) was significantly below that for the other tree species: Almond trees (7.53 ± 1.23%; 199 parasitized juveniles; n = 300 trees sampled), oak trees (11.96 ± 1.66%; 74 parasitized juveniles; n = 300 trees sampled) and pine trees (6.54 ± 1.29%; 56 parasitized juveniles; n = 300 trees sampled); almond, oak and pine trees did not show any significant inter-species differences (Table 3, Table S2).
With regard to the temporal evolution of the parasitism rate, juvenile chrysopids collected in almond trees were found to be affected by parasitism between the months of July and September, reaching a maximum of 34.8% in August. A similar tendency was detected in pine trees, with a maximum of 26.5% recorded in August. On the other hand, juvenile chrysopids in olive and oak trees were affected by parasitism virtually throughout the whole period of the study, with oak trees displaying a maximum rate of 28% in January (Figure 2).
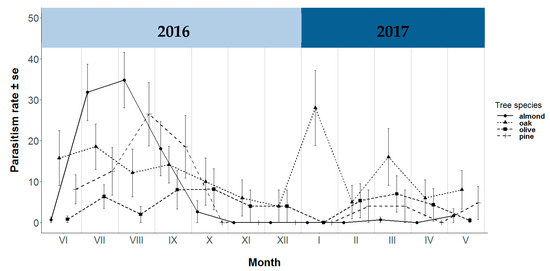
Figure 2.
Temporal evolution of parasitism rate (%) in almond, oak, olive and pine trees by month sampled.
With respect to the sites sampled, the average rate of parasitism was found to be significantly higher in the Los Almendros farm (12.24 ± 2%; n = 216 trees sampled) as compared to the Norberto farm (8.49 ± 1.23%; n = 336 trees sampled), although differences in relation to the other farms (Píñar (right), La Pedriza and Píñar (left)) or with respect to inter-farm rates were not significant (Table 3, Table S2).
On the other hand, the parasitism rate of naked juveniles (5.08 ± 0.55%; 287 juveniles; n = 1200 trees sampled) was significantly higher than that for trash-bearing juveniles (3.69 ± 0.51%; 70 juveniles; n = 1200 trees sampled) (Kruskal–Wallis χ² = 11.64, d.f. = 1, p < 0.001).
A total of 1033 parasitoids belonging to five species from five different families of the Order Hymenoptera emerged in the laboratory from 174 parasitized juveniles: Baryscapus impeditus (Nees, 1834) (Chalcidoidea: Eulophidae), Gelis ilicicola (Seyrig, 1927) (Ichneumonoidea: Ichneumonidae), Helorus ruficornis Förster, 1856 (Proctotrupoidea: Heloridae), Isodromus puncticeps (Howard, 1885) (Chalcidoidea: Encyrtidae) and Perilampus minutalis Steffan, 1952 (Chalcidoidea: Perilampidae) (Table 5).

Table 5.
Abundance of juvenile chrysopids parasitized (mean ± SE) by the parasitoid species complex in almond, oak, olive and pine trees by season.
Baryscapus impeditus was the most numerous species (903 individuals from 84 parasitized juveniles). The number of parasitoids per parasitized juvenile ranged from one to 30 (10.75 ± 0.65; n = 84 parasitized juveniles), which emerged through one, two or three unevenly edged circular apertures with an average diameter of 0.42 ± 0.02 mm (n = 5 cocoon apertures) (Figure 1c). Helorus ruficornis was the second most abundant species (64 individuals from 64 parasitized chrysopids). A single parasitoid emerged from each cocoon through a single helicoidal-shaped aperture with a clearly defined edge and an average diameter of 1.72 ± 0.04 mm (n = 5 cocoon apertures) (Figure 1b). With respect to Isodromus puncticeps (52 individuals from 12 parasitized chrysopids), the number of individuals per parasitized chrysopid, which emerged, through a single unevenly edged circular aperture with an average diameter of 0.77 ± 0.04 mm (n = 5 cocoon apertures), ranged from one to ten (4.33 ± 0.85; n = 12 parasitized juveniles) (Figure 1d). The following species were much less abundant: Nine Gelis iliciola and five Perilampus minutalis individuals emerged through an unevenly edged aperture with a diameter of 1.11 ± 0.05 mm (n = 5 cocoon apertures) and 1.58 ± 0.26 mm (n = 5 cocoon apertures), respectively; in both species, each parasitoid emerged from a single parasitized juvenile.
3.4. Multivariate Analysis of the Relationship between Parasitoid and Chrysopid Species, Tree Species and Season
Using RDA analysis, we determined that tree species and season accounted for 14.1% of the variation in the parasitoid and chrysopid community. The first two RDA axes accounted for 79% of this variation and adjusted R² for 12.8%, suggesting that other variables were not captured by the model.
The RDA correlation tri-plot (Figure 3) showed that three groups of species were positively inter-correlated. The first group was composed of three chrysopids (C. baetica, P. flavifrons and P. picteti) and one parasitoid (H. ruficornis). The abundance of C. baetica reached maximum levels in oak trees in autumn, with a similar pattern being observed for P. flavifrons and P. picteti only in spring, while the parasitoid H. ruficornis recorded maximum abundance in oak trees in all seasons (Table 4 and Table 5).
