Influence of Abscisic Acid-Biosynthesis Inhibitor Fluridone on the Feeding Behavior and Fecundity of Nilaparvata lugens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rice Cultivars and Insects
2.2. Analysis of BPH Feeding Behavior with EPG
2.3. Effects of FLU on BPH Eggs and Oviposition Period
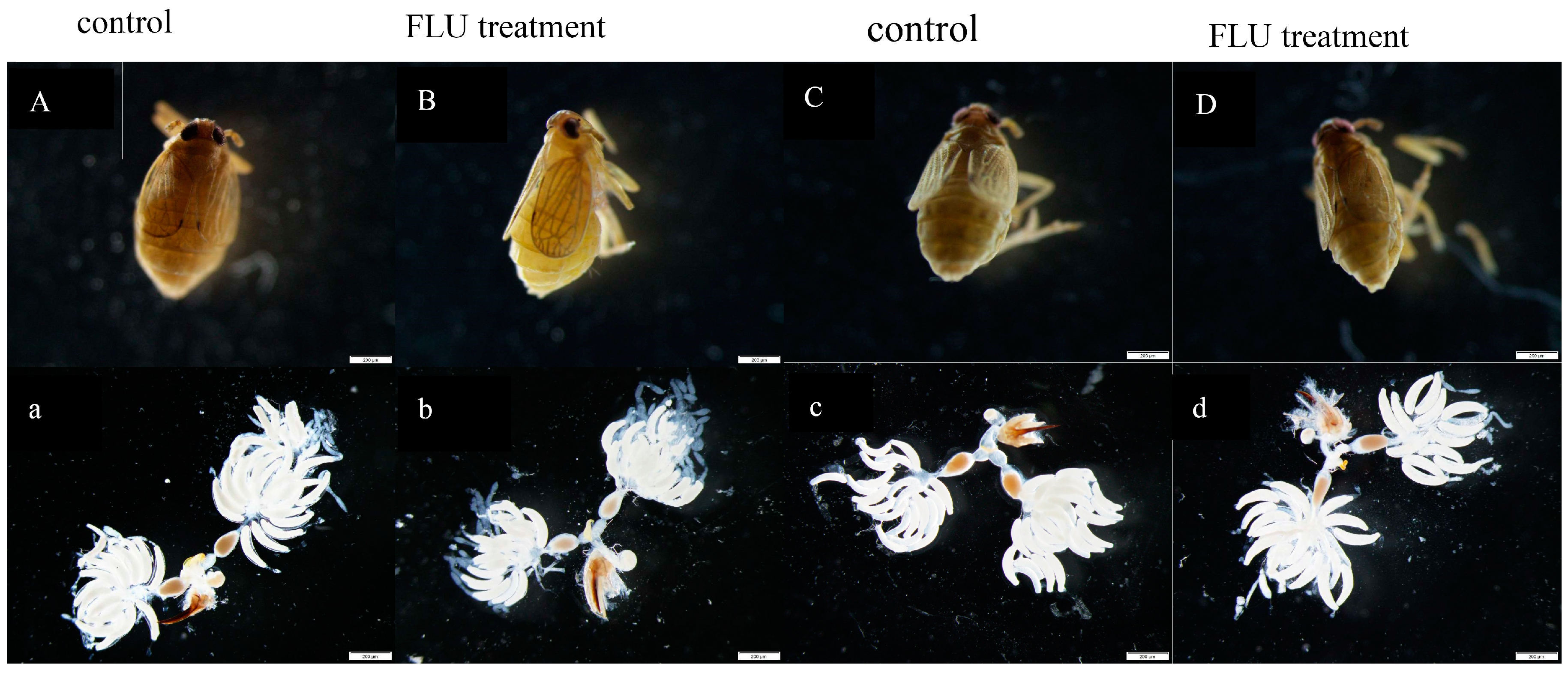
2.4. Ovarian Anatomy
2.5. Influences of FLU on the Relative Expression of Nlvg
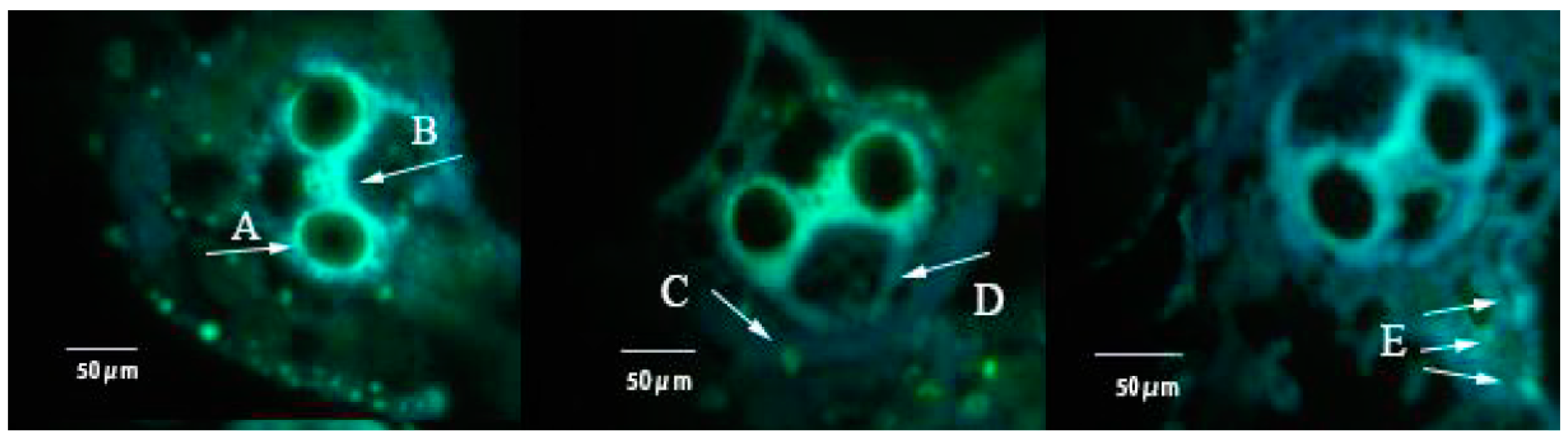
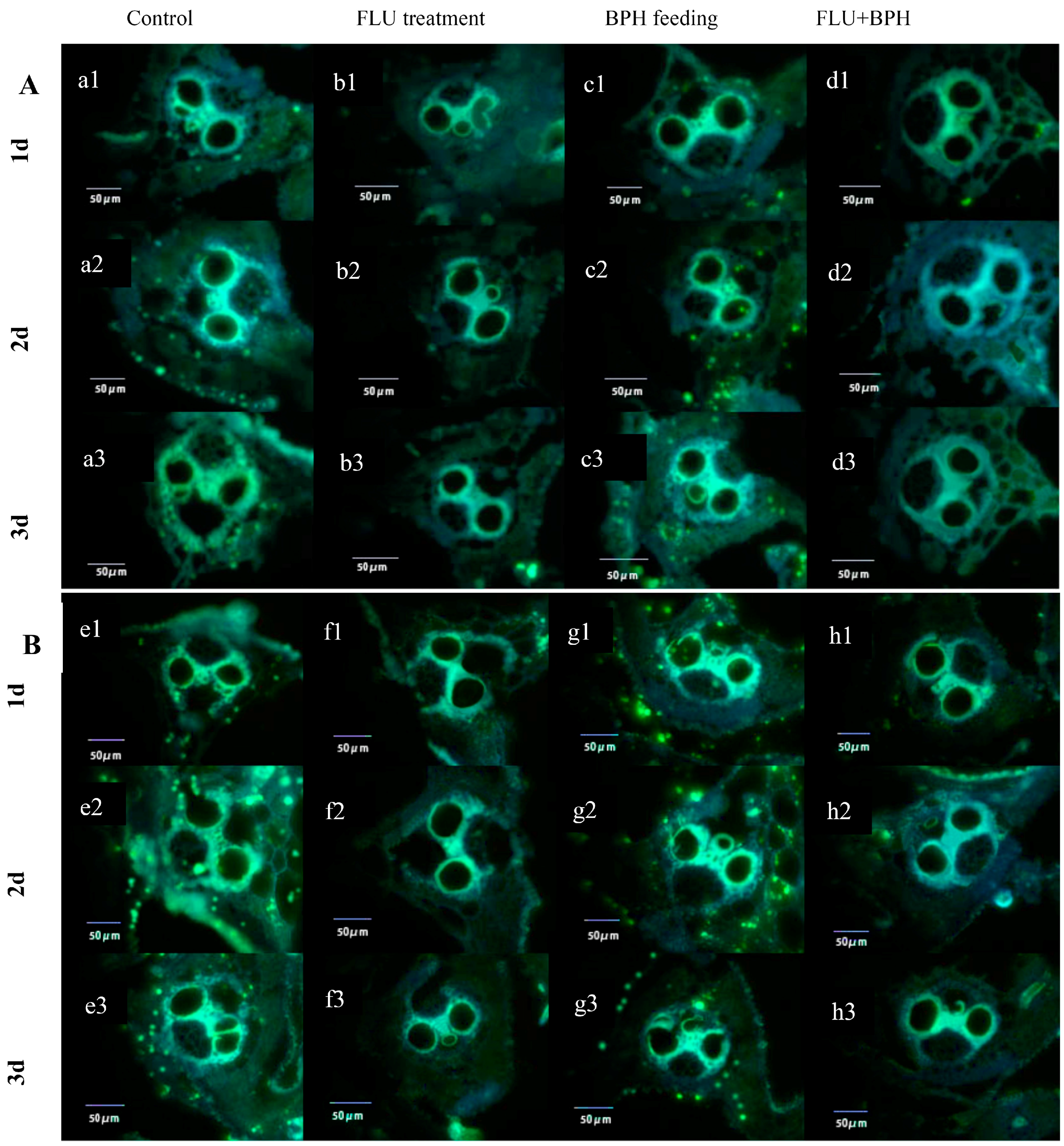
2.6. Callose Deposition Area in Rice after Treated with FLU
2.7. Data Analysis
3. Results
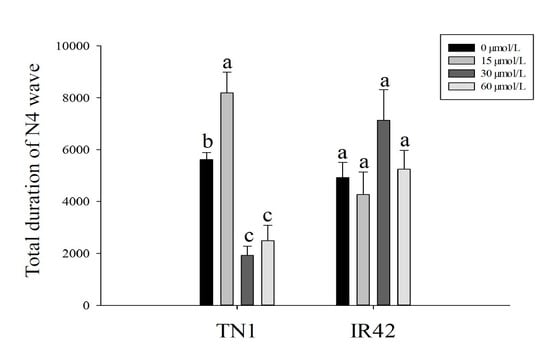
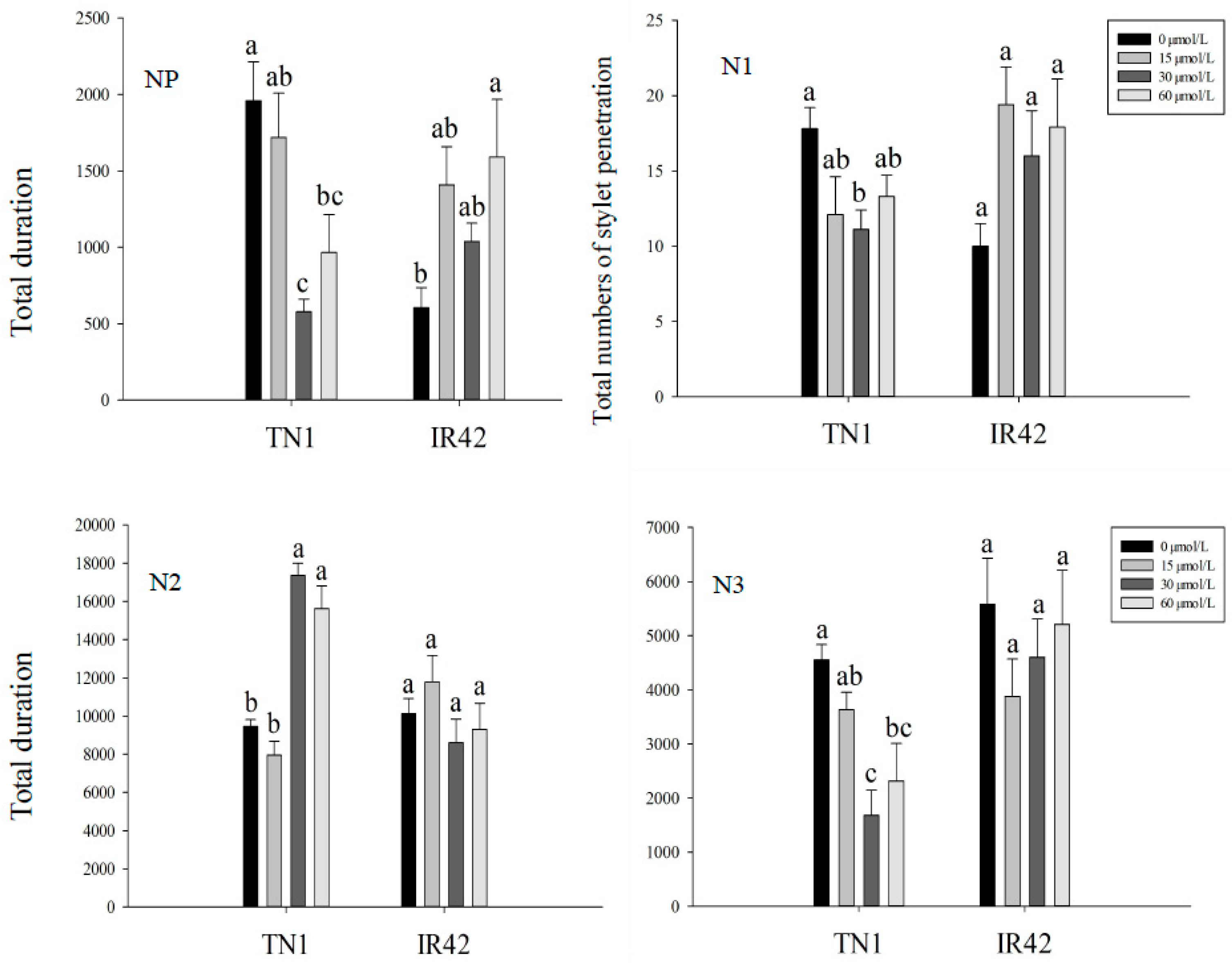
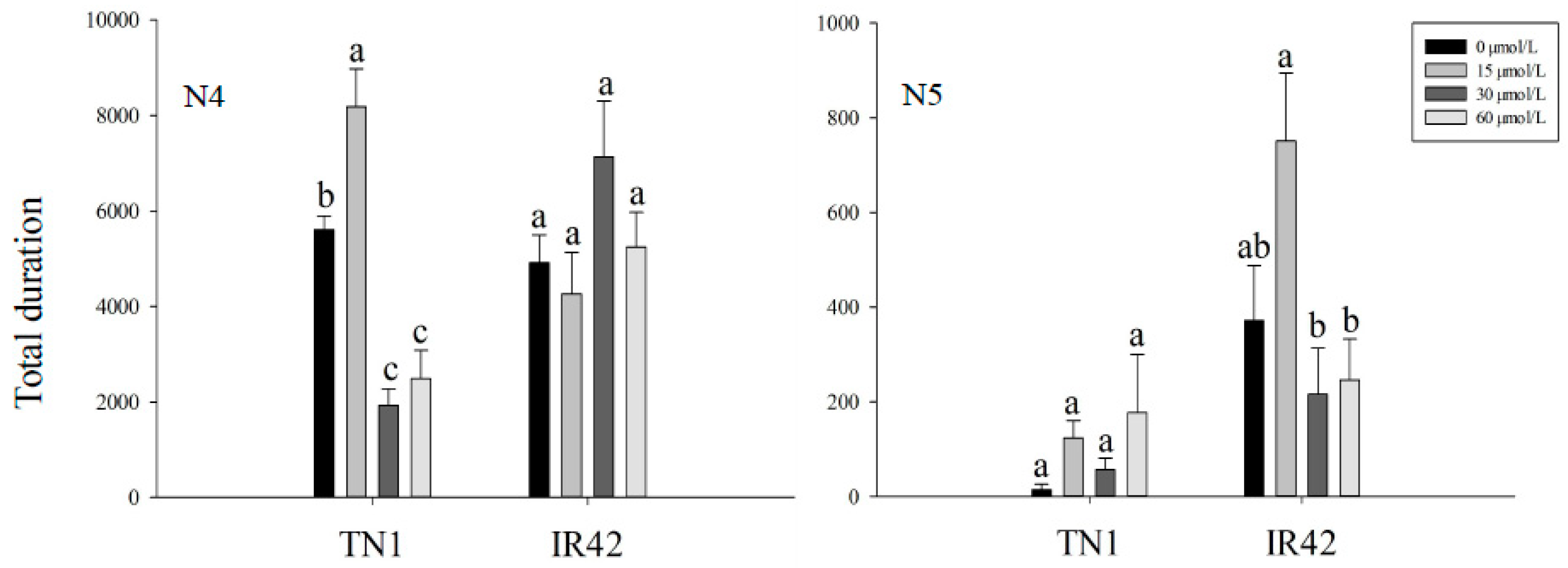
3.1. EPG Analysis of BPH Feeding Behavior
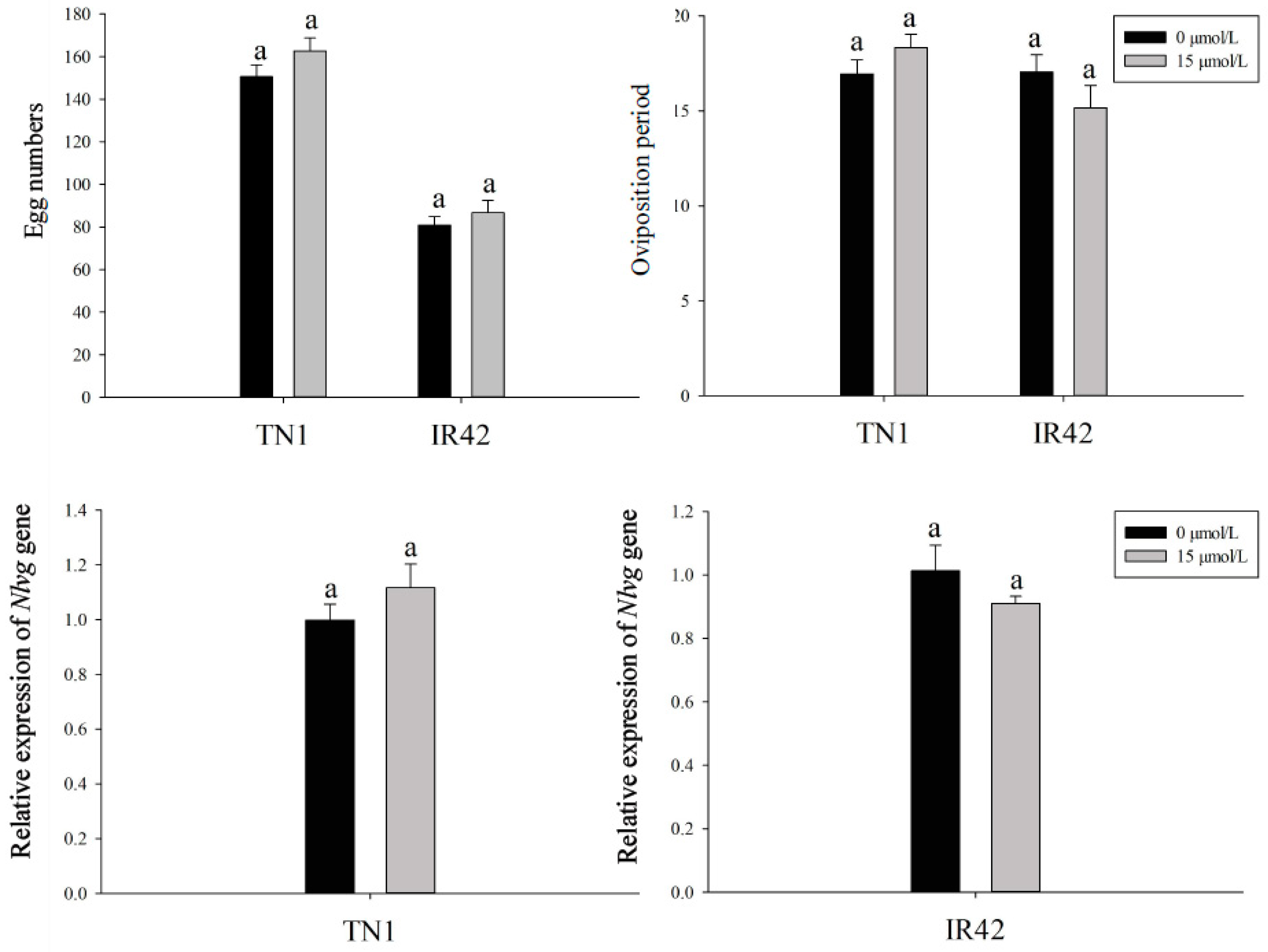
3.2. Effects of FLU on the Fecundity of BPH
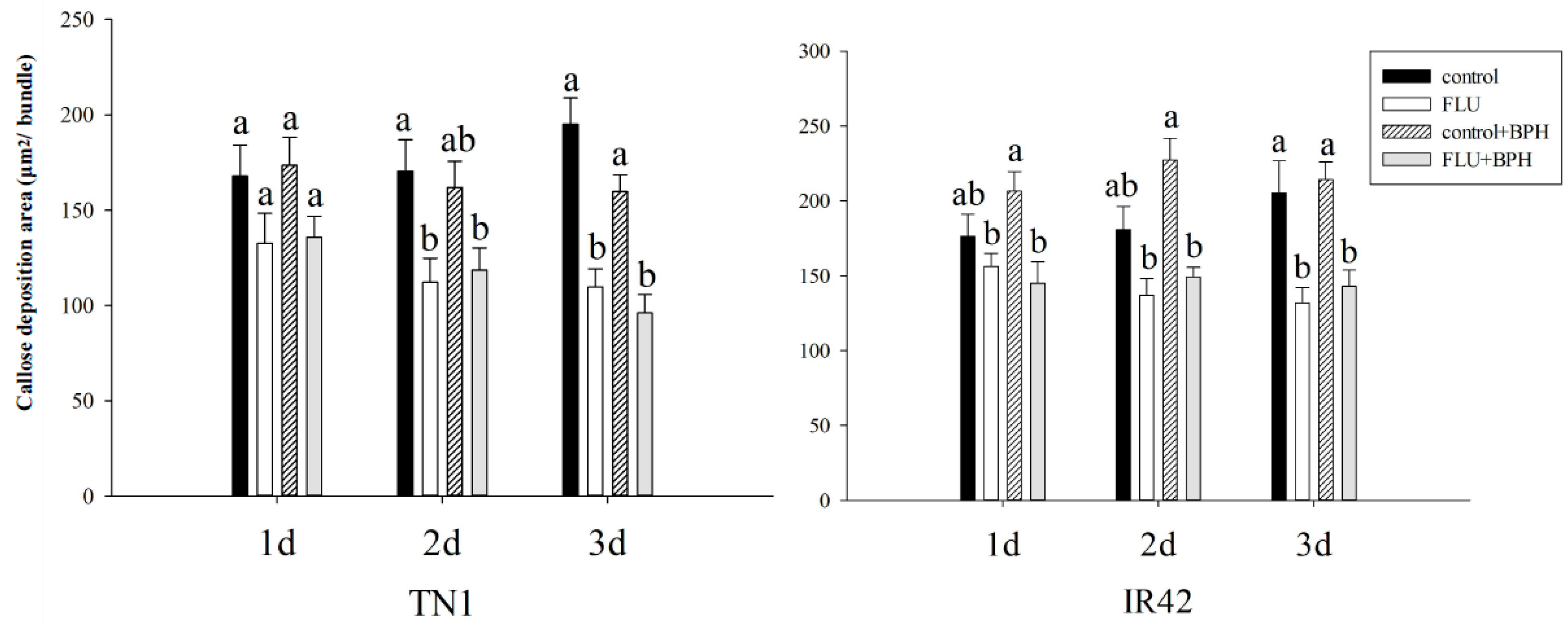
3.3. Effect of FLU on Callose Deposition in Rice Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, L.X.; Lv, B.S.; Wang, M.M.; Ma, H.Y.; Yang, H.Y.; Liu, X.L.; Jiang, C.J.; Liang, Z.W. Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol. Biochem. 2015, 90, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Long, H.T.; Li, L.; Wan, X.R. Relationship between ABA-inducible gene and stress. Sub. Trop. Plant Sci. 2004, 33, 74–77. [Google Scholar]
- Liu, H.J.; Liu, Y.; Liu, L. Progress of research on the influence of abscisic acid in plant resistance. Biotechnol. Bull. 2008, 6, 7–9. [Google Scholar]
