How Cabbage Aphids Brevicoryne brassicae (L.) Make a Choice to Feed on Brassica napus Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aphids and Plants Rearing
2.2. Investigation of Aphid Population in Greenhouse
2.3. Microscopic Observation of Leaf Surface
2.4. EPG Experiments
2.5. Statistical Analysis
3. Results
3.1. Aphid Performance in Greenhouse
3.2. Leaf Surface Characteristic
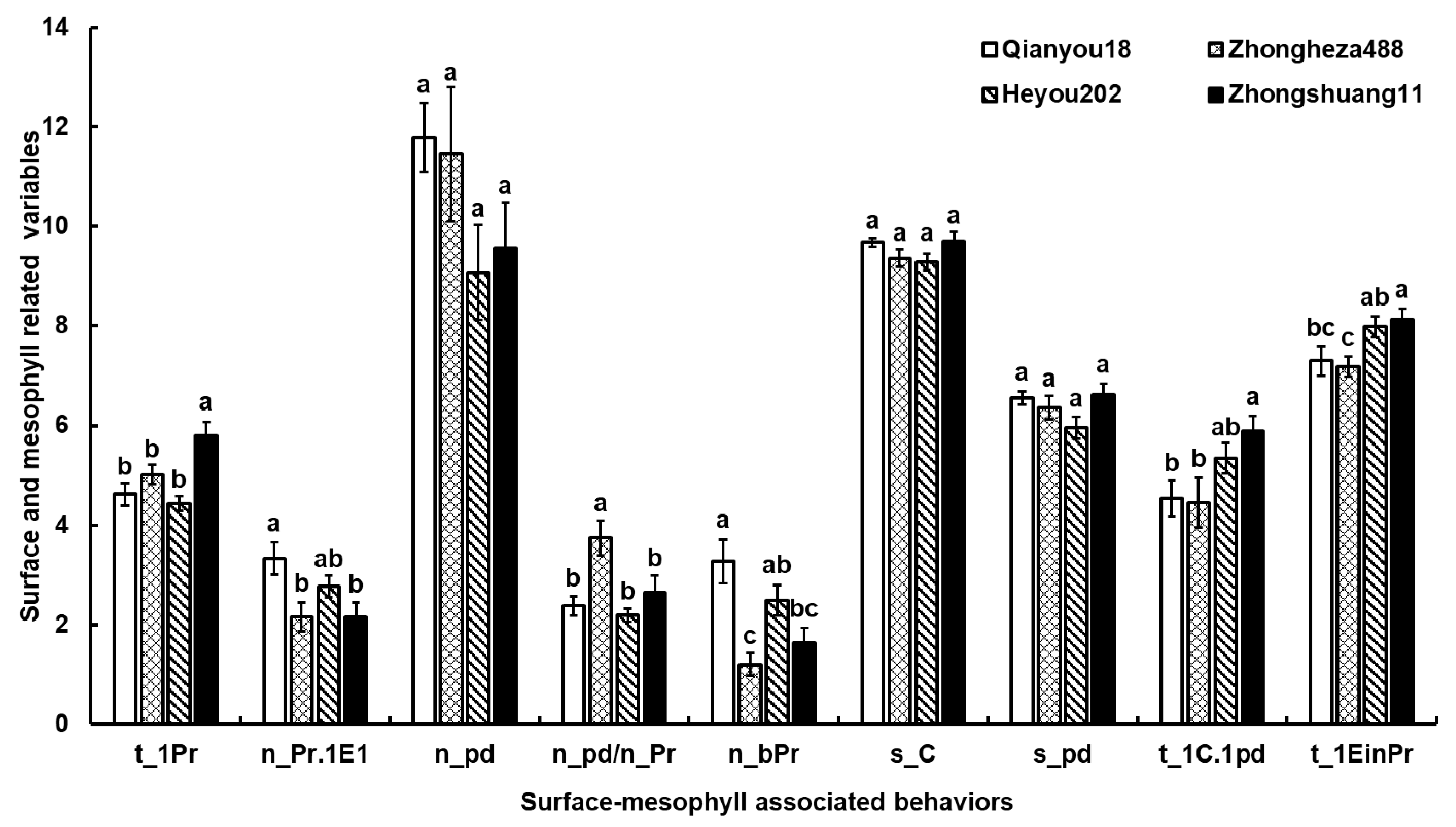
3.3. Aphid Probing and Feeding Behavior
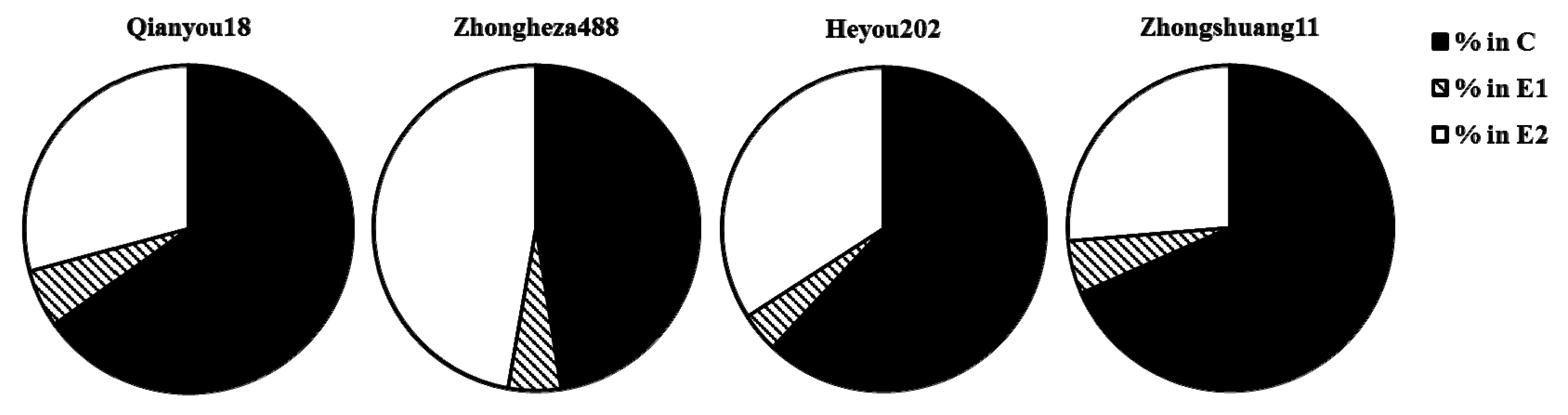
3.4. Relative Average Duration of Main Waveforms over 6 h
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prado, E.; Tjallingii, W.F. Aphid activities during sieve element punctures. Entomol. Exp. Appl. 1994, 72, 157–165. [Google Scholar] [CrossRef]
- Klingler, J.; Creasy, R.; Gao, L.; Nair, R.M.; Calix, A.S.; Jacob, H.S.; Edwards, O.R.; Singh, K.B. Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol. 2005, 137, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, F.; Opitz, S.E.W.; Inselsbacher, E.; Ganeteg, U.; Nasholm, T.; Ninkovic, V. Exploring the nitrogen ingestion of aphids-a new method using electrical penetration graph and 15N labelling. PLoS ONE 2013, 8, e83085. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.; Tjallingii, W.F.; Hardie, J. Host-plant selection and feeding. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CAB International, Cromwell Press: Trowbridge, UK, 2007; pp. 87–114. [Google Scholar]
- Alvarez, A.E.; Tjallingii, W.F.; Garzo, E.; Vleeshouwers, V.; Dicke, M.; Vosman, B. Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomol. Exp. Appl. 2006, 121, 145–157. [Google Scholar] [CrossRef]
- Le Roux, V.; Dugravot, S.; Campan, E.; Dubois, F.; Vincent, C.; Giordanengo, P. Wild Solanum resistance to aphids: Antixenosis or antibiosis? J. Econ. Entomol. 2008, 101, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Pompon, J.; Quiring, D.; Giordanengo, P.; Pelletier, Y. Role of host-plant selection in resistance of wild Solanum species to Macrosiphum euphorbiae and Myzus persicae. Entomol. Exp. Appl. 2010, 137, 73–85. [Google Scholar] [CrossRef]
- Escudero-Martinez, C.; Leybourne, D.J.; Bos, J.I.B. Non-host and poor-host resistance against aphids may reside in different plant cell layers depending on the plant species-aphid species interaction. bioRxiv 2018. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Zhang, L.; Shen, S.X.; Ma, Z.Y. Correlation analysis of physical and chemical characteristics with resistance to Sorghum aphid (Melanaphis sacchari) in different Sorghum genotypes. J. Plant Genet. Resour. 2008, 9, 55–61. [Google Scholar]