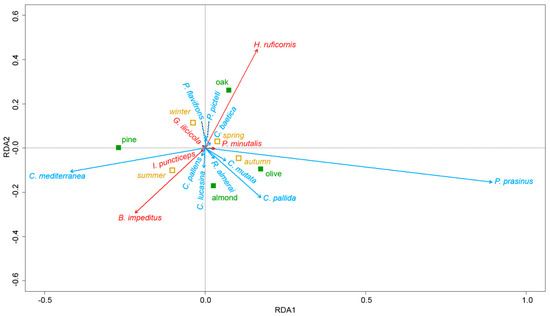
Figure 3.
Redundancy analysis (RDA) tri-plot ordination showing variations in the abundance of the parasitoid complex (in red) and chrysopid species community (in blue) with respect to two nominal variables; tree species (in green) and season (in yellow).
The second group was composed of three chrysopids (C. pallens, C. lucasina and C. mediterranea) collected in spring and summer and two parasitoids (B. impeditus and I. puncticeps) (Figure 3). C. lucasina appeared in spring in almond trees and then spread to the four tree species, while C. pallens was only detected in almond trees and C. mediterranea reached maximum abundance in pine trees in summer (Table 4 and Table 5). B. impeditus was mainly observed in almond trees and dispersed to pine trees in summer, though with a lower level of abundance, while the other parasitoid species I. puncticeps appeared in spring in almond and pine trees and had a preference for almond trees in summer (Table 4 and Table 5).
The third group is composed of C. pallida, R. almerai, C. mutata, P. prasinus and the parasitoid P. minutalis. R. almerai only appeared in olive trees in spring and summer, while C. pallida was reported in olive trees throughout the year, reaching maximum levels in almond trees in summer. C. mutata was mainly recorded in summer and autumn. Finally, P. prasinus, though collected from olive and almond trees throughout the year, reached maximum abundance in olive trees in autumn, with the parasitoid P. minutalis showing a similar pattern (Table 4 and Table 5).
4. Discussion
This study provides an insight into the abundance of chrysopid populations in olive groves, as well as almond, oak and pine trees adjacent to the crop, in addition to population decline factors. Juvenile stages of chrysopids were more abundant in almond, oak and olive trees than in pine trees. We found that parasitoids and chrysopids shared a similar temporal pattern in our study area. Additionally, the period of parasitoid incidence was found to extend beyond the April to November period previously reported [78,93]. We observed that parasitoid abundance was highest in the summer months in olive trees, which is in line with the findings of Neuenschwander and Michelakis [80] and Campos [78].
The presence of “predated chrysopids” and “unknown factors” had a marked seasonal character, with the largest number in both categories recorded in summer, when the environment is less humid and temperatures are higher than in other seasons. This concurs with the results of previous studies which demonstrate that conditions, such as low humidity and high temperatures lead to increased mortality and slower development in the preimaginal stages [35,59,94]. This slower development could also render the juvenile stages more vulnerable to predators.
Overall, we found that mortality caused by parasitism (26.5%) constitutes a major chrysopid population decline factor. Although this is very similar to the level (27.7%) determined by Campos [78] in olive groves in southern Spain, it is quite low compared to the levels (80% and 54.9%, respectively) reported in olive groves by Alrouechdi et al. [50] in France and Neuenschwander and Michelakis [80] in Crete.
With regard to tree species, the parasitism rate per tree in olive trees was very low as compared to previous studies [50,78,80,93] and considerably lower than that in the three arboreal species (almond, oak and pine) studied. This, together with predation and unknown factors, make olive trees the most important arboreal species with regard to the number of viable next-generation adult chrysopids.
The highest rate of parasitism recorded in almond, oak and pine trees could be due to their location in semi-natural areas bordering the crop. The semi-natural habitats and landscape bordering the crop are characterized by greater species richness and parasitoid diversity than other types of habitat such as crop and vegetation cover [95]. Few data are available on the seasonality of parasitism in these trees. However, we demonstrated that the parasitism rate in pine and almond trees is higher in the summer months, which is similar to the pattern found by Judd [58] in pine trees. Oak trees showed a more-or-less constant rate of parasitism throughout the year, which is similar to the rate of close to 15% recorded in other studies [96]. Additionally, oak trees become a parasitoid bank in winter due to their high rate of parasitism. This could have a negative effect on the next chrysopid generation and enable parasitoids to move into olive groves in spring. However, low rates of parasitism in olive trees and high rates in oak trees in spring suggest that parasitoids remain in oak trees. As almond trees have a high rate of parasitism in summer and are a good reservoir of juvenile chrysopids, they could play an important role in increasing chrysopid populations in olive groves in the summer months, when P. oleae are especially harmful to olive trees.
The chrysopid community is composed of ten species in our biotope, with, as already noted in previous studies, P. prasinus and the C. carnea complex accounting for the majority of individuals [21,29,97]. On the other hand, studies focusing on the parasitoid complex of chrysopids have reported that a relationship exists between chrysopid species and their associated parasitoids [45,49,56]. The parasitoid complex is composed of five species: Three primary parasitoids (B. impeditus, H. ruficornis and I. puncticeps), with the highest levels of abundance, and two primary parasitoids, which also could act as hyperparasitoids (G. ilicicola and P. minutalis), with the lowest levels of abundance.