- You, M.K.; Shin, H.Y.; Kim, Y.J.; Ok, S.H.; Cho, S.K.; Jeung, J.U.; Yoo, S.D.; Kim, J.K.; Shin, J.S. Novel bifunctional nucleases, OmBBD and AtBBD1, are involved in abscisic acid-mediated callose deposition in Arabidopsis. Plant Physiol. 2010, 152, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Chen, X.; Zhang, H.M.; Yang, X.; Wong, A. Effects of exogenous plant growth regulator abscisic acid-induced resistance in rice on the expression of vitellogenin mRNA in Nilaparvata lugens (Hemiptera: Delphacidae) adult females. J. Insect Sci. 2014, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.J.; Sun, J.Y.; Dai, B.J. HPLC Analysis of fluridone. Fine Chem. Intermed. 2013, 43, 71–73. [Google Scholar]
- Bartels, P.G.; Watson, C.W. Inhibition of carotenoid synthesis by fluridone and norflurazon. Weed Sci. 1978, 26, 198–203. [Google Scholar]
- Bartley, G.E.; Scolnik, P.K.; Giuliano, G. Molecular biology of carotenoid biosynthesis in plants. Annu. Rev. Plant. Biol. 1994, 45, 287–301. [Google Scholar] [CrossRef]
- Belefant-Miller, H.; Fong, F.; Smith, J.D. Abscisic acid biosynthesis during corn embryo development. Planta 1994, 195, 17–21. [Google Scholar] [CrossRef]
- Cowan, A.K.; Richardson, G.R. Carotenogenic and abscisic acid biosynthesizing activity in a cell-free system. Plant Physiol. 1997, 99, 371–378. [Google Scholar] [CrossRef]
- Rasmussen, R.D.; Hole, D.; Hess, J.R.; Carman, J.G. Wheat kernel dormancy and abscisic acid level following exposure to fluridone. J. Plant Physiol. 1997, 150, 440–445. [Google Scholar] [CrossRef]
- Perales, L.; Arbona, V.; Gomez-Cadenas, A.; Cornejo, M.J.; Sanz, A. A relationship between tolerance to dehydration of rice cell lines and ability for ABA synthesis under stress. Plant Physiol. Biochem. 