Scanning Electron Microscopy of the Antennal Sensilla and Their Secretion Analysis in Adults of Aromia bungii (Faldermann, 1835) (Coleoptera, Cerambycidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Beetles
2.2. Scanning Electron Microscopy (SEM)
2.3. Preparation of Antennal Secretion Extracts
2.4. Gas Chromatography-Mass Spectrometry (GC-MS)
3. Results and Discussion
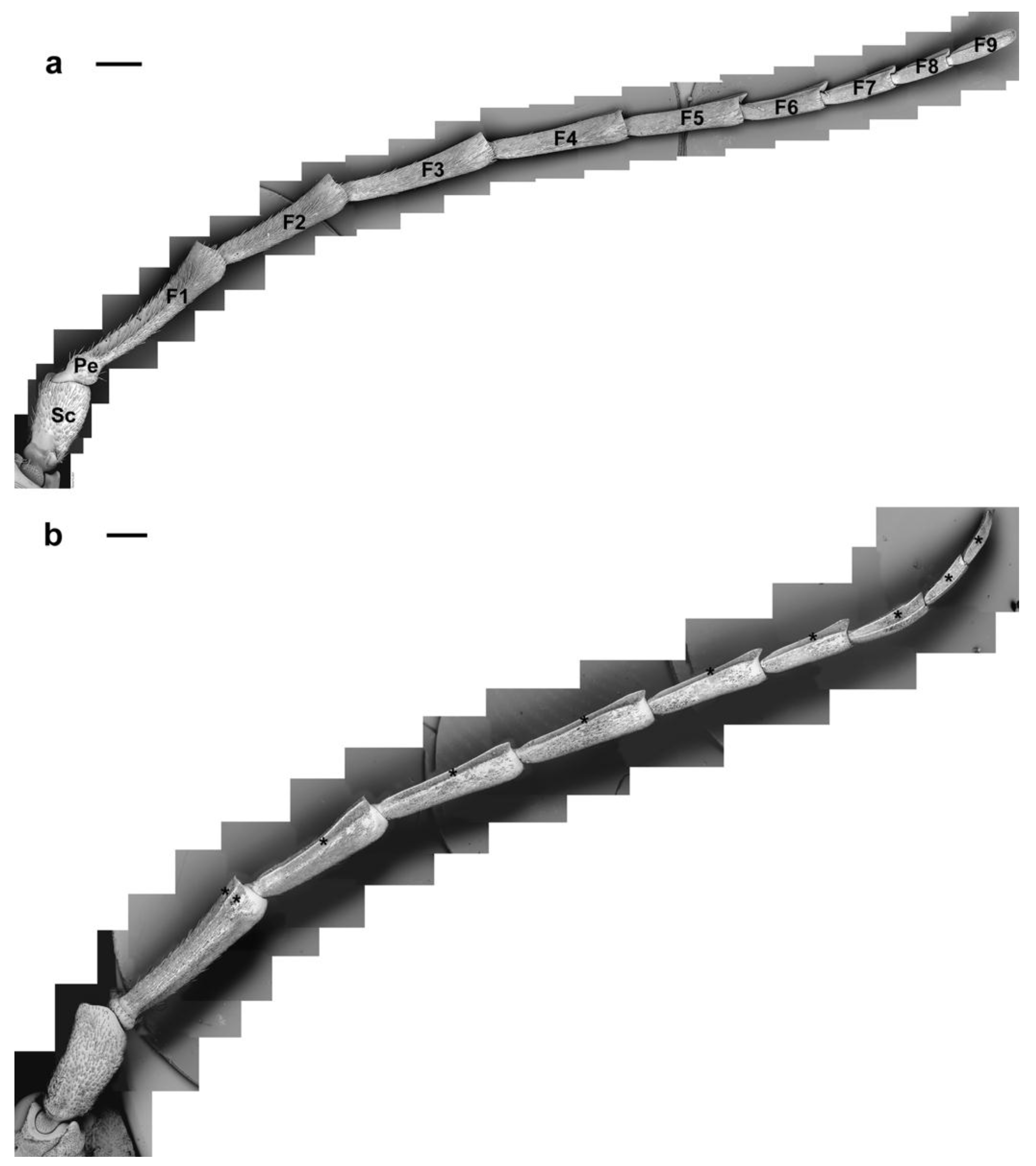
3.1. Antennal General Morphology
3.2. Antennal Sensilla and Their Secretion Analysis
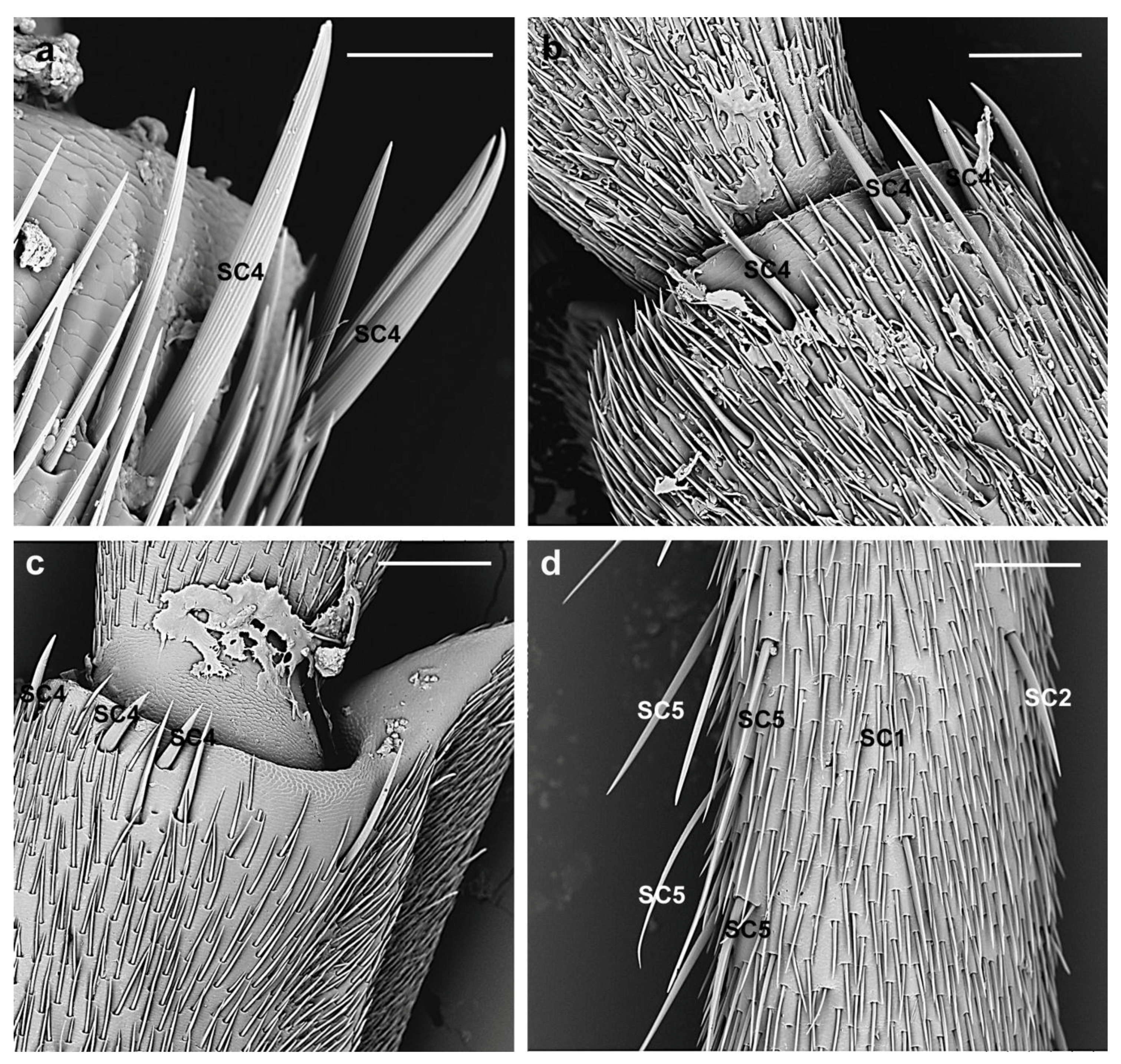
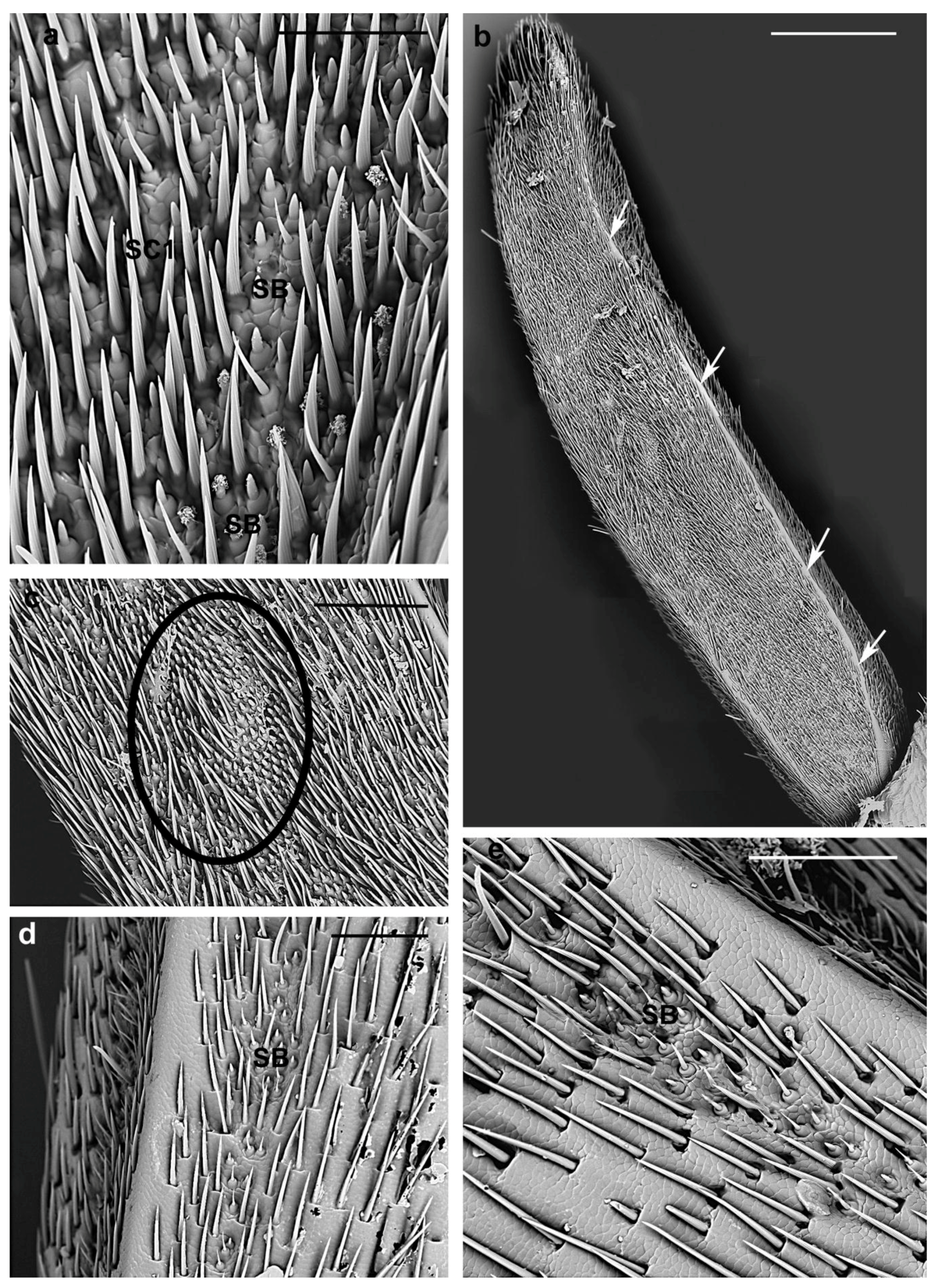
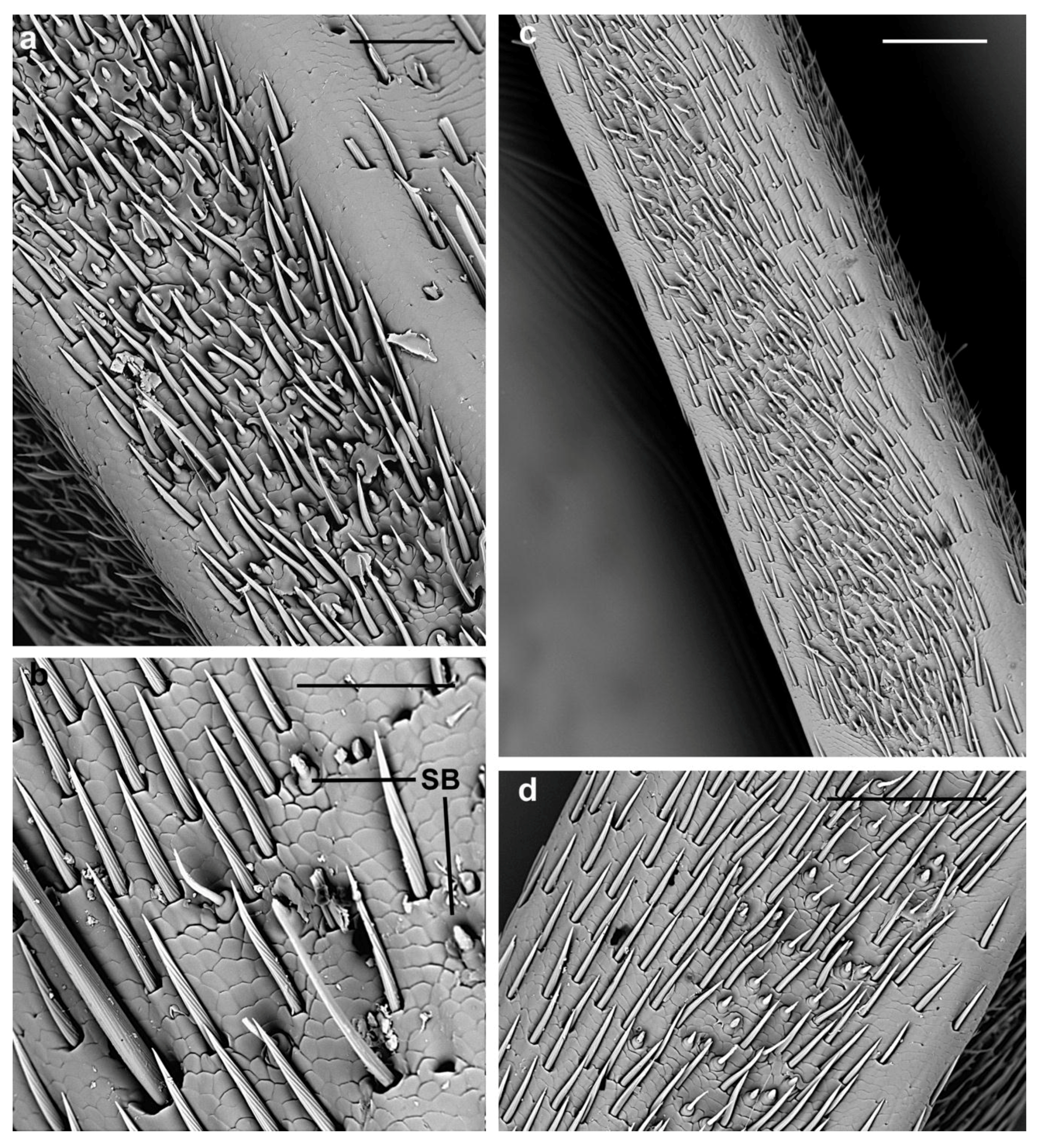
3.2.1. Sensilla Chaetica
3.2.2. Sensilla Trichodea
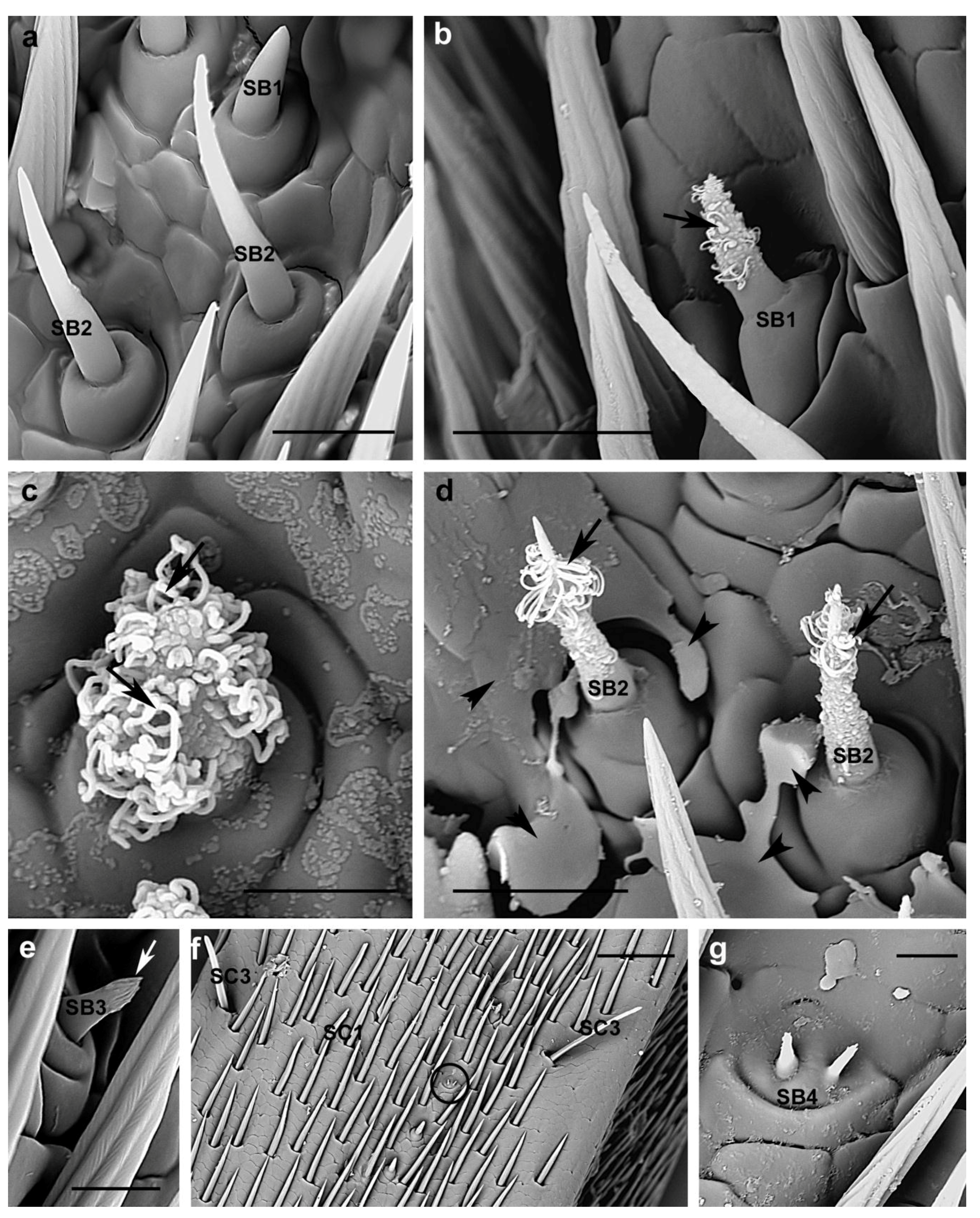
3.2.3. Sensilla Basiconica