B. impeditus, the most abundant species, affected a large number of chrysopids, mainly juveniles of the species C. mediterranea, C. lucasina and C. pallens, which were collected in almond and pine trees. Our results regarding this parasitoid, which is characterized by gregarious behaviour and emerges from the host through various orifices, corroborate the findings of previous studies [45,50]. Although the period of activity of B. impeditus was similar to that in olive groves in Crete and France, the number of parasitoids per host was larger in our study [47,80].
The second most important parasitoid was H. ruficornis, which is found in Palearctic, Nearctic and Afrotropical regions [98,99,100]. This species has been previously cited in the Iberian Peninsula [101], specifically in olive groves [78,93]. Our findings would appear to contradict those of New [56], who has stated that H. ruficornis is in a minority among species in the chrysopid parasitoid complex in Europe due to competition from other parasitoids for hosts. In our study, the second most abundant parasitoid H. ruficornis, which competed with four parasitoid species, plays a similar role to that observed by New [56]. Although little is known about its biology, H. ruficornis can, in our view, be classified as a solitary parasitoid, as only one parasitoid exits in the host cocoon. This behaviour resembles that of other species of the same genus and concurs with other studies which suggest that all species of the genus Helorus are biologically similar [45,48,51,56,98]. H. ruficornis has also been shown to parasitize species of the genera Chrysoperla, Pseudomallada, Chrysopa, and Nineta [45,46,51,56]. We observed that H. ruficornis parasitizes the juvenile stages of the genera Pseudomallada (P. picteti, P. flavifrons and P. prasinus) and C. baetica which have a preference for oak trees in the Iberian Peninsula [21,102].
Of the two species from the genus Isodromus that parasitize chrysopids [48], we collected I. puncticeps, which is in a minority in the parasitoid complex studied. Although this resembles the pattern observed in Greek olive groves [56,78,80,96], I. puncticeps plays an important role in French olive groves [47,50,103]. With the aid of RDA analysis, although we found a positive relationship between the abundance of B. impeditus and I. puncticeps, given the insufficient number of individuals of the latter, we were unable to shed any light on this relationship. Nevertheless, as previously described by Clancy [45] and Campos [78], we found I. puncticeps to be a gregarious parasitoid.
While the characteristics that enable chrysopids to protect against natural enemies include the use of exogenous trash by juveniles as a defensive shield against predation [72], evidence with regard to parasitism is less clear [49,71,104]. In our study, the rate of parasitism was found to be higher in naked chrysopid species (C. lucasina, C. mediterranea, C. mutata, C. pallida and C. pallens) as compared to trash-bearing species (C. baetica, P. flavifrons, P. picteti, P. prasinus and R. almerai); however Muma [49] found that the rate of parasitism is lower in naked chrysopids than in more abundant trash-bearing chrysopids. Therefore, depending on chrysopid assemblage and abundance, as well as the parasitoid complex associated with each geographical area, rates of parasitism will, in our view, be affected by whether juvenile chrysopids are trash-bearing or naked. However further research is required to cast light on this relationship.
5. Conclusions
We have demonstrated that chrysopid abundance in almond and oak tree species in the arboreal stratum adjacent to olive groves is comparable to that in olive trees. With regard to population dynamics, the combined effect of three decline factors (parasitism, predation and unknown factors) of chrysopid populations over the short term needs to be taken into account when habitat management is being considered to conserve these populations. Additionally, in the biotope studied, we found that ten chrysopid species use the arboreal stratum to develop their biological cycle, in which P. prasinus is the most abundant species. We also found that three out of the five species in the parasitoid complex of the family Chrysopidae are primary parasitoids, with B. impeditus showing a preference for C. pallens, C. lucasina and C. mediterranea; and H. ruficornis being associated with C. baetica, P. flavifrons and P. picteti, representing the majority of parasitoid species. A knowledge of chrysopid population decline factors in semi-natural habitats could be crucial for an effective habitat management program aimed at conserving and expanding chrysopid populations to boost the presence of chrysopids and the natural pressure on pests and to contribute to olive grove sustainability.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4450/10/5/134/s1, Table S1: Multiple comparisons of generalized linear mixed model (GLMM) abundance of juvenile stages of chrysopids in relation to tree species, site and month sampled including estimate, standard error (SE) and p value. Significance codes: *** p < 0.001, ** p < 0.01, * p < 0.05, Table S2: Multiple comparisons of GLMM parasitism in relation to tree species, site and month sampled including estimate, standard error (SE) and p value. Significance codes: *** p < 0.001, ** p < 0.01, * p < 0.05.
Author Contributions
M.C. and F.R. obtained funding. M.C., R.A.H. and F.R. conceived and designed the study. R.A.H. and F.R. carried out the sampling, identified the chrysopids and parasitoids and formal analyses. M.G.-S. participated in three monthly sampling and identified parasitoids up to superfamily-family level. R.A.H., M.C. and F.R. wrote, reviewed and edited the manuscript. The manuscript was revised and approved by all the authors.