2005, 43, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Kuluev, B.; Mikhaylova, E.; Berezhneva, Z.; Nikonorov, Y.; Postrigan, B.; Kudoyarova, G.; Chemeris, A. Expression profiles and hormonal regulation of tobacco NtEXGT gene and its involvement in abiotic stress response. Plant Physiol. Biochem. 2017, 111, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.Y.; Liu, C.X.; Wang, Y.Y.; Chen, R.Z.; Tang, M.; Du, B.; Zhu, L.; He, G. Herbivore-induced callose deposition on the sieve plates of rice: An important mechanism for host resistance. Plant Physiol. 2008, 146, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Du, H.T.; Ding, X.; Zhou, Y.D.; Xie, P.F.; Wu, J. Mechanisms of callose deposition in rice regulated by exogenous abscisic acid and its involvement in rice resistance to Nilaparvata lugens Stål (Hemiptera: Delphacidae). Pest Manag. Sci. 2017, 73, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Aist, J.R. Papillae and related wound plugs of plant cells. Annu. Rev. Phytopathol. 1976, 14, 145–163. [Google Scholar] [CrossRef]
- Ton, J.; Mauch-Mani, B. Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J. 2004, 38, 119–130. [Google Scholar] [CrossRef]
- Flors, V.; Ton, J.; Jakab, G.; Mauch-Mani, B. Abscisic acid and callose: Team players in defence against pathogens? J. Phytopathol. 2005, 153, 377–383. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Mauch, F. The role of abscisic acid in plant-pathogen interactions. Curr. Opin. Plant. Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef]
- Ingle, R.A.; Carstens, M.; Denby, K.J. PAMP recognition and the plant-pathogen arms race. Bioessays 2006, 28, 880–889. [Google Scholar] [CrossRef]
- Asselbergha, B.; Hofte, M. Basal tomato defences to Botrytis cinerea include abscisic acid-dependent callose formation. Physiol. Mol. Plant. Pathol. 2007, 71, 33–40. [Google Scholar] [CrossRef]