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, J.W. NPAG Report: Aromia bungii (Faldermann): Redneck Longhorned Beetle Coleoptera/Cerambycidae; New Pest Advisory Group (NPAG), Plant Epidemiology and Risk Analysis Laboratory, Center for Plant Health Science & Technology, APHIS, USDA: Washington, DC, USA, 2009. [Google Scholar]
- Ostojá-Starzewski, J.O.; Baker, R.H.A. Red-necked Longhorn: Aromia bungii. Plant Pest Factsheet. FERA. 2012. Available online: http://www.fera.defra.gov.uk/plants/publications/documents/factsheets/aromiaBungii.pdf (accessed on 10 December 2018).
- EPPO. First Report of Aromia bungii in Germany: Addition to the EPPO Alert List. EPPO Reporting Service, September 2012. [Google Scholar]
- Burmeister, E.G. Der asiatische moschusbock in Bayern ausgerottet!? Ein Kafer, neu fur Deutschland, im Paragraphendschungel (Coleoptera: Cerambycidae, Aromia bungii (Faldermann, 1835)). Nachrichtenbl. Bayer. Entomol. 2012, 61, 80–82. (In German) [Google Scholar]
- Schrader, G.; Schröder, T. Express PRA for Aromia bungii; Vogt-Arndt, E., Translator; Institut fur Nationale und International Angelegenheiten der Pflanzengesundheit: Braunschweig, Germany, 2012; 7p. [Google Scholar]
- Garonna, A.P. Aromia bungii: Un Nuovo Fitofago delle Drupacee in Campania. Seminario-Workshop: Nuovi Pericolosi Insetti di Recente Introduzione in Campania. 27 November 2012. Available online: http://www.agricoltura.regione.campania.it/difesa/files/aromia_garonna.pdf (accessed on 7 December 2018). (In Italian).