Funding
This research was funded by the Junta de Andalucía (excellence project P12-AGR-1419).
Acknowledgments
We wish to thank the olive grove owners for access to their farms and the following experts for their assistance: Víctor Monserrat, who identified issues regarding emerged adult chrysopids from the juvenile stages, as well as Juli Pujade (Heloridae), Dirk Askew (Eulophidae), Chris Darling (Perilampidae) and J. Vicent Falcó (Ichneumonidae) who helped with the identification of the parasitoid complex. We would also like to thank Ariel Turchak, Ignacio Martín, Jesús Domingo and Luis Plaza for field, laboratory and sampling logistics assistance, Belén Cotes for statistical advice, María Luisa Fernández for image processing and laboratory assistance and Michael O´Shea for translating the manuscript. The Spanish Council for Scientific Research CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI) assisted with the payment of the publication fee.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Monserrat, V.J. Los crisópidos de la Península Ibérica y Baleares (Insecta, Neuropterida, Neuroptera: Chrysopidae). Graellsia 2016, 72, 1–123. [Google Scholar] [CrossRef]
- Duelli, P. Lacewings in field crops. In Lacewings in the Crop Environment; Whittington, A.E., McEwen, P.K., New, T.R., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 158–171. [Google Scholar] [CrossRef]
- Szentkirályi, F. Lacewings in vegetables, forests, and other crops. In Lacewings in the Crop Environment; Whittington, A.E., McEwen, P.K., New, T.R., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 239–292. [Google Scholar]
- Pappas, M.L.; Broufas, G.D.; Koveos, D.S. Chrysopid predators and their role in biological control. J. Entomol. 2011, 8, 301–326. [Google Scholar] [CrossRef]
- Principi, M.M.; Canard, M. Feeding habits. In Biology of Chrysopidae; Canard, M., Séméria, Y., New, T.R., Eds.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1984; Volume 27, pp. 76–92. [Google Scholar]
- New, T.R. A review of the biology of Neuroptera Planipennia. Neuroptera Int. Suppl. 1986, 1, 1–57. [Google Scholar]
- Oswald, J.D. LDL NSW: Neuropterida Species of the World (Version June 2017). Available online: www.catalogueoflife.org/col (accessed on 18 September 2018).
- Alrouechdi, K. Relations comportementales et trophiques entre Chrysoperla carnea (Stephens) (Neuroptera; Chrysopidae) et trois principaux ravageurs de l’olivier. I. La Teigne de L’olivier Prays oleae Bern. (Lep. Hyponomeutidae). Neuroptera Int. 1981, 1, 122–134. [Google Scholar]
- Tauber, M.J.; Tauber, C.A.; LopezArroyo, J.I. Life-history variation in Chrysoperla carnea: Implications for rearing and storing a Mexican population. Biol. Control 1997, 8, 185–190. [Google Scholar] [CrossRef]
- Gerling, D.; Kravchenko, V.; Lazare, M. Dynamics of common green lacewing (Neuroptera: Chrysopidae) in Israeli cotton fields in relation to whitefly (Homoptera: Aleyrodidae) populations. Environ. Entomol. 1997, 26, 815–827. [Google Scholar] [CrossRef]
- Daane, K.M.; Yokota, G.Y.; Zheng, Y.; Hagen, K.S. Inundative release of common green lacewings (Neuroptera: Chrysopidae) to suppress Erythroneura variabilis and E. elegantula (Homoptera: Cicadellidae) in vineyards. Environ. Entomol. 1996, 25, 1224–1234. [Google Scholar] [CrossRef]
- New, T.R. Lacewings (Neuroptera) as biological control agents. Vic. Entomol. 1975, 5, 102–103. [Google Scholar]
- Henry, C.S. The proliferation of cryptic species in Chrysoperla green lacewings through song divergence. Florida Entomol. 1985, 68, 18–38. [Google Scholar] [CrossRef]
- Tauber, C.A.; Tauber, M.J. Ecophysiological responses in life-history evolution: Evidence for their importance in a geographically widespread insect species complex. Can. J. Zool.-Rev. Can. Zool. 1986, 64, 875–884. [Google Scholar] [CrossRef]
- Henry, C.S.; Brooks, S.J.; Johnson, J.B.; Duelli, P. Chrysoperla lucasina (Lacroix): A distinct species of green lacewing, confirmed by acoustical analysis (Neuroptera: Chrysopidae). Syst. Entomol. 1996, 21, 205–218. [Google Scholar] [CrossRef]
- Henry, C.S.; Brooks, S.J.; Duelli, P.; Johnson, J.B. Discovering the true Chrysoperla carnea (Insecta: Neuroptera: Chrysopidae) using song analysis, morphology, and ecology. Ann. Entomol. Soc. Am. 2002, 95, 172–191. [Google Scholar] [CrossRef]
- Canard, M.; Thierry, D. A historical perspective on nomenclature within the genus Chrysoperla Steinmann, 1964 in Europe: The carnea-complex (Neuroptera Chrysopidae). Ann. Museo Civ. Storia Nat. Ferrara 2007, 8, 173–179. [Google Scholar]