- Garcia-Andrade, J.; Ramírez, V.; Flors, V.; Vera, P. Arabidopsis ocp3 mutant reveals a mechanism linking ABA and JA to pathogen-induced callose deposition. Plant J. 2011, 67, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Oide, S.; Bejai, S.; Staalm, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Cheng, S.N.; Yan, L.M.; Yin, H.T. The ovarial development of the brown planthopper (Nilaparvata lugens Stal) and it’s relation to migration. Acta Entomol. Sin. 1979, 22, 280–288. [Google Scholar]
- Lu, F.; Qi, G.J.; Qin, R.R.; Hu, G.; Wang, Z.; Zhang, X.X.; Cheng, X.N.; Zhai, B.P. The processes of morphological change and grading criteria for ovarian development in the brown planthopper. J. Appl. Entomol. 2011, 48, 1394–1400. [Google Scholar]
- Wang, X.; Xu, H.; Xu, Y.; Liu, Y.; Zhou, Z. The structures and developmental progress of reproductive system of beet armyworm, Spodoptera exigua (Hübner), and their use in forecast. J. Plant Protec. 2003, 30, 261–266. [Google Scholar]
- Ge, L.Q.; Gu, H.T.; Huang, B.; Song, Q.S.; Stanley, D.; Liu, F.; Yang, G.Q.; Wu, J.C. An adenylyl cyclase like-9 gene (NlAC9) influences growth and fecundity in the brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). PLoS ONE 2017, 12, e0189214. [Google Scholar] [CrossRef]
- SPSS Inc. SPSS 11 for Mac OS X; SPSS Inc.: Chicago, IL, USA, 2002. [Google Scholar]
- Lei, H.; Xu, R.M. EPG-An effective way to study spiking behavior of phytophagous insects. Entomol. Knowl. 1996, 33, 116–120. [Google Scholar]