- EPPO. First Report of Aromia bungii in Italy. EPPO Reporting Service, 1 October 2012. [Google Scholar]
- EPPO. Aromia bungii Found for the First Time in Lombardia Region, Italy. EPPO Reporting Service, September 2013. [Google Scholar]
- Garonna, A.P.; Nugnes, F.; Epinosa, B.; Griffo, R.; Benchi, D. Aromia bungii, nuovo tarlo asiatico ritrovato in Camapania [Aromia bungii, a new Asian worm found in Campania]. Inf. Agrar. 2013, 69, 60–62. (In Italian) [Google Scholar]
- Cocquempot, C. Aromia bungii. EPPO datasheet on pests recommended for regulation. EPPO Bull. 2015, 45, 4–8. [Google Scholar]
- EPPO. PRA for Aromia bungii. EPPO, September 2014. [Google Scholar]
- European Union. Commission Implementing Decision (EU) 2018/1503 of 8 October 2018 as regards measures to prevent the introduction into and the spread within the Union of Aromia bungii (Faldermann). Off. J. Eur. Union 2018, L 254, 9–18. [Google Scholar]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef]
- Iwabuchi, K. Mating Behavior of Xylotrechus pyrrhoderus BATES (Coleoptera: Cerambycidae) I. Behavioral sequences and existence of the male sex pheromone. Appl. Entomol. Zool. 1982, 17, 494–500. [Google Scholar] [CrossRef]
- Iwabuchi, K. Mating behavior of Xylotrechus pyrrhoderus Bates (Coleoptera; Cerambycidae) II. Female recognition by male and existence of a female sex pheromone. Appl. Entomol. Zool. 1985, 20, 416–423. [Google Scholar] [CrossRef]
- Iwabuchi, K. Mating behavior of Xylotrechus pyrrhoderus Bates (Coleoptera: Cerambycidae) III. Pheromone secretion by male. Appl. Entomol. Zool. 1986, 21, 606–612. [Google Scholar] [CrossRef]
- Iwabuchi, K. Mating behavior of Xylotrechus pyrrhoderus Bates (Coleoptera: Cerambycidae). VI mating system. J. Ethol. 1988, 6, 69–76. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Zeng, W.; Yin, X. Sex recognition by males and evidence for a female sex pheromone in Paraglenea fortunei (Coleoptera: Cerambycidae). Ann. Entomol. Soc. Am. 1991, 84, 107–110. [Google Scholar] [CrossRef]
- Hanks, L.M. Influence of the larval host plant on the reproductive strategies of cerambycid beetles. Annu. Rev. Entomol. 1999, 44, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Reagel, P.F.; Ginzel, M.D.; Hanks, L.M. Aggregation and mate location in the red milkweed beetle (Coleoptera: Cerambycidae). J. Insect Behav. 2002, 15, 811–830. [Google Scholar] [CrossRef]
- Allison, J.D.; Borden, J.H.; Seybold, S.J. A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 2004, 14, 123–150. [Google Scholar] [CrossRef]
- Ginzel, M.D.; Hanks, L.M. Role of host plant volatiles in mate location for three species of longhorned beetles. J. Chem. Ecol. 2005, 31, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Ammagarahalli, B.; Hall, D.R.; Pajares, J.A.; Gemeno, C. Smoke, pheromone and kairomone olfactory receptor neurons in males and females of the pine sawyer Monochamus galloprovincialis (Olivier) (Coleoptera: Cerambycidae). J. Insect Physiol. 2015, 82, 46–55. [Google Scholar] [CrossRef]
- Collignon, R.M.; Swift, I.P.; Zou, Y.; McElfresh, J.S.; Hanks, L.M.; Millar, J.G. The influence of host plant volatiles on the attraction of longhorn beetles to pheromones. J. Chem. Ecol. 2016, 42, 215–229. [Google Scholar] [CrossRef]
- Kuboki, M.; Akutsu, K.; Sakai, A.; Chuman, T. Bioassay of the sex pheromone of the udo longicorn beetle, Acalolepta luxuriosa Bates (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1985, 20, 88–89. [Google Scholar] [CrossRef]
- Fauziah, B.A.; Tabata, K.; Ito, K.; Takahashi, S.; Hidaka, T. Mating behavior of the cryptomeria bark borer, Semanotus japonicus Lacordaire (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1992, 27, 19–30. [Google Scholar] [CrossRef]
- Fukaya, M.; Honda, H. Reproductive biology of the yellow-spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae) I. Male mating behaviors and female sex pheromones. Appl. Entomol. Zool. 1992, 27, 89–97. [Google Scholar] [CrossRef]
- Hanks, L.M.; Millar, J.G.; Paine, T.D. Mating behavior of the eucalyptus longhorned borer (Coleoptera: Cerambycidae) and the adaptive significance of long “horns”. J. Insect Behav. 1996, 9, 383–393. [Google Scholar] [CrossRef]