- Henry, C.S.; Brooks, S.J.; Duelli, P.; Johnson, J.B.; Wells, M.M.; Mochizuki, A. Obligatory duetting behaviour in the Chrysoperla carnea-group of cryptic species (Neuroptera: Chrysopidae): Its role in shaping evolutionary history. Biol. Rev. 2013, 88, 787–808. [Google Scholar] [CrossRef]
- Price, B.W.; Henry, C.S.; Hall, A.C.; Mochizuki, A.; Duelli, P.; Brooks, S.J. Singing from the Grave: DNA from a 180 Year Old Type Specimen Confirms the Identity of Chrysoperla carnea (Stephens). PLoS ONE 2015, 10, 11. [Google Scholar]
- Campos, M. Observaciones sobre la bioecologia de Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) en el sur de España. Neuroptera Int. 1989, 5, 159–164. [Google Scholar]
- Monserrat, V.J.; Marín, F. Plant substrate-specifity of Iberian Chrysopidae (Insecta, Neuroptera). Acta Oecol.-Int. J. Ecol. 1994, 15, 119–131. [Google Scholar]
- Porcel, M. Estudio de la Bioecología de la Familia Chrysopidae (Insecta: Neuroptera) Desde la Perspectiva de su Incremento y Conservación en el Olivar. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2012. [Google Scholar]
- Szentkirályi, F. Lacewings in fruit and nut crops. In Lacewings in the Crop Environment; Whittington, A.E., McEwen, P.K., New, T.R., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 172–238. [Google Scholar]
- Alrouechdi, K. Les Chrysopides en Verger D’oliviers: Bio-écologie de Chrysoperla carnea (Steph.) (Neuroptera, Chrysopidae); Relations Comportementales et Trophiques Avec Certaines Espèces Phytophages. Ph.D. Thesis, L‘Université Pierre et Marie Curie, Paris, France, 1980. [Google Scholar]
- Ramos, P.; Campos, M.; Ramos, J. Estabilización del ataque de Prays oleae Bern. y de la actividad de los depredadores oófagos sobre el fruto del olivo. Bol. Sanidad Vegetal. Plagas 1984, 10, 239–243. [Google Scholar]
- Campos, M. Lacewings in Andalusian olive orchards. In Lacewings in the Crop Environment; Whittington, A.E., McEwen, P.K., New, T.R., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 492–497. [Google Scholar]
- McEwen, P.K.; Jervis, M.A.; Kidd, N.A.C. Use of a sprayed L-tryptophan solution to concentrate numbers of the green lacewing Chrysoperla carnea in olive tree canopy. Entomol. Exp. Appl. 1994, 70, 97–99. [Google Scholar] [CrossRef]
- McEwen, P.K.; Ruiz, J. Relationship between non-olive vegetation and green lacewing eggs in a Spanish olive orchard. Antenna 1994, 18, 148–150. [Google Scholar]
- Porcel, M.; Cotes, B.; Castro, J.; Campos, M. The effect of resident vegetation cover on abundance and diversity of green lacewings (Neuroptera: Chrysopidae) on olive trees. J. Pest Sci. 2017, 90, 195–196. [Google Scholar] [CrossRef]
- McEwen, P.K.; New, T.R.; Whittington, A.E. Lacewings in the Crop Environment; Cambridge University Press: Cambridge, UK, 2001; p. 546. [Google Scholar]
- Canard, M.; Séméria, Y.; New, T.R. Biology of Chrysopidae; Dr. W. Junk Publishers: The Hague, The Netherlands, 1984; Volume 27, pp. 1–294. [Google Scholar]
- Canard, M.; Volkovich, T.A. Outlines of lacewing development. In Lacewings in the Crop Environment; Whittington, A.E., McEwen, P.K., New, T.R., Eds.; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Chang, Y.F.; Tauber, M.J.; Tauber, C.A. Storage of the mass-produced predator Chrysoperla carnea (Neuroptera: Chrysopidae): Influence of photoperiod, temperature, and diet. Environ. Entomol. 1995, 24, 1365–1374. [Google Scholar] [CrossRef]
- Nadeem, S.; Hamed, M.; Ishfaq, M.; Nadeem, M.K.; Hasnain, M.; Saeed, N.A. Effect of storage duration and low temperatures on the developmental stages of Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). J. Anim. Plant Sci. 2014, 24, 1569–1572. [Google Scholar]
- Pappas, M.L.; Broufas, G.D.; Koveos, D.S. Effect of relative humidity on development, survival and reproduction of the predatory lacewing Dichochrysa prasina (Neuroptera: Chrysopidae). Biol. Control 2008, 46, 234–241. [Google Scholar] [CrossRef]
- Pappas, M.L.; Karagiorgou, E.; Papaioannou, G.; Koveos, D.S.; Broufas, G.D. Developmental temperature responses of Chrysoperla agilis (Neuroptera: Chrysopidae), a member of the European carnea cryptic species group. Biol. Control 2013, 64, 291–298. [Google Scholar] [CrossRef]
- Morris, T.I.; Campos, M.; Jervis, M.A.; McEwen, P.K.; Kidd, N.A.C. Potential effects of various ant species on green lacewing, Chrysoperla carnea (Stephens) (Neuropt., Chrysopidae) egg numbers. J. Appl. Entomol.-Z. Angew. Entomol. 1998, 122, 401–403. [Google Scholar] [CrossRef]