- Backus, E.A.; Rangasamy, M.; Stamm, M.; Mcauslane, H.J.; Cherry, R. Waveform library for chinch bugs (Hemiptera: Heteroptera: Blissidae): Characterization of electrical penetration graph waveforms at multiple input impedances. Ann. Entomol. Soc. Am. 2013, 106, 524–539. [Google Scholar] [CrossRef]
- Chuche, J.; Backus, E.A.; Thiéry, D.; Sauvion, N. First finding of a dual-meaning X wave for phloem and xylem fluid ingestion: Characterization of Scaphoideus titanus (hemiptera: Cicadellidae) EPG waveforms. J. Insect Physiol. 2017, 102, 50–61. [Google Scholar] [CrossRef]
- Luo, C.; Yue, M.; Xu, H.F.; Zhang, Z.L. Application of electrical penetration graph (EPG) in entomological studies and new findings. Acta. Entomol. Sin. 2005, 48, 437–443. [Google Scholar]
- Duan, F.; Li, Y.; Liang, C. Effects of different concentrations of herbicide (Liberty) and spraying time on agronomical characters of Bar-transgenic rice. Hybrid Rice 2001, 16, 44–46. [Google Scholar]
- Xu, S.C.; Xiao, Q.Q.; Lai, G.Z.; Hu, C.H. Study on the effects of fluridone in seed germination rate and chlorophyll content of the maize. J. Fuyang Teach. Coll. 2010, 27, 55–59. [Google Scholar]
- Huckaba, R.M.; Duyn, J.W.V. Joint effects of acifluorfen applications and soybean thrips (Sericothrips variabilis) feeding on soybean (Glycine max). Weed Sci. 1988, 36, 667–670. [Google Scholar]
- Wu, J.C.; XU, J.F.; Feng, X.M.; Liu, J.L.; Qiu, H.M.; Luo, S.S. Impacts of pesticides on physiology and biochemistry of rice. Sci. Agric. Sin. 2003, 36, 536–541. [Google Scholar]
- Yuan, S.Z.; Wu, J.C.; Xu, J.X.; Li, G.S. Influences of herbicides on physiology and biochemistry of rice. J. Plant Prot. 2001, 28, 274–278. [Google Scholar]
