Seasonal Assessment of Supercooling Points for Two Introduced and One Native Laricobius spp. (Coleoptera: Derodontidae), Predators of Adelgidae
Abstract
:1. Introduction
2. Methods
2.1. Colony Rearing and Acclimating Laricobius Osakensis and Laricobius Nigrinus to Field Conditions
2.2. Field Collection of Laricobius rubidus
2.3. Supercooling Point Measurement
3. Statistical Analysis
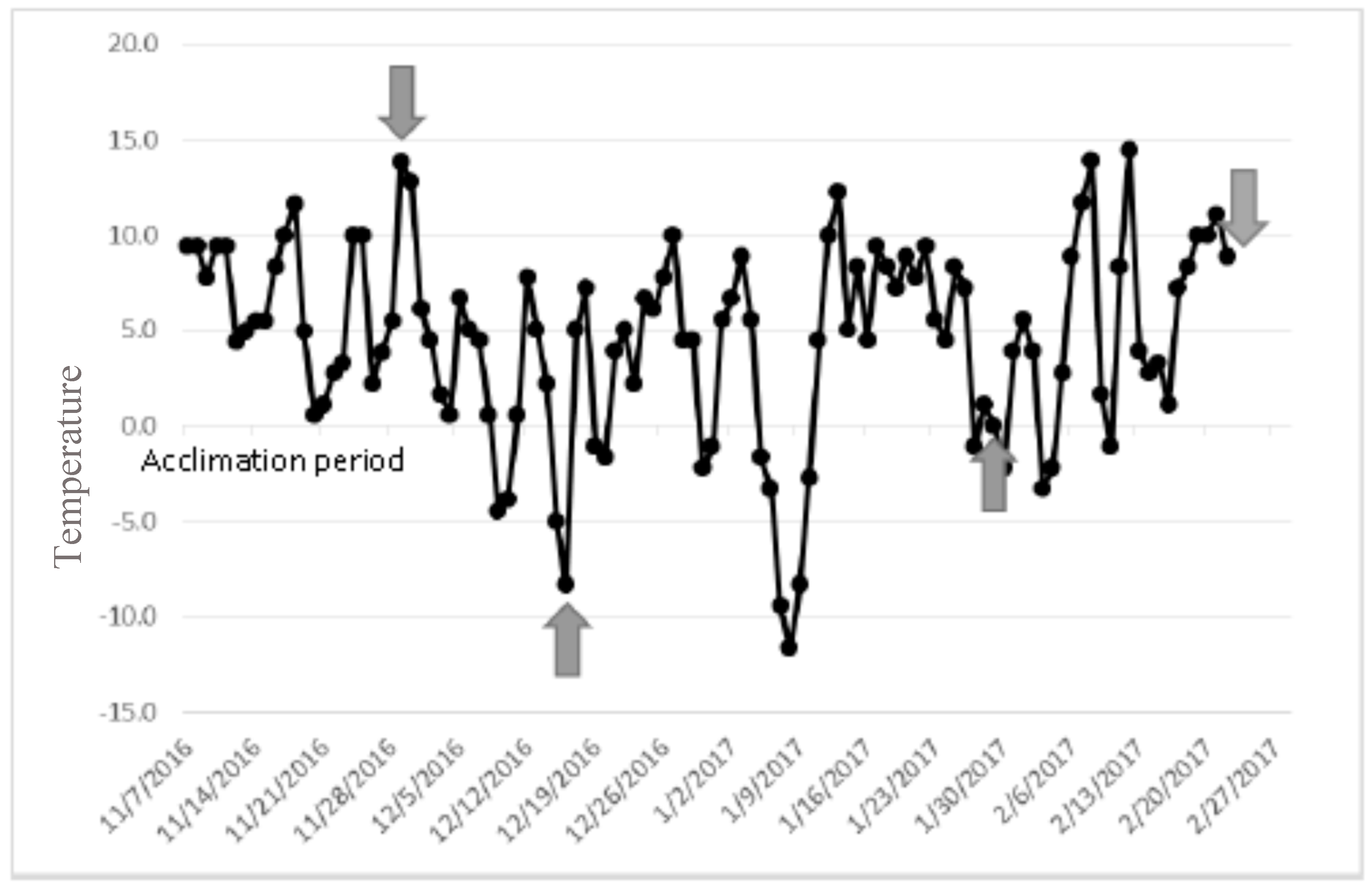
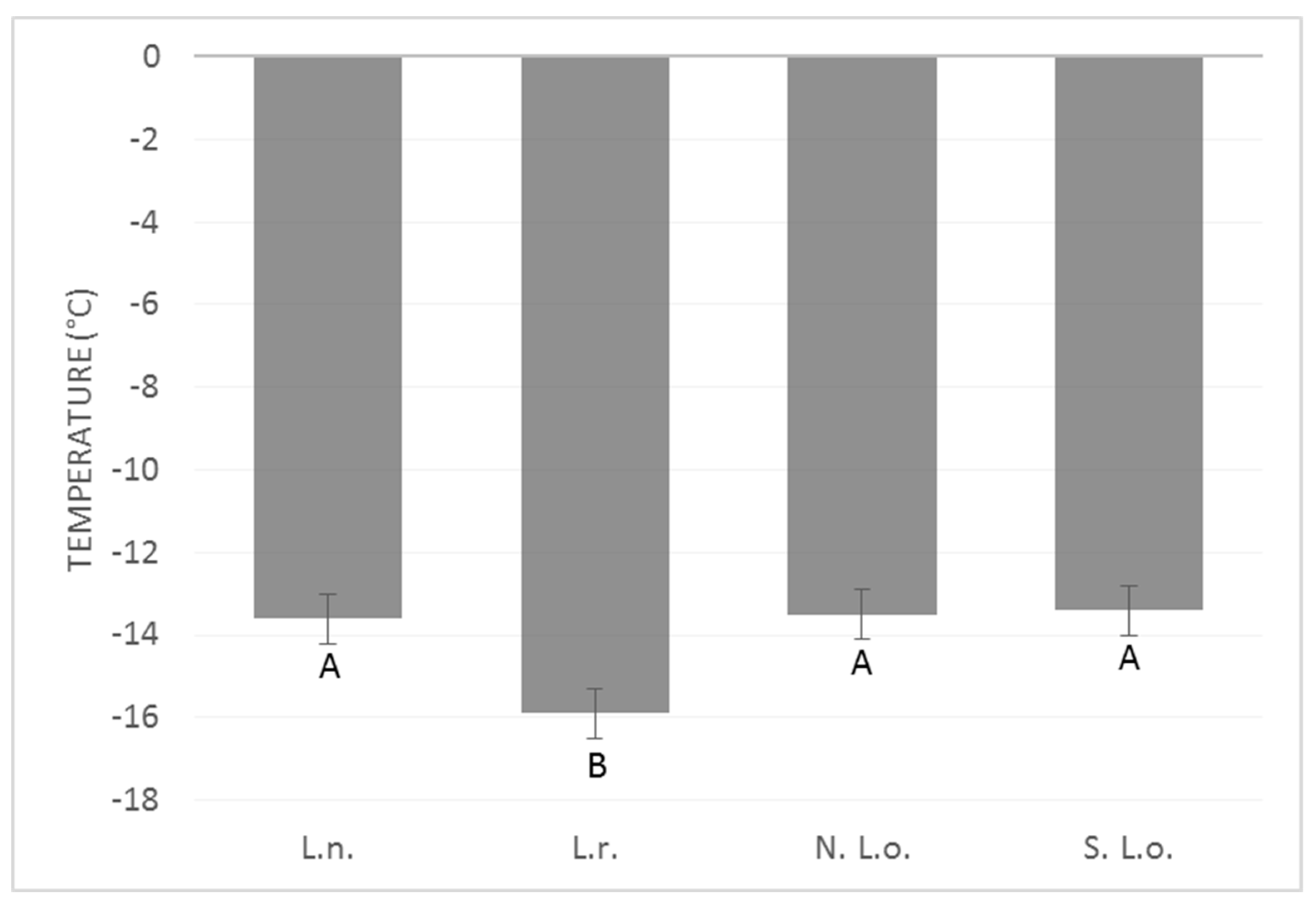
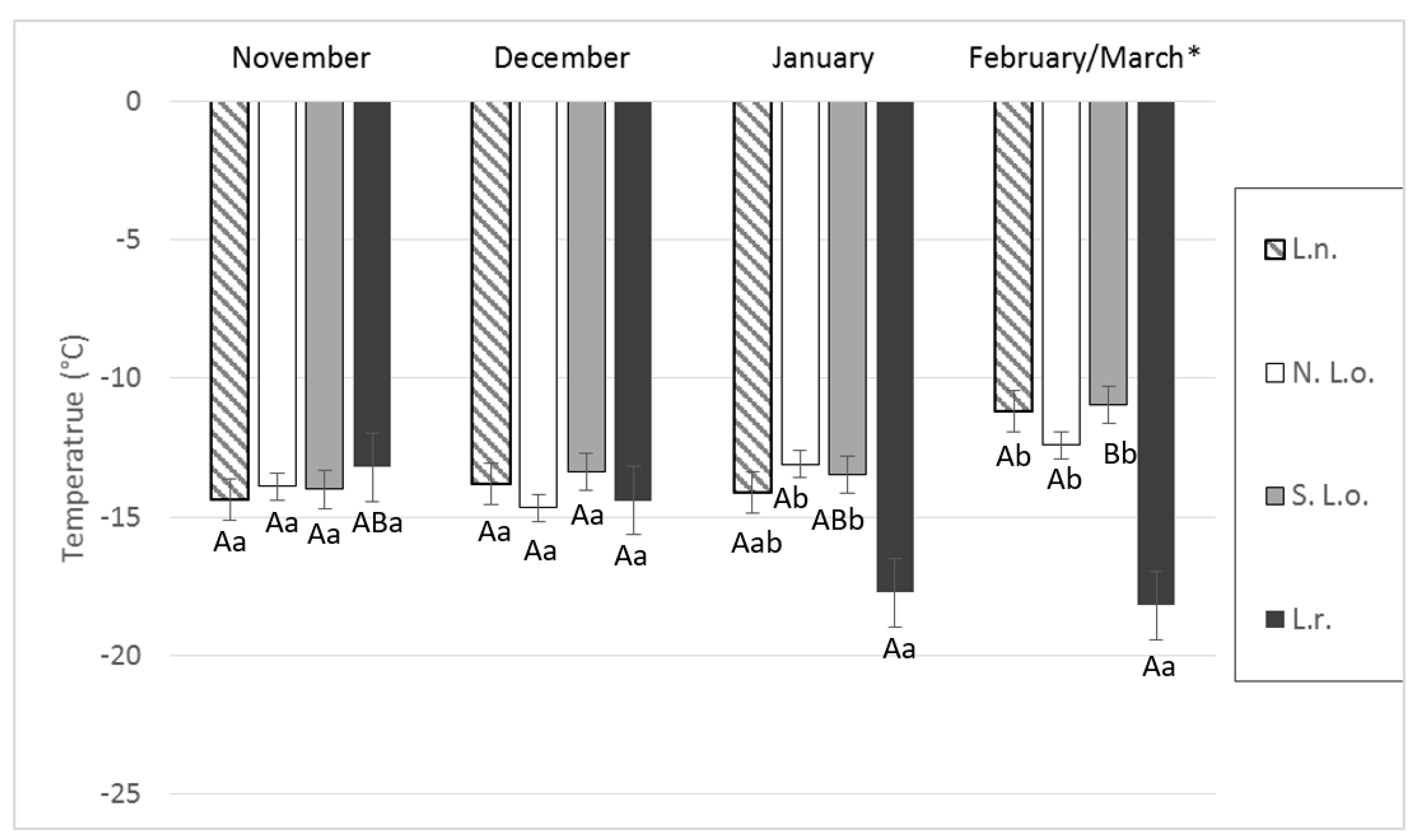
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stadler, B.; Müller, T.; Orwig, D.; Cobb, R. Hemlock woolly adelgid in New England forests: Canopy impacts transforming ecosystem processes and landscapes. Ecosystems 2005, 8, 233–247. [Google Scholar] [CrossRef]
- Nuckolls, A.E.; Wurzburger, N.; Ford, C.R.; Hendrick, R.L.; Vose, J.M.; Kloepell, B.D. Hemlock declines rapidly with hemlock woolly adelgid infestation: Impacts on the carbon cycle of southern Appalachian forests. Ecosystems 2009, 12, 179–190. [Google Scholar] [CrossRef]
- Spaulding, H.L.; Rieske, L.K. The aftermath of an invasion: Structure and composition of Central Appalachian hemlock forests following establishment of the hemlock woolly adelgid, Adelges tsugae. Biol. Invasions 2010, 12, 3135–3143. [Google Scholar] [CrossRef]
- Evans, A.M.; Gregoire, T.G. A geographically variable model of hemlock woolly adelgid spread. Biol. Invasions 2007, 9, 369–382. [Google Scholar] [CrossRef]
- McAvoy, T.J.; Régnière, J.; St-Amant, R.; Schneeberger, N.F.; Salom, S.M. Mortality and recovery of hemlock woolly adelgid (Adelges tsugae) in response to winter temperatures and predictions for the future. Forests 2017, 8, 497. [Google Scholar] [CrossRef]