- Vinson, S.B.; Scarborough, T.A. Impact of the imported fire ant on laboratory populations of cotton aphid (Aphis gossypii) predators. Fla. Entomol. 1989, 72, 107–111. [Google Scholar] [CrossRef]
- Dinter, A. Intraguild predation between erigonid spiders, lacewing larvae and carabids. J. Appl. Entomol. 1998, 122, 163–167. [Google Scholar] [CrossRef]
- Cisneros, J.; Rosenheim, J.A.Y. Ontogenetic change of prey preference in the generalist predator Zelus renardii and its influence on predator–predator interactions. Ecol. Entomol. 1997, 22, 399–407. [Google Scholar] [CrossRef]
- Lucas, E.; Coderre, D.; Brodeur, J. Instar-specific defense of Coleomegilla maculata lengi (Col.: Coccinellidae): Influence on attack success of the intraguild predator Chrysoperla rufilabris (Neur.: Chrysopidae). Entomophaga 1997, 42, 3–12. [Google Scholar] [CrossRef]
- Canard, M.; Duelli, P. Predatory behavior of larvae and cannibalism. In Biology of Chrysopidae; Canard, M., Séméria, Y., New, T.R., Eds.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1984; Volume 27, pp. 92–100. [Google Scholar]
- Sidor, C. A polyhedral virus disease of Chrysopa perla (L.). Virology 1960, 10, 551–552. [Google Scholar] [CrossRef]
- Ventura, M.A.; Garcia, V.; Canard, M. Antibiosis effect caused by the entomopathogenic fungus Metarhizium anisopliae (Metschnikoff) Sorokin variety anisopliae Tulloch, to a “common green lacewing” Chrysoperla kolthoffi (Navás) (Neuroptera: Chrysopidae). J. Neuropterol. 2000, 3, 33–41. [Google Scholar]
- Clancy, D.W. The insect parasites of the Chrysopidae (Neuroptera). Univ. Calif. Publ. Entomol. 1946, 7, 403–496. [Google Scholar]
- Killington, F.J. The parasites of Neuroptera with special reference to those attacking British species. Trans. Entomol. Soc. South England 1932, 8, 84–91. [Google Scholar]
- Alrouechdi, K.; Panis, A. Les parasites de Chrysoperla carnea Steph. (Neuroptera, Chrysopidae) sur Olivier en Provence. Agronomie 1981, 1, 139–141. [Google Scholar] [CrossRef]
- Alrouechdi, K.; Séméria, Y.; New, T.R. Natural enemies. In Biology of Chrysopidae; Canard, M., Séméria, Y., New, T.R., Eds.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1984; Volume 27, pp. 187–204. [Google Scholar]
- Muma, M.H. Hymenopterous parasites of Chrysopidae on Florida citrus. Fla. Entomol. 1959, 42, 149–153. [Google Scholar] [CrossRef]
- Alrouechdi, K.; Canard, M.; Pralavorio, R.; Arambourg, Y. Influence du complexe parasitaire sur les populations de chrysopides (Neuroptera) dans un verger d’oliviers du Sud-Est de la France. Z. Angew. Entomol.-J. Appl. Entomol. 1981, 91, 411–417. [Google Scholar] [CrossRef]
- Principi, M.M. Contributi allo studio dei Neurotteri Italiani. VII. Osservazioni su alcuni parassiti di crisopidi. Boll. dell’Istituto Entomol. Univ. Studi Bol. 1948, 17, 93–121. [Google Scholar]
- Mehra, B.P. Biology of Chrysopa lacciperda Kimmins. J. Bombay Nat. Hist. Soc. 1966, 63, 215–218. [Google Scholar]
- Ickert, G. Beiträge zur Biologie einheimischer Chrysopiden (Planipennia, Chrysopidae). Entomol. Abhandlungen Staatliches Museum Tierkunde Dresden 1968, 36, 123–192. [Google Scholar]
- New, T.R. A recent host record of Helorus coruscus Hal. (Hym., Heloridae). Entomol. Mon. Mag. 1967, 102, 86. [Google Scholar]
- New, T.R. An Australian species of Helorus Latreille (Hymenoptera: Heloridae). Aust. J. Entomol. 1975, 14, 15–17. [Google Scholar] [CrossRef]
- New, T.R. Hymenopterous parasites of some larvae Chrysopidae (Neuroptera) near Melbourne, Australia. Neuroptera Int. 1982, 2, 33–36. [Google Scholar]
- New, T.R. Trap-banding as a collecting method for Neuroptera and their parasites, and some results obtained. Entomol. Gazette 1967, 18, 37–44. [Google Scholar]
- Judd, W. Emergence of the lacewing, Chrysopa harrisii Fitch (Neuroptera) and three hymenopterous parasites from the cocoon. Ann. Entomol. Soc. Am. 1949, 42, 461–464. [Google Scholar] [CrossRef]
- Daane, K.M. Ecological studies of released lacewings in crops. In Lacewings in the Crop Environment; Whittington, A.E., McEwen, P.K., New, T.R., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 338–350. [Google Scholar] [CrossRef]
- Withycombe, C.L. Notes on the biology of some British Neuroptera (Planipennia). Trans. R. Entomol. Soc. Lond. 1923, 70, 501–594. [Google Scholar] [CrossRef]