- Yazawa, K.; Jiang, C.J.; Kojima, M.; Sakakibara, H.; Takatsuji, H. Reduction of abscisic acid levels or inhibition of abscisic acid signaling in rice during the early phase of Magnaporthe oryzae infection decreases its susceptibility to the fungus. Physiol. Mol. Plant Pathol. 2012, 78, 1–7. [Google Scholar] [CrossRef]
- Xu, J.; Audenaert, K.; Hofte, M.; Vleesschauwer, D.D. Abscisic acid promotes susceptibility to the rice leaf blight pathogen Xanthomonas oryzae pv oryzae by suppressing salicylic acid-mediated defenses. PLoS ONE 2013, 8, e67413. [Google Scholar]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef]
- Cai, S.L.; Jiang, G.B.; Ye, N.H.; Chu, Z.Z.; Xu, X.Z.; Zhang, J.; Zhu, G. A key ABA catabolic gene, OsABA8ox3, is involved in drought stress resistance in rice. PLoS ONE 2015, 10, e0116646. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Hamachi, L.; Mioto, P.T.; Purgatto, E.; Mercier, H. Implications of leaf ontogeny on drought-induced gradients of CAM expression and ABA levels in rosettes of the epiphytic tank bromeliad Guzmania monostachia. Plant Physiol. Biochem. 2016, 108, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Jiang, T.Z.; Xu, L.X.; Liu, H.; Mao, H.; Wang, X.; Jiao, B.; Duan, Y.; Wang, Q.; Dong, Q.; et al. A salt-stress-regulator from the Poplar R2R3 MYB family integrates the regulation of lateral root emergence and ABA signaling to mediate salt stress tolerance in Arabidopsis. Plant Physiol. Biochem. 2017, 114, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.T.; Li, T.; Zhu, T.H. The multiple roles of ABA in plant-pathogen interactions. J. Anhui Agric. 