- Tobin, P.C.; Turcotte, R.M.; Blackburn, L.M.; Juracko, J.A.; Simpson, B.T. The big chill: Quantifying the effect of the 2014 North American cold wave on hemlock woolly adelgid populations in the central Appalachian Mountains. Popul. Ecol. 2017, 59, 251–258. [Google Scholar] [CrossRef]
- Mooneyham, K.; Kok, L.T.; Salom, S.M. Release and colonization of Laricobius osakensis (Coleoptera: Derodontidae), a predator of the hemlock woolly adelgid, Adelges tsugae. Northeast. Nat. 2016, 23, 141–150. [Google Scholar] [CrossRef]
- Heminger, A. Establishment of Laricobius nigrinus (Coleoptera: Derodontidae) in Virginia and Assessment of Its Impact on Hemlock Woolly Adelgid, Adelges tsugae (Hemiptera: Adelgidae), throughout the Eastern US. Master’s Thesis, Virginia Tech, Blacksburg, VA, USA, 2017; 93p. [Google Scholar]
- Sumpter, K.L.; McAvoy, T.J.; Brewster, C.C.; Mayfield, A.E.; Salom, S.M. Assessing an integrated biological and chemical control strategy for managing hemlock woolly adelgid in southern Appalachian forests. For. Ecol. Manag. 2018, 411, 12–19. [Google Scholar] [CrossRef]
- Clark, R.C.; Brown, N.R. Studies of predators of the balsam woolly aphid, Adelges piceae (Ratz.) (Homoptera: Adelgidae) VII Laricobius rubidus Lec. (Coleoptera: Derodontidae) a predator Pineus strobi (Htg.) (Homoptera: Adelgidae). Can. Entomol. 1960, 92, 237–240. [Google Scholar] [CrossRef]
- Doane, C.C. Taxonomy and biology of Pineus strobi (Hartig) and P. coloradensis (Gillette) (Homoptera: Adelgidae). Can. Entomol. 1961, 93, 553–560. [Google Scholar] [CrossRef]