- Monserrat, V.J. Estrategias de defensa visual en los Neuropterida Ibéricos (Megaloptera, Raphidioptera, Neuroptera). Bol. Soc. Entomol. Aragon. 2015, 57, 459–480. [Google Scholar]
- Smith, R.C. The trash-carrying habit of certain lacewing larvae. Sci. Mon. N. Y. 1926, 23, 265–267. [Google Scholar]
- Duelli, P. A missing link in the evolution of the egg pedicel in lacewings. Experientia 1986, 42, 624. [Google Scholar] [CrossRef]
- Duelli, P. Oviposition. In Biology of Chrysopidae; Canard, M., Séméria, Y., New, T.R., Eds.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1984; Volume 27, pp. 129–133. [Google Scholar]
- Monserrat, V.J. Contribución al conocimiento de los neurópteros de Toledo. Graellsia 1980, 34, 177–193. [Google Scholar]
- Güsten, R.; Dettner, K. The prothoracic gland of the Chrysopidae (Neuropteroidea: Planipennia). In Proceedings of the 4th European Congress of Entomology and the XIII Internationale Symposium für die Entomofaunistik Mitteleuropas, Gödöllö, Hungary, 1–6 September 1991; Zombori, L., Peregovits, L., Eds.; 1991; Volume 1, pp. 60–65. [Google Scholar]
- Blum, M.S.; Wallace, J.D.; Fales, H.M. Skatole and tricedene: Identification and possible role in a chrysopid secretion. Insect Biochem. 1973, 3, 353–357. [Google Scholar] [CrossRef]
- Rothschild, M.; Euw, J.V.; Reichstein, T. Cardiac glycosides in a scale insect (Aspidiotus), a ladybird (Coccinella) and a lacewing (Chrysopa). Physiol. Entomol. 1973, 48, 89–90. [Google Scholar] [CrossRef]
- Kennett, C.E. Defense mechanism exhibited by larvae of Chrysopa californica Coq. (Neuroptera: Chrysopidae). Pan-Pac. Entomol. 1948, 24, 209–211. [Google Scholar]
- Smith, R.C. A study of the biology of the Chrysopidae. Ann. Entomol. Soc. Am. 1921, 14, 27–35. [Google Scholar] [CrossRef]
- New, T.R. Note on the debris-carrying habit in larvae of British Chrysopidae (Neuroptera). Entomol. Gazette 1969, 20, 119–124. [Google Scholar]
- Eisner, T.; Eisner, M. Coiling into a sphere: Defensive behavior of a trash-carrying chrysopid larva Leucochrysa (Nodita) pavida (Neuroptera: Chrysopidae). Entomol. News 2002, 113, 6–10. [Google Scholar]
- Monserrat, V.J.; Diaz-Aranda, L.M. Larval stages of the Iberian green-lacewings (Insecta, Neuroptera, Chrysopidae), new data on larval morphology applicable to the family systematics. Graellsia 2012, 68, 31–158. [Google Scholar] [CrossRef]
- González, R.; Al-Asaad, S.; Bozsik, A. Influencia de las masas forestales en la diversidad y abundancia de los crisópidos (Neur. Chrysopidae) del olivar. Cuaderno Soc. Española Cienc. For. 2008, 26, 33–38. [Google Scholar]
- Alcalá Herrera, R.; Campos, M.; Ruano, F. Late summer oviposition of green lacewings (Neuroptera: Chrysopidae) on olive groves and adjacent trees. Environ. Entomol. 2019. [Google Scholar] [CrossRef]
- Principi, M.M. Contributi allo studio dei Neurotteri Italiani. V. Ricerche su Chrysopa formosa Brauer e su alcuni suoi parassiti. Boll. dell’Inst. Entomol. Univ. Bol. 1947, 16, 134–175. [Google Scholar]
- Principi, M.M. Contributi allo studio dei Neurotteri italiani. XIII. Studio morfologico, etologico e sistematico di un gruppo omogeneo di specie del Gen. Chrysopa Leach (C. flavifrons Brauer, prasina Burm. e clathrata Schn.). Boll. dell’Istituto Entomol. Univ. Studi Bol. 1956, 21, 319–410. [Google Scholar]
- Campos, M. Influencia del complejo parasitario sobre las poblaciones de Chrysoperla carnea (Neuroptera, Chrysopidae) en olivares del sur de España. Neuroptera Int. 1986, 4, 97–105. [Google Scholar]
- Putman, W.L. Biological notes on the Chrysopidae. Can. J. Res. 1937, 15, 29–37. [Google Scholar] [CrossRef]
- Neuenschwander, P.; Michelakis, S. The seasonal and spatial distribution of adult and larval chrysopids on olive-trees in Crete. Acta Oecol. Oecol. Appl. 1980, 1, 93–102. [Google Scholar]
- Junta de Andalucía. Análisis de las Plantaciones de Olivar en Andalucía. Encuesta sobre Superficies y Rendimientos de Cultivos en España (ESYRCE); Secretaria General de Agricultura y Alimentación, Servicios de Estudios y Estadísticas; Consejería de Agricultura, Pesca y Desarrollo Rural: Sevilla, Spain, 2015. [Google Scholar]
- European Union. Reglamento (CE) no. 834/2007 del Consejo, de 28 de junio de 2007, sobre producción y etiquetado de los productos ecológicos y por el que se deroga el Reglamento (CEE) no. 2092/91. Luxemburgo: Diario Oficial de la Unión Europea, de 20 de julio de 2007, no. 189. 2007, pp. 1–23. Available online: https://eur-lex.europa.eu/legal-content/ES/ALL/?uri=celex:32007R0834 (accessed on 1 March 2018).