- Lapointe, S.L.; Tingey, W.M. Feeding response of the green peach aphid (Homoptera: Aphididae) to potato glandular trichomes. J. Econ. Entomol. 1984, 77, 386–389. [Google Scholar] [CrossRef]
- Gregory, P.; Tingey, W.M.; Ave, D.A.; Bouthyette, P.Y. Potato glandular trichomes: A physicochemical defense mechanism against insects. ACS Sym. Ser. 1986, 296, 160–167. [Google Scholar]
- Ave, D.A.; Gregory, P.; Tingey, W.M. Aphid repellent sesquiterpenes in glandular trichomes of Solanum berthaultii and S. tuberosum. Entomol. Exp. Appl. 1987, 44, 131–138. [Google Scholar] [CrossRef]
- Sauge, M.H.; Kervella, J.; Rahbe, Y. Probing behaviour of the green peach aphid Myzus persicae on resistant Prunus genotypes. Entomol. Exp. Appl. 1998, 89, 223–232. [Google Scholar] [CrossRef]
- Niemeyer, H.M. Secondary plant chemicals in aphid-host interactions. In Aphid-Plant Interactions: Population to Molecules; Peters, D.C., Webster, J.A., Chlouber, C.S., Eds.; Stillwater: Warner Robins, OK, USA, 1991; pp. 101–111. [Google Scholar]
- Dreyer, D.L.; Campbell, B.C. Association of the degree of methylation of intercellular pectin with plant resistance to aphids and with induction of aphid biotypes. Experientia 1984, 40, 224–226. [Google Scholar] [CrossRef]
- Givovich, A.; Niemeyer, H.M. Hydroxamic acids affecting barley yellow dwarf virus transmission by the aphid Rhopalosiphum padi. Entomol. Exp. Appl. 1991, 59, 79–85. [Google Scholar] [CrossRef]
- van Helden, M.; Tjallingii, W.F. Tissue localisation of lettuce resistance to the aphid Nasonovia ribisnigri using electrical penetration graphs. Entomol. Exp. Appl. 1993, 68, 269–278. [Google Scholar] [CrossRef]
- Chen, J.Q.; Rahbe, Y.; Delobel, B.; Sauvion, N.; Guillaud, J.; Febvay, G. Melon resistance to the aphid Aphis gossypii: Behavioural analysis and chemical correlations with nitrogenous compounds. Entomol. Exp. Appl. 1997, 85, 33–44. [Google Scholar] [CrossRef]
- Liang, L.Y.; Liu, L.F.; Yu, X.P.; Han, B.Y. Evaluation of the resistance of different tea cultivars to tea aphids by EPG technique. J. Integr. Agr. 2012, 11, 2028–2034. [Google Scholar] [CrossRef]
- Gabrys, B.; Pawluk, M. Acceptability of different species of Brassicaceae as hosts for the cabbage aphid. Entomol. Exp. Appl. 1999, 91, 105–109. [Google Scholar] [CrossRef]
- Hao, Z.P.; Hou, S.M.; Hu, B.C.; Huang, F.; Dang, X.L. Assessment of probing behavior of the cabbage aphid, Brevicoryne brassicae (Hemiptera: Aphididae), on three Brassica napus cultivars at three developmental stages using Electropenetrography (EPG). J. Kansas Entomol. Soc. 2017, 90, 11–23. [Google Scholar] [CrossRef]
- Yan, F.M.; Wang, M.Q. Insect Electrical Penetration Graph and Its Application, 1st ed.; Henan Science and Technology Press: Zhengzhou, China, 2017; p. 88. [Google Scholar]