- Brown, W.J. Some new and poorly known species of Coleoptera. Can. Entomol. 1944, 76, 4–10. [Google Scholar] [CrossRef]
- Wantuch, H.W.; Kuhar, T.P.; Salom, S.M. Phenology of the pine bark adelgid, Pineus strobi (Hemiptera: Adelgidae), in white pine forests of southwestern Virginia. Environ. Entomol. 2017, 46, 1195–1201. [Google Scholar] [CrossRef]
- Annand, P.N. A Contribution toward a Monograph of the Adelginae (Phylloxeridae) of North America; University series; Biological Sciences; Stanford University Publications: Standford, CA, USA, 1928; p. 6. [Google Scholar]
- Bale, J.S. Classes of insect cold hardiness. Funct. Ecol. 1993, 7, 751–753. [Google Scholar]
- Sinclair, B.J.; Alvarado, L.E.C.; Ferguson, L.V. An invitation to measure insect cold tolerance: Methods, approaches, and workflow. J. Therm. Biol. 2015, 53, 180–197. [Google Scholar] [CrossRef]
- Ramløv, H.; Westh, P. Ice formation in the freeze tolerant alpine weta Hemideina maori Hutton (Orthoptera; Stenopelmatidae). Cryoletters 1993, 14, 169–176. [Google Scholar]
- Block, W.; Wharton, D.A.; Sinclair, B.J. Cold tolerance of a New Zealand alpine cockroach, Celatoblatta quinquemaculata (Dictyoptera, Blattidae). Physiol. Entomol. 1998, 23, 1–6. [Google Scholar] [CrossRef]
- Storey, K.B.; Storey, J.M. Molecular biology of freezing tolerance. Compr. Physiol. 2013, 3, 1283–1308. [Google Scholar]
- Dunman, J.G. Antifreeze and ice nucleator proteins in terrestrial arthropods. Ann. Rev. Physiol. 2001, 63, 327–357. [Google Scholar] [CrossRef]
- Crosthwaite, J.C.; Sobek, S.; Lyons, D.B.; Bernards, M.A.; Sinclair, B.J. The overwintering physiology of the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). J. Insect Physiol. 2011, 57, 166–173. [Google Scholar] [CrossRef]