- European Union. Reglamento (CE) no. 889/2008 de la Comisión, de 5 de septiembre de 2008, por el que se establecen disposiciones de aplicación del Reglamento (CE) no. 834/2007 del Consejo sobre producción y etiquetado de los productos ecológicos, con respecto a la producción ecológica, su etiquetado y su control. Luxemburgo: Diario Oficial de la Unión Europea, de 18 de septiembre de 2008, no. 250. 2008, pp. 1–84. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/?uri=celex%3A32008R0889 (accessed on 1 March 2018).
- Goulet, H.; Huber, J.T. Hymenoptera of the World: An Identification Guide to Families; Canada, A., Ed.; Centre for Land and Biological Resources Research: Ottawa, ON, Canada, 1993. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, AT, USA, 2017. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer Science & Business Media: New York, NY, USA, 2002. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, R Package version 0.2.0; 2018; Available online: http://CRAN.Rproject.org/package=DHARMa (accessed on 7 April 2019).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R Package version 1.2-8; 2017; Available online: http://CRAN.Rproject.org/package=agricolae (accessed on 7 April 2019).
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package, R Package version 2.5-2; 2018; Available online: http://CRAN.Rproject.org/package=vegan (accessed on 7 April 2019).
- Alrouechdi, K. Les chrysopides (Neuroptera) en oliveraie. In Progress in World’s Neuropterology; Proceedings of the 1st International Symposium on Neuropterology, Graz, Austria, 22–26 September 1980; Gepp, J., Ed.; Osterreichischen Akademie der Wissenschaften: Vienna, Austria, 1984. [Google Scholar]
- El-Taeif, H.A.; El-Ghariani, I.M.; Bataw, A.A. Some biological data on the predator Green Lacewing Chrysoperla carnea (Steph.) (Neuropera: Chrysopidae). Egypt. J. Biol. Pest Control 2008, 18, 243–248. [Google Scholar]
- Inclan, D.J.; Cerretti, P.; Marini, L. Landscape composition affects parasitoid spillover. Agric. Ecosyst. Environ. 2015, 208, 48–54. [Google Scholar] [CrossRef]
- Pantaleoni, R.A. Distribuzione spaziale di alcuni Neurotteri Planipenni su piante arboree. Boll. dell’Istituto Entomol. “Guido Grandi” dell’Univ. Bol. 1996, 50, 133–141. [Google Scholar]
- Campos, M.; Ramos, P. Crisópidos (Neuroptera) capturados en un olivar del sur de España. Neuroptera Int. 1983, 2, 219–227. [Google Scholar]
- Townes, H. A revision of the Heloridae (Hymenoptera). Contrib. Am. Entomol. Inst. 1977, 15, 1–12. [Google Scholar]
- Van Achterberg, C. European species of the genus Helorus Latreille (Hymenoptera: Heloridae), with description of a new species from Sulawesi (Indonesia). Zool. Meded. 2006, 80, 1. [Google Scholar]
- Buffington, M.L.; Copeland, R.S. Redescription of Helorus ruficornis Förster (Hymenoptera: Heloridae), with a New Synonymy and New Afrotropical Specimen Records. Proc. Entomol. Soc. Wash. 2016, 118, 330–344. [Google Scholar] [CrossRef]
- Algarra, A.; Segade, C.; Ventura, D.; Pujade, J. Dos citas nuevas para la Península Ibérica y Andorra de Helorus Latreille, 1802 (Hymenoptera: Proctotrupoidea: Heloridae). Bol. Asoc. Española Entomol. 1996, 20, 262–263. [Google Scholar]
- Marín, F. Las comunidades de neurópteros de la provincia de Alabcete (Insecta: Neuropteroidea). In Al-Basit Revista de Estudios Albacetenses; Diputación Provincial de Albacete: Albacete, Spain, 1994; pp. 247–304. [Google Scholar]
- Alrouechdi, K.; Lyon, J.P.; Canard, M.; Fournier, D. Les chrysopids (Neuroptera) récoltés dans une oliveraie du sud-est de la France. Acta Oecol.-Oecol. Appl. 1980, 1, 173–180. [Google Scholar]
- Smith, R.C. The Biology of the Chrysopidae; Cornell University Agricultural Experimental Station: Ithaca, NY, USA, 1922; Volume 58. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).