- Tjallingii, W.F. Electronic recording of penetration behaviour by aphids. Entomol. Exp. Appl. 1978, 24, 721–730. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Salivary secretions by aphids interacting with proteins of phloem wound responses. J. Exp. Bot. 2006, 57, 739–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarria, E.; Cid, M.; Garzo, E.; Fereres, A. Excel workbook for automatic parameter calculation of EPG data. Comput. Electron. Agr. 2009, 67, 35–42. [Google Scholar] [CrossRef]
- Jiang, G.H.; Xie, M.; Lv, Z.X.; Wang, H.R.; Wang, L.P.; Wu, Y.J.; Zhang, H.Q.; Huang, P.L. Mechanism of resistance to aphids in strawberry cultivars. J. Fruit Sci. 2006, 23, 728–731. [Google Scholar]
- van Emden, H.F. Host-plant resistance. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CAB International, Cromwell Press: Trowbridge, UK, 2007; pp. 447–468. [Google Scholar]
- Martin, B.; Collar, J.L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78, 2701–2705. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.W. Aphid salivary secretions and their involvement in plant toxicoses. In Aphid-Plant Genotype Interactions; Campbell, R.K., Eikenbary, R.D., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1990; pp. 131–147. [Google Scholar]
- Dreyer, D.L.; Jones, K.C.; Jurd, L.; Campbell, B.C. Feeding deterrency of some 4-hydroxycoumarins and related compounds relationship to host-plant resistance of alfalfa towards pea aphid Acyrthosiphon pisum. J. Chem. Ecol. 1987, 13, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Goławska, S.; Leszczyński, B.; Oleszek, W. Effect of low and high-saponin lines of alfalfa on pea aphid. J. Insect Physiol. 2006, 52, 737–743. [Google Scholar]
- Goławska, S.; Łukasik, I. Acceptance of low-saponin lines of alfalfa with varied phenolic concentrations by pea aphid (Homoptera: Aphididae). Biologia 2009, 64, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Gururani, M.A.; Venkatesh, J.; Upadhyaya, C.P.; Nookaraju, A.; Pandey, S.K.; Park, S.W. Plant disease resistance genes: Current status and future directions. Physiol. Mol. Plant P. 2012, 78, 51–65. [Google Scholar] [CrossRef]
- Sandstrom, J.; Pettersson, J. Amino acid composition of phloem sap and the relation to intraspecific variation in pea aphid (Acyrthosiphon pisum) performance. J. Insect Physiol. 1994, 40, 947–955. [Google Scholar] [CrossRef]
- Karley, A.J.; Douglas, A.E.; Parker, W.E. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J. Exp. Biol. 2002, 205, 3009–3018. [Google Scholar] [PubMed]