2014, 42, 3278–3279. [Google Scholar]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Cao, F.Y.; Yoshioka, K.; Desveaux, D. The roles of ABA in plant-pathogen interactions. J. Plant Res. 2011, 124, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Alazem, M.; He, M.H.; Moffett, P.; Lin, N.S. Abscisic acid induces resistance against bamboo mosaic virus through argonaute 2 and 3. Plant Physiol. 2017, 174, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Kyndt, T.; Nahar, K.; Haeck, A.; Verbeek, R.; Demeestere, K.; Gheysen, G. Interplay between carotenoids, abscisic acid and jasmonate guides the compatible rice-meloidogyne graminicola interaction. Front. Plant Sci. 2017, 8, 951. [Google Scholar] [CrossRef]
- Abe, H.; Urao, T.T.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003, 15, 63–78. [Google Scholar] [CrossRef]
- Vos, I.A.; Verhage, A.; Schuurink, R.C.; Watt, L.G.; Pieterse, C.M.J.; Van-Wees, S.C. Onset of herbivore-induced resistance in systemic tissue primed for jasmonate-dependent defenses is activated by abscisic acid. Front. Plant Sci. 2013, 4, 539. [Google Scholar] [CrossRef]
- Nguyen, D.; D’Agostino, N.; Tytgat, T.O.G.; Sun, P.L.; Lortzing, T.; Visser, E.J.; Cristescu, S.M.; Steppuhn, A.; Mariani, C.; van-Dam, N.M.; et al. Drought and flooding have distinct effects on herbivore-induced responses and resistance in Solanum dulcamara. Plant Cell Environ. 2016, 39, 1485–1499. [Google Scholar] [CrossRef]
- Klingler, J.P.; Nair, R.M.; Edwards, O.R.; Singh, K.B. A single gene, ain, in Medicago truncatula mediates a hypersensitive response to both bluegreen aphid and pea aphid, but confers resistance only to bluegreen aphid. J. Exp. Bot. 2009, 60, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, R.; Arimura, G.; Takabayashi, J.; Shimoda, T.; Nishioka, T. Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol. 2000, 41, 391–398. [Google Scholar] [CrossRef]