- Mausel, D.L.; Van Driesche, R.G.; Elkinton, J.S. Comparative cold tolerance and climate matching of coastal and inland Laricobius nigrinus (Coleoptera: Derodontidae), a biological control agent of hemlock woolly adelgid. Biol. Control. 2011, 58, 96–102. [Google Scholar] [CrossRef]
- Zachariassen, K.E. Physiology of cold tolerance in insects. Physiol. Rev. 1985, 65, 799–832. [Google Scholar] [CrossRef]
- Ramlov, H.; Lee, R.E. Extreme resistance to desiccation in overwintering larvae of the gall fly Eurosta solidaginis (Diptera, Tephritidae). J. Exp. Biol. 2000, 203, 783–789. [Google Scholar]
- Holmstrup, M.; Hedlund, K.; Boriss, H. Drought acclimation and lipid composition in Folsomia candida: Implications for cold shock, heat shock and acute desiccation stress. J. Insect Physiol. 2002, 48, 961–970. [Google Scholar] [CrossRef]
- Sinclair, B.J.; Vernon, P.; Klok, C.J.; Chown, S.L. Insects at low temperatures: An ecological perspective. Trends Ecol. Evol. 2003, 18, 257–262. [Google Scholar] [CrossRef]
- Zachariassen, K.E.; Kristiansen, E. Ice nucleation and antinucleation in nature. Cryobiology 2000, 41, 257–279. [Google Scholar] [CrossRef]
- Andersen, J.L.; Manenti, T.; Sorensen, J.G.; MacMillan, H.A.; Loeschcke, V.; Overgaard, J. How to assess Drosophila cold tolerance: Chill coma temperature and lower lethal temperature are the best predictors of cold distribution limits. Funct. Ecol. 2015, 29, 55–65. [Google Scholar] [CrossRef]
- Salt, R.W. Principles of insect cold hardiness. Ann. Rev. Entomol. 1961, 6, 55–74. [Google Scholar] [CrossRef]