- Ginzel, M.D.; Hanks, L.M. Contact pheromones as mate recognition cues of four species of longhorned beetles (Coleoptera: Cerambycidae). J. Insect Behav. 2003, 16, 181–187. [Google Scholar] [CrossRef]
- Ginzel, M.D.; Blomquist, G.J.; Millar, J.G.; Hanks, L.M. Role of contact pheromones in mate recognition in Xylotrechus colonus. J. Chem. Ecol. 2003, 29, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Ginzel, M.D.; Millar, J.G.; Hanks, L.M. Z9-pentacosene-contact sex pheromone of the locust borer Megacyllene robinae. Chemoecology 2003, 13, 135–141. [Google Scholar] [CrossRef]
- Zhang, A.; Oliver, J.E.; Chauhan, K.; Zhao, B.; Xia, L.; Xu, Z. Evidence for contact sex recognition pheromone of the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Naturwissenschaften 2003, 90, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Ginzel, M.D.; Moreira, J.A.; Ray, A.M.; Millar, J.G.; Hanks, L.M. (Z)-9-Nonacosene-major component of the contact sex pheromone of the beetle Megacyllene caryae. J. Chem. Ecol. 2006, 32, 435–451. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, J.-S.; Hant, J.-H.; Kim, Y.-J.; Yoon, C.; Kim, G.-H. Mating behavior of pine sawyer, Monochamus saltuarius Gebler (Coleoptera: Cerambycidae). J. Asia-Pac. Entomol. 2006, 9, 275–280. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Q.; Tian, M.Y.; He, X.Z.; Zeng, X.L.; Zhong, Y.X. Mate location and recognition in Glenea cantor (Fabr.) (Coleoptera: Cerambycidae: Lamiinae): Roles of host plant health, female sex pheromone, and vision. Environ. Entomol. 2007, 36, 864–870. [Google Scholar] [CrossRef]
- Ibeas, F.; Díez, J.J.; Pajares, J.A. Olfactory sex attraction and mating behaviour in the pine sawyer Monochamus galloprovincialis (Coleoptera: Cerambycidae). J. Insect Behav. 2008, 21, 101–110. [Google Scholar] [CrossRef]
- Lacey, E.S.; Ginzel, M.D.; Millar, J.G.; Hanks, L.M. 7-Methylheptacosane is a major component of the contact sex pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. Physiol. Entomol. 2008, 33, 209–216. [Google Scholar] [CrossRef]
- Fonseca, M.G.; Zarbin, P.H.G. Mating behaviour and evidence for sex-specific pheromones in Hedypathes betulinus (Coleoptera: Cerambycidae: Lamiinae). J. Appl. Entomol. 2009, 133, 695–701. [Google Scholar] [CrossRef]
- Rutledge, C.E.; Millar, J.G.; Romero, C.M.; Hanks, L.M. Identification of an important component of the contact sex pheromone of Callidiellum rufipenne (Coleoptera: Cerambycidae). Environ. Entomol. 2009, 38, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-L.; Zhuge, P.-P.; Wang, M.-Q. Mating behavior and contact pheromones of Batocera horsfieldi (Hope) (Coleoptera: Cerambycidae). Entomol. Sci. 2011, 14, 359–363. [Google Scholar] [CrossRef]
- Silk, P.J.; Sweeney, J.; Wu, J.; Sopow, S.; Mayo, P.D.; Magee, D. Contact sex pheromones identified for two species of longhorned beetles (Coleoptera: Cerambycidae) Tetropium fuscum and T. cinnamopterum in the Subfamily Spondylidinae. Environ. Entomol. 2011, 40, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Lopes, O.; Marques, P.C.; Araújo, J. The Role of Antennae in Mate Recognition in Phoracantha semipunctata (Coleoptera: Cerambycidae). Insect Behav. 2005, 18, 243–257. [Google Scholar] [CrossRef]
- Hughes, G.P.; Spikes, A.E.; Holland, J.D.; Ginzel, M.D. Evidence for the stratification of hydrocarbons in the epicuticular wax layer of female Megacyllene robiniae (Coleoptera: Cerambycidae). Chemoecology 2011, 21, 99–105. [Google Scholar] [CrossRef]
- Dyer, L.J.; Seabrook, W.D. Sensilla on the antennal flagellum of the sawyer beetles Monochamus notatus (Drury) and Monochamus scutellatus (Say) (Coleoptera: Cerambycidae). J. Morphol. 1975, 146, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, M.J. Antennal sensilla of the yellow longicorn beetle Phoracantha recurva Newman, 1840: Distribution and comparison with Phoracantha semipunctata (Fabricius, 1775) (Coleoptera: Cerambycidae). Bull. Inst. Sci. 2011, 33, 19–29. [Google Scholar]