- Powell, G.; Hardie, J.; Pickett, J.A. Effects of the antifeedant polygodial on plant penetration by aphids, assessed by video and electrical recording. Entomol. Exp. Appl. 1993, 68, 193–200. [Google Scholar] [CrossRef]
- Gabrys, B.; Tjallingii, W.F.; van Beek, T.A. Analysis of EPG recorded probing by cabbage aphid on host plant parts with different glucosinolate contents. J. Chem. Ecol. 1997, 23, 1661–1673. [Google Scholar] [CrossRef]
- Pettersson, J. Olfactory reactions of Brevicoryne brassicae (L.) (Hom.: Aph.). Swed. J. Agric. Res. 1973, 3, 95–103. [Google Scholar]
- Pettersson, J.; Pickett, J.A.; Pye, B.J.; Quiroz, A.; Smart, L.E.; Wadhams, L.J.; Woodcock, C.M. Winter host component reduces colonization of summer hosts by the bird-cherry–oat aphid, Rhopalosiphum padi (L.) and other aphids in cereal fields. J. Chem. Ecol. 1994, 20, 2565–2574. [Google Scholar] [CrossRef] [PubMed]
- Nottingham, S.F.; Hardie, J.; Dawson, G.W.; Hick, A.J.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Behavioural and electrophysiological responses of aphids to host- and non-host plant volatiles. J. Chem. Ecol. 1991, 17, 1231–1242. [Google Scholar] [CrossRef] [PubMed]


| Acronym | Variable Type | Definition |
|---|---|---|
| General | ||
| n_Pr | Frequency | Number of probes |
| s_Pr | Time | Total probing time |
| s_nE | Time | Total duration of the no phloematic phase |
| s_np | Time | Total time of the non-probing intervals |
| s_np.1E | Time | Duration of the nonprobe period before the 1st E |
| t_1E2rec | Time | Time from the start of EPG to the 1st E2 |
| t_1Erec | Time | Time from the start of EPG to the 1st E |
| Surface-mesophyll (Leaf) | ||
| t_1Pr | Time | Time to the first probe from the start of EPG |
| n_bPr | Frequency | Number of short probes (C < 3 min) |
| n_Pr.1E1 | Frequency | Number of probes before the 1st E1 (first phloem contact) |
| s_C | Time | Total C duration with pd |
| n_pd | Frequency | Number of pd |
| s_pd | Time | Total duration of pd |
| n_pd/n_Pr | Frequency | Average number of pd per probe |
| t_1C.1pd | Time | Time from the beginning of the 1st probe to the first pd |
| t_1EinPr | Time | Time from the beginning of that probe to the 1st E |
| Phloem | ||
| s_E | Time | Total duration of the E phases |
| n_E1 | Frequency | Number of E1 periods |
| s_E1 | Time | Total duration of E1 |
| d_E1followedby1sE2 | Time | Duration of the E1 followed by the first sustained E2 (>10 min) |
| s_E1followedbysE2 | Time | Total duration of E1 followed by sustained E2 (>10 min) |
| t_endLpd.E1followedbysE2 | Time | Time from the end of the last pd to the beginning of the E1 followed by the sustained E2 (>10 min) |
| rel_E1_E12 | Index | Relative amount of E1 on E12 |