- Cira, T.M.; Venette, R.C.; Aigner, J.; Kuhar, T.; Mullins, D.E.; Gabbert, S.E. Cold tolerance of Halyomorpha halys (Hemiptera: Pentatomidae) across geographic and temporal scales. Environ. Entomol. 2015, 45, 484–491. [Google Scholar] [CrossRef]
- Danks, H.V. Key themes in the study of seasonal adaptations in insects I. Patterns of cold hardiness. Appl. Entomol. Zool. 2005, 40, 199–211. [Google Scholar] [CrossRef]
- Hopper, K.R.; Roush, R.T.; Powell, W. Management of genetics of biological control introductions. Ann. Rev. Entomol. 1993, 38, 27–51. [Google Scholar] [CrossRef]
- Zilahi-Balogh, G.M.G.; Kok, L.T.; Salom, S.M. Host specificity of Laricobius nigrinus Fender (Coleoptera: Derodontidae), a potential biological control agent of the hemlock woolly adelgid, Adelges tsugae Annand (Homoptera: Adelgidae). Biol. Control. 2002, 24, 192–198. [Google Scholar] [CrossRef]
- Vieira, L.C.; Mcavoy, T.J.; Chantos, J.; Lamb, A.B.; Salom, S.M.; Kok, L.T. Host range of Laricobius osakensis (Coleoptera: Derodontidae), a new biological control agent of hemlock woolly adelgid (Hemiptera: Adelgidae). Environ. Entomol. 2011, 40, 324–332. [Google Scholar] [CrossRef]
- Overgaard, J.; Sørensen, J.G. Rapid thermal adaptation during field temperature variations in Drosophila melanogaster. Cryobiology 2008, 56, 159–162. [Google Scholar] [CrossRef]
- Teets, N.M.; Denlinger, D.L. Physiological mechanisms of seasonal and rapid coldhardening in insects. Physiol. Entomol. 2013, 38, 105–116. [Google Scholar] [CrossRef]
- Worland, M.R.; Convey, P. Rapid cold hardening in Antarctic microarthropods. Funct. Ecol. 2001, 15, 515–524. [Google Scholar] [CrossRef]
- Elkinton, J.S.; Lombardo, J.A.; Roehrig, A.D.; McAvoy, T.J.; Mayfield