- Kovats, E. Characterization of organic compounds by gas chromatography. Part 1. Retention indices of aliphatic halides, alcohols, aldehydes and ketones. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Nelson, D.R. Methyl-branched lipids in insects. In Insect Lipids: Chemistry, Biochemistry, and Biology; Stanley-Samuelson, D.W., Nelson, D.R., Eds.; University of Nebraska Press: Lincoln, NE, USA, 1993; pp. 271–315. [Google Scholar]
- Carlson, D.A.; Bernier, R.U.; Sutton, B.D. Elution patterns from capillary GC for methyl-branched alkanes. J. Chem. Ecol. 1998, 24, 1845–1865. [Google Scholar] [CrossRef]
- Guédot, C.; Millar, J.G.; Horton, D.R.; Landolt, P.J. Identification of a sex attractant pheromone for male winter form pear psylla, Cacopsylla pyricola. J. Chem. Ecol. 2009, 35, 1437–1447. [Google Scholar] [CrossRef]
- Mullen, S.P.; Millar, J.G.; Schal, C.; Shaw, K.L. Identification and characterization of cuticular hydrocarbons from a rapid species radiation of Hawaiian swordtailed crickets (Gryllidae: Trigonidiinae: Laupala). J. Chem. Ecol. 2008, 34, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- McIver, S.B. Structure of cuticular mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Akutsu, K.; Kuboki, M. Analysis of mating behavior of udo longicorn beetle, Acalolepta luxuriosa Bates (Coleoptera: Cerambycidae). Jpn. J. Appl. Entomol. Zool. 1983, 27, 247–251. [Google Scholar] [CrossRef]
- Slifer, E.H. The structure of Arthropod chemoreceptors. Annu. Rev. Entomol. 1970, 15, 121–142. [Google Scholar] [CrossRef]
- Keil, T.A.; Steinbrecht, R.A. Mechanosensitive and olfactory sensilla of insects. In Insect Ultrastructure; King, R.C., Akai, H., Eds.; Plenum Press: New York, NY, USA, 1984; Volume 25, pp. 477–516. [Google Scholar]
- Zacharuk, R.Y. Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Antennae and sensilla. In Comprehensive Insect Physiology Biochemistry and Pharmacology. Nervous System: Sensory; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 1–69. [Google Scholar]
- Lopes, O.; Barata, E.N.; Mustaparta, H.; Araúyo, J. Fine structure of antennal sensilla basiconica and their detection of plant volatiles in the eucalyptus woodborer, Phoracantha semipunctata Fabricius (Coleoptera: Cerambycidae). Arthropod Struct. Dev. 2002, 31, 1–13. [Google Scholar] [CrossRef]
- MacKay, C.A.; Sweeney, J.D.; Hillier, N.K. Morphology of antennal sensilla of the brown spruce longhorn beetle, Tetropium fuscum (Fabr.) (Coleoptera: Cerambycidae). Arthropod Struct. Dev. 2014, 43, 469–475. [Google Scholar] [CrossRef]
- Smith, C.M.; Frazier, J.L.; Coons, L.B.; Knight, W.E. Antennal sensilla of the clover head weevil Hypera Meles (F.) (Coleoptera: Curculionidae). Int. J. Insect Morphol. Embryol. 1976, 5, 349–355. [Google Scholar] [CrossRef]
- Inouchi, J.; Shibuya, T.; Matsuzaki, O.; Hatanaka, T. Distribution and fine structure of antennal olfactory sensilla in Japanese dung beetles, Geotrupes auratus Mtos. (Coleoptera: Geotrupidae) and Copris pecuarius Lew. (Coleoptera: Scarabaeidae). Int. J. Insect Morphol. Embryol. 1987, 16, 177–187. [Google Scholar] [CrossRef]
- Dai, H.-G.; Honda, H. Sensilla on the antennal flagellum of the yellow spotted longicorn beetle, Psacothea hilaris (Pascoe) (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 1990, 25, 273–282. [Google Scholar] [CrossRef]
- Ritcey, G.M.; Mciver, S.B. External morphology of antennal sensilla of four species of adult flea beetles (Coleoptera:Chrysomelidae:Alticinae). Int. J. Insect Morphol. Embryol. 1990, 19, 141–153. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, L.; Ren, B. Fine structure and distribution of antennal sensilla of longicorn beetles Leptura arcuata and Leptura aethiops (Coleoptera: Cerambycidae). Ann. Entomol. Soc. Am. 2011, 104, 778–787. [Google Scholar] [CrossRef]
- Chen, J.-M.; Qiao, H.-L.; Chen, J.; Xu, C.-Q.; Liu, S.; Lian, Z.-M.; Guo, K. Observation of antennal sensilla in Xylotrechus grayii (Coleoptera: Cerambycidae) with scanning electron microscopy. Microsc. Res. Tech. 2014, 77, 264–273. [Google Scholar] [CrossRef] [PubMed]
- De Marzo, L.; Vit, S. Contributo alla conoscenza delle Batrisinae paleartiche (Coleoptera, Pselaphidae). Le ghiandole antennali nei maschi di Batrisus Aubè e Batrisoides Reitter: Variazioni morfologiche, istologia e valore tassonomico. Entomologica 1983, XVIII, 77–110. [Google Scholar]