| s_E2 | Time | Total duration of E2 periods |
| s_longestE2 | Time | Duration of the longest E2 |
| E2index | Index | phloemian index: % of the time of the E2 after the start of the 1st E2 |
| %sE2/E2 | Index | Relative amount of sE2 on E2 |
| Variables 1 | Qianyou18 | Zhongheza488 | Heyou202 | Zhongshuang11 |
|---|---|---|---|---|
| n_Pr | 5.30 ± 0.46a | 3.38 ± 0.50b | 4.33 ± 0.47ab | 3.67 ± 0.50b |
| s_Pr | 10.04 ± 0.04b | 10.24 ± 0.01a | 10.16 ± 0.02a | 10.19 ± 0.02a |
| s_nE | 9.90 ± 0.08a | 9.45 ± 0.17a | 9.67 ± 0.12a | 9.71 ± 0.23a |
| s_np | 8.18 ± 0.22a | 6.50 ± 0.24c | 7.54 ± 0.26ab | 7.24 ± 0.35bc |
| s_np.1E | 7.22 ± 0.24a | 6.04 ± 0.31b | 6.88 ± 0.28ab | 7.02 ± 0.34a |
| t_1Erec | 9.08 ± 0.23a | 8.40 ± 0.20b | 8.81 ± 0.07ab | 9.37 ± 0.22a |
| t_1E2rec | 9.19 ± 0.24a | 8.71 ± 0.22a | 8.82 ± 0.07a | 9.54 ± 0.24a |
| Variables 1 | Qianyou18 | Zhongheza488 | Heyou202 | Zhongshuang11 |
|---|---|---|---|---|
| n_E1 | 1.70 ± 0.29a | 2.20 ± 0.39a | 1.16 ± 0.19a | 1.42 ± 0.28a |
| s_E | 8.75 ± 0.20a | 9.24 ± 0.30a | 8.74 ± 0.44a | 8.29 ± 0.63a |
| s_E1 | 5.38 ± 0.22a | 5.47 ± 0.41a | 4.83 ± 0.22a | 5.64 ± 0.29a |
| s_E2 | 8.85 ± 0.15a | 9.06 ± 0.38a | 8.64 ± 0.51a | 8.46 ± 0.66a |
| s_longestE2 | 8.49 ± 0.22a | 8.80 ± 0.45a | 8.42 ± 0.50a | 8.31 ± 0.66a |
| d_E1followedby1sE2 | 4.34 ± 0.04a | 4.10 ± 0.02a | 4.26 ± 0.11a | 4.41 ± 0.15a |
| s_E1followedbysE2 | 4.94 ± 0.20a | 4.15 ± 0.04b | 4.73 ± 0.18a | 4.98 ± 0.17a |
| t_endLpd.E1followedbysE2 | 6.60 ± 0.75a | 5.30 ± 0.69a | 6.05 ± 0.58a | 3.91 ± 0.04a |
| rel_E1_E12 | 0.20 ± 0.02b | 0.16 ± 0.06b | 0.14 ± 0.01b | 0.54 ± 0.19a |
| E2index | 0.82 ± 0.13a | 0.99 ± 0.15a | 0.88 ± 0.13a | 0.86 ± 0.19a |
| %sE2/E2 | 1.16 ± 0.16a | 1.06 ± 0.19a | 1.26 ± 0.11a | 0.83 ± 0.24a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.-P.; Zhan, H.-X.; Wang, Y.-L.; Hou, S.-M. How Cabbage Aphids Brevicoryne brassicae (L.) Make a Choice to Feed on Brassica napus Cultivars. Insects 2019, 10, 75. https://doi.org/10.3390/insects10030075
Hao Z-P, Zhan H-X, Wang Y-L, Hou S-M. How Cabbage Aphids Brevicoryne brassicae (L.) Make a Choice to Feed on Brassica napus Cultivars. Insects. 2019; 10(3):75. https://doi.org/10.3390/insects10030075
Chicago/Turabian StyleHao, Zhong-Ping, Hai-Xia Zhan, Yu-Long Wang, and Shu-Min Hou. 2019. "How Cabbage Aphids Brevicoryne brassicae (L.) Make a Choice to Feed on Brassica napus Cultivars" Insects 10, no. 3: 75. https://doi.org/10.3390/insects10030075
APA StyleHao, Z.-P., Zhan, H.-X., Wang, Y.-L., & Hou, S.-M. (2019). How Cabbage Aphids Brevicoryne brassicae (L.) Make a Choice to Feed on Brassica napus Cultivars. Insects, 10(3), 75. https://doi.org/10.3390/insects10030075