- Cipollini, D.; Enright, S.M.; Traw, M.B.; Bergelson, J. Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol. Ecol. 2004, 13, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Ederli, L.; Brunetti, C.; Centritto, M.; Colazza, S.; Frati, F.; Loreto, F.; Marino, G.; Salerno, G.; Pasqualini, S. Infestation of broad bean (Vicia faba) by the green stink bug (Nezara viridula) decreases shoot abscisic acid contents under well-watered and drought conditions. Front. Plant Sci. 2017, 8, 959. [Google Scholar] [CrossRef] [PubMed]
- Moeder, W.; Ung, H.; Mosher, S.; Yoshioka, K. SA-ABA antagonism in defense responses. Plant Signal Behav. 2010, 5, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Huang, X.; Sun, L.; Wu, J.; Liu, J. Influence of Abscisic Acid-Biosynthesis Inhibitor Fluridone on the Feeding Behavior and Fecundity of Nilaparvata lugens. Insects 2019, 10, 57. https://doi.org/10.3390/insects10020057
Ding X, Huang X, Sun L, Wu J, Liu J. Influence of Abscisic Acid-Biosynthesis Inhibitor Fluridone on the Feeding Behavior and Fecundity of Nilaparvata lugens. Insects. 2019; 10(2):57. https://doi.org/10.3390/insects10020057
Chicago/Turabian StyleDing, Xu, Xi Huang, Litong Sun, Jincai Wu, and Jinglan Liu. 2019. "Influence of Abscisic Acid-Biosynthesis Inhibitor Fluridone on the Feeding Behavior and Fecundity of Nilaparvata lugens" Insects 10, no. 2: 57. https://doi.org/10.3390/insects10020057
APA StyleDing, X., Huang, X., Sun, L., Wu, J., & Liu, J. (2019). Influence of Abscisic Acid-Biosynthesis Inhibitor Fluridone on the Feeding Behavior and Fecundity of Nilaparvata lugens. Insects, 10(2), 57. https://doi.org/10.3390/insects10020057