- Bin, F.; Vinson, S.B. Morphology of the antennal sex-gland in male Trissolcus basalis (Woll.) (Hymenoptera: Scelionidae), an egg parasitoid of the green stink bug, Nezara viridula (Hemiptera: Pentatomidae). Int. J. Insect Morphol. Embryol. 1986, 15, 129–138. [Google Scholar] [CrossRef]
- Bin, F.; Colazza, S.; Isidoro, N.; Solinas, M.; Vinson, S.B. Antennal chemosensilla and glands, and their possible meaning in the reproductive behaviour of Trissolcus basalis (Woll.) (Hym.: Scelionidae). Entomologica 1989, 30, 33–97. [Google Scholar]
- Bartlet, E.; Isidoro, N.; Williams, I.H. Antennal glands in Psylliodes chrysocephala, and their possible role in reproductive behaviour. Physiol. Entomol. 1994, 19, 241–250. [Google Scholar] [CrossRef]
- Skilbeck, C.A.; Anderson, M. The fine structure of glandular units on the antennae of two species of the parasitoid Aleochara (Coleoptera: Staphylinidae). Int. J. Insect Morphol. Embryol. 1994, 23, 319–328. [Google Scholar] [CrossRef]
- Isidoro, N.; Bin, F. Male antennal gland of Amitus spiniferus (Brethes) (Hymenoptera: Platygastridae) likely involved in courtship behavior. Int. J. Insect Morphol. Embryol. 1995, 24, 365–373. [Google Scholar] [CrossRef]
- Isidoro, N.; Bin, F.; Colazza, S.; Vinson, S.B. Morphology of antennal gustatory sensilla and glands in some parasitoids Hymenoptera with hypothesis on their role in sex and host recognition. J. Hymenopt. Res. 1996, 5, 206–239. [Google Scholar]
- Isidoro, N.; Romani, R.; Bin, F. Antennal multiporous sensilla; their gustatory features for host recognition in female wasps (Insecta, Hymenoptera: Platygastroidea). Microsc. Res. Tech. 2001, 55, 350–358. [Google Scholar] [CrossRef]
- Bin, F.; Isidoro, N.; Romani, R. Antennal structures of Hymenoptera: Sensilla or glands? Atti dell’Accademia Nazionale Italiana di Entomologia. Rendiconti 1999, XLVIII, 251–263. [Google Scholar]
- Weis, A.; Schonitzer, K.; Melzer, R.R. Exocrine glands in the antennae of the carabid beetle, Platynus assimilis (Paykull) (Coleoptera, Carabidae, Pterostichinae). I. Int. J. Insect Morphol. Embryol. 1999, 28, 311–335. [Google Scholar] [CrossRef]
- Giglio, A.; Ferrero, E.A.; Brandmayr, T.Z. Ultrastructural identification of the antennal gland complement in Siagona europaea Dejean 1826, a myrmecophagous carabid beetle. Acta Zool. 2005, 86, 195–203. [Google Scholar] [CrossRef]
- Belcari, A.; Kozánek, M. Secretory material from antennal organs and its possible role in mating behaviour of Pipunculidae (Diptera). Can. J. Zool. 2006, 84, 1727–1732. [Google Scholar] [CrossRef]
- Romani, R.; Rosi, M.C.; Isidoro, N.; Bin, F. The role of the antennae during courtship behaviour in the parasitic wasp Trichopria drosophilae. J. Exp. Biol. 2008, 211, 2486–2491. [Google Scholar] [CrossRef]
- Crook, D.J.; Higgins, R.A.; Ramaswamy, S.B. Antennal morphology of the soybean stemborer Dectes texanus texanus LeConte (Coleoptera: Cerambycidae). J. Kans. Entomol. Soc. 2003, 76, 397–405. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. 2018. Available online: http://www.pherobase.com (accessed on 6 December 2018).
- Ginzel, M.D. Hydrocarbons as contact pheromones of longhorned beetles (Coleoptera: Cerambycidae). In Insect Hydrocarbons; Blomquist, G.J., Bagneres, A.G., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 375–389. [Google Scholar]
- Fukaya, M.; Kiriyama, S.; Yasui, H. Mate-location flight of the red-necked longicorn beetle, Aromia bungii (Coleoptera: Cerambycidae): An invasive pest lethal to Rosaceae trees. Appl. Entomol. Zool. 2017, 52, 559–565. [Google Scholar] [CrossRef]
- Xu, T.; Yasui, H.; Teale, S.A.; Fujiwara-Tsujii, N.; Wickham, J.D.; Fukaya, M.; Hansen, L.; Kiriyama, S.; Hao, D.; Nakano, A.; et al. Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle, Aromia bungii. Sci. Rep. 2017, 7, 7330. [Google Scholar] [CrossRef] [PubMed]





| Sensillum Type | Location | Length | Shaft Aspect | Angle | Tips |
|---|---|---|---|---|---|
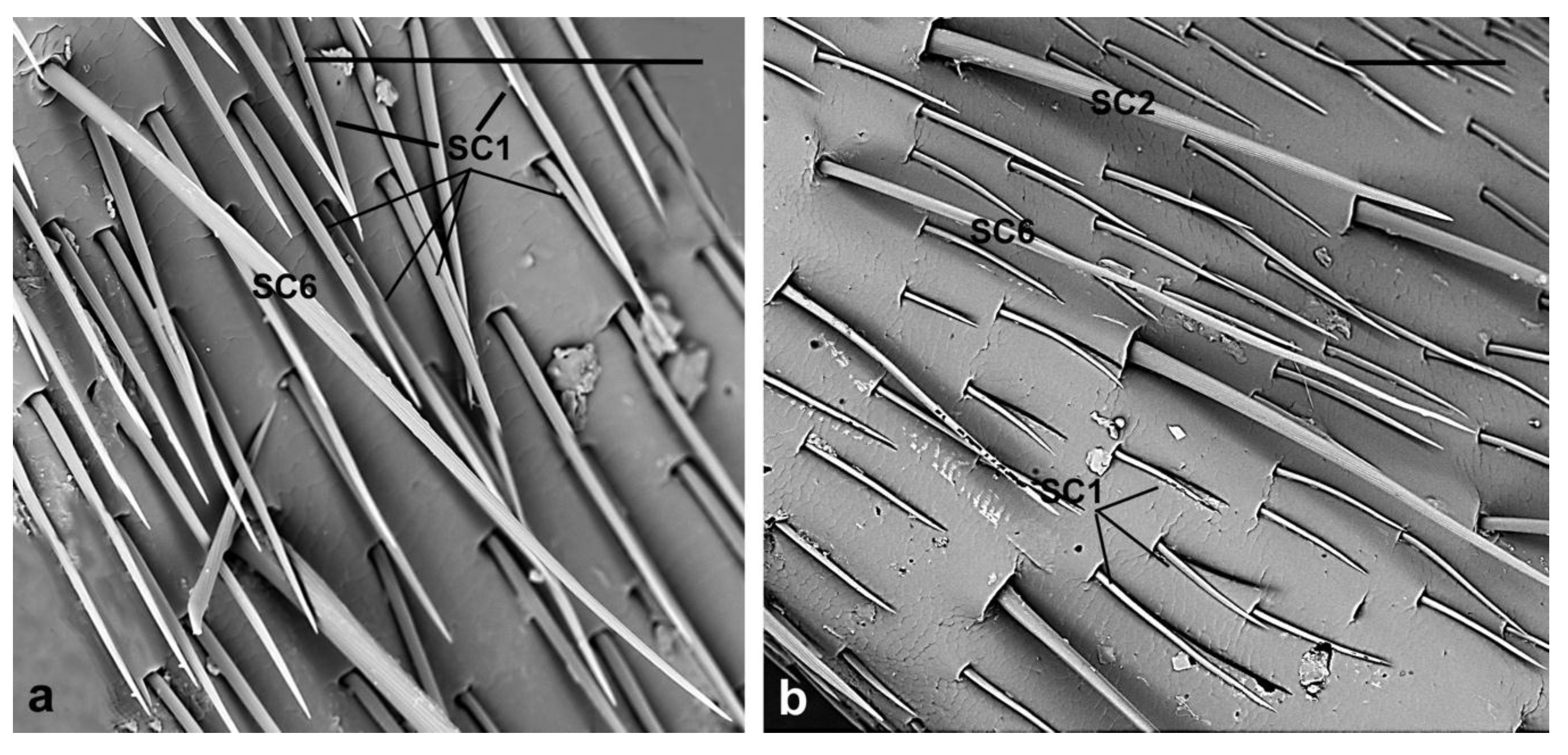
| SC1 | Evenly distributed around the circumference of each antennomere | Variable from 45 µm to 140 µm | V-shaped grooves | Parallel to the antennal surface | Sharp |
| SC2 | Around the circumference of scape, pedicel and flagellomeres | female 233.9 ± 35.4 µm; male 225.2 ± 51.6 µm | Longitudinal grooves | Shaft slightly diverging from the antennal surface | Tapering |
| SC3 | Around the circumference of flagellomeres and among the distal tuft of setae | female 68.9 ± 9.5 µm; male 67.9 ± 15 µm | Thin parallel grooves | Shaft at an angle of about 45° on the antennal surface | Blunt |
| SC4 | Arranged in a horizontal line on the distal region of the flagellomeres | female 209.4 ± 50.6 µm; male 277.3 ± 84 µm | Longitudinal grooves converging towards the tips | Parallel to the antennal surface | Sharp |
| SC5 | Dorsal side of the flagellomeres 1st–6th | female 329.4 ± 35.2 µm; male 384.4 ± 82.3 µm | Longitudinal grooves | Shaft diverging from the antennal surface | Tapering |
| SC6 | Dorsal side of flagellomeres 1st–5th | female 367 ± 53.2 µm; male 411 ± 68.3 µm | Longitudinal grooves | Shaft irregularly curved | Pointed |
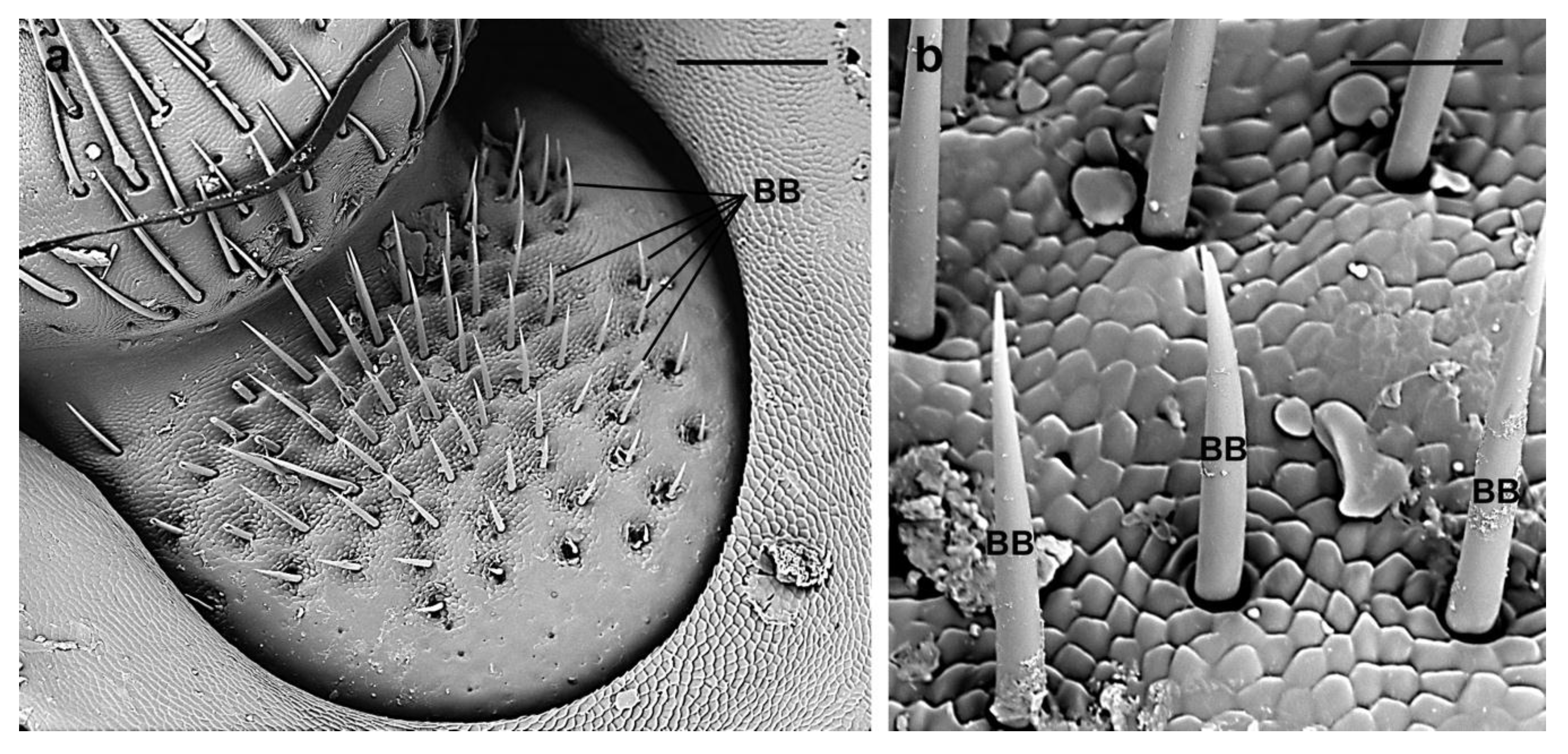
| BB | In groups between the scape and the head and between the scape and the pedicel | female 64.8 ± 17 µm; male 62.5 ± 13 µm | Smooth cuticle | Almost perpendicular to the antennal surface | Sharp |
| ST1 | Present only in males on the 1st and 2nd flagellomeres | 57.5 ± 4.4 µm | Grooved straight shaft | Parallel to the antennal surface | Pointed |
| SB1 | Present in all flagellomeres concentrated in two lateral bands | female 9.5 ± 1 µm; male 8.5 ± 0.7 µm | Curved, smooth | Arising from an elevated base | Blunt-tipped |
| SB2 | Present in all flagellomeres concentrated in two lateral bands | female 20.3 ± 3.8 µm; male 18.1 ± 3.3 µm | Thin, bent shaft with smooth surface | Emerging from an elevated socket | Pointed |
| SB3 | Few, scattered among SB1 and SB2 | female 5.9 ± 0.8 µm; male 5.3 ± 0.8 µm | Smooth at the base, finger like-structures at the tip | Insert into a wide dome | Blunt |
| SB4 | 1 sensillum ventral side of the 9th flagellomere | Smooth shaft | Raising from a common base | Jointed sensilla with blunt tip |
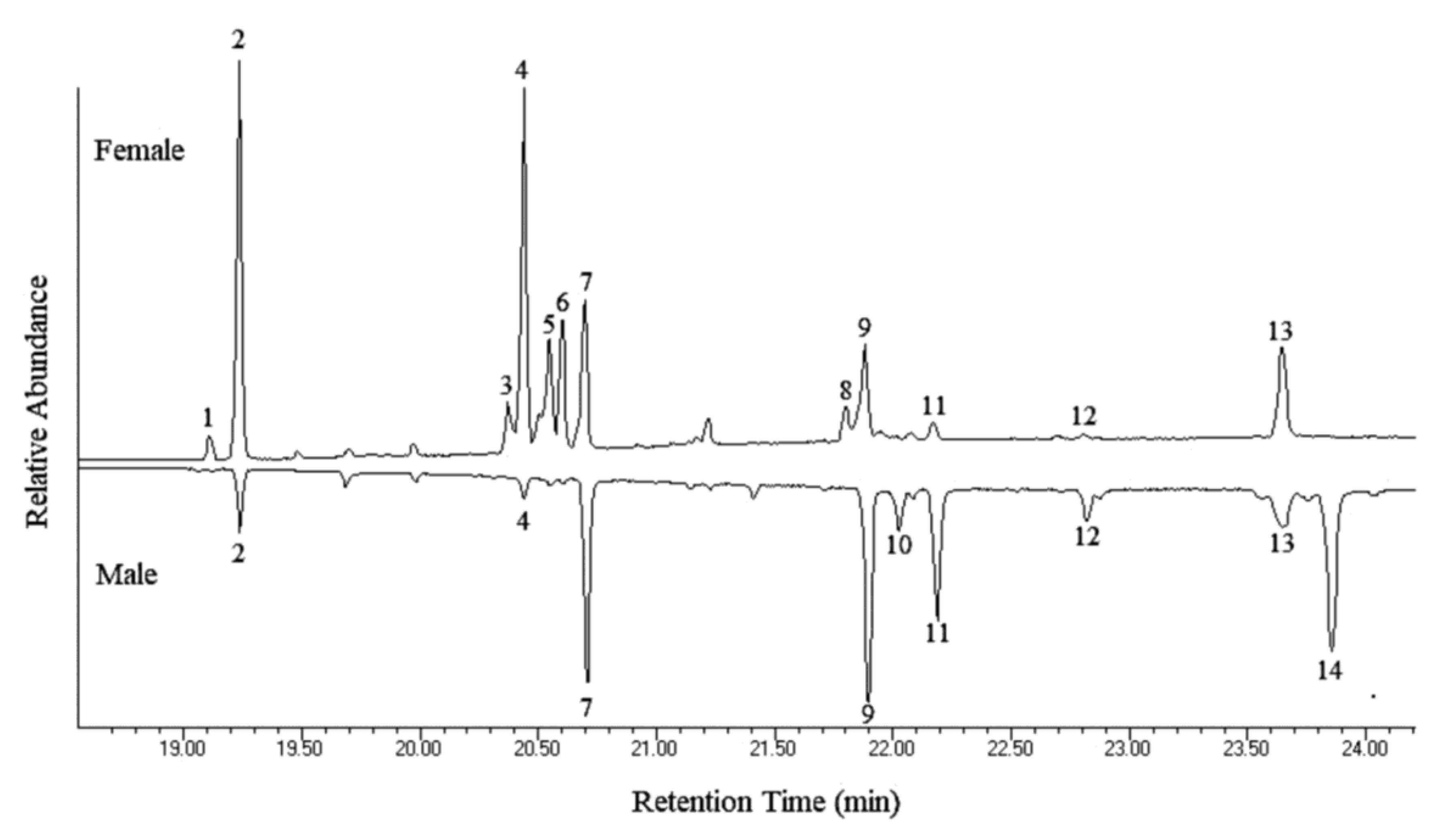
| Peak No. | Hydrocarbon | Percent of Total Hydrocarbons ± S.E. 1 | Diagnostic Ions | |
|---|---|---|---|---|
| Male | Female | |||
| 1 | 9:C23 | N.D. 2 | 1.69 ± 0.37 | 83, 97, 111 (322) |
| 2 | C25 | 1.87 ± 0.25 | 25.45 ± 5.71 | 352 |
| 3 | C27-diene | N.D. | 4.00 ± 0.66 | 376 |
| 4 | 2-Me-C26 | 0.54 ± 0.33 | 21.24 ± 4.43 | 365, 337 (380) |
| 5 | C27-monoene | N.D. | 10.36 ± 2.25 | 378 |
| 6 | C27-monoene | N.D. | 11.39 ± 1.81 | 378 |
| 7 | C27 | 11.27 ± 1.55 | 9.63 ± 0.96 | 380 |
| 8 | C29-diene | N.D. | 2.41 ± 0.38 | 404 |
| 9 | 2-Me-C28 | 20.51 ± 4.73 | 5.62 ± 0.88 | 365, 393 (408) |
| 10 | C29-monoene | 3.52 ± 0.55 | N.D. | 406 |
| 11 | C29 | 15.41 ± 2.88 | 1.56 ± 0.56 | 408 |
| 12 | 2-Me-C28 | 3.52 ± 0.31 | 0.26 ± 0.04 | 365, 393 (408) |
| 13 | 2-Me-C30 | 7.11 ± 0.77 | 6.39 ± 0.61 | 393, 421 (436) |
| 14 | C31-monoene | 36.24 ± 5.97 | N.D. | 434 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Palma, A.; Pistillo, M.; Griffo, R.; Garonna, A.P.; Germinara, G.S. Scanning Electron Microscopy of the Antennal Sensilla and Their Secretion Analysis in Adults of Aromia bungii (Faldermann, 1835) (Coleoptera, Cerambycidae). Insects 2019, 10, 88. https://doi.org/10.3390/insects10040088
Di Palma A, Pistillo M, Griffo R, Garonna AP, Germinara GS. Scanning Electron Microscopy of the Antennal Sensilla and Their Secretion Analysis in Adults of Aromia bungii (Faldermann, 1835) (Coleoptera, Cerambycidae). Insects. 2019; 10(4):88. https://doi.org/10.3390/insects10040088
Chicago/Turabian StyleDi Palma, Antonella, Marco Pistillo, Raffaele Griffo, Antonio P. Garonna, and Giacinto S. Germinara. 2019. "Scanning Electron Microscopy of the Antennal Sensilla and Their Secretion Analysis in Adults of Aromia bungii (Faldermann, 1835) (Coleoptera, Cerambycidae)" Insects 10, no. 4: 88. https://doi.org/10.3390/insects10040088
APA StyleDi Palma, A., Pistillo, M., Griffo, R., Garonna, A. P., & Germinara, G. S. (2019). Scanning Electron Microscopy of the Antennal Sensilla and Their Secretion Analysis in Adults of Aromia bungii (Faldermann, 1835) (Coleoptera, Cerambycidae). Insects, 10(4), 88. https://doi.org/10.3390/insects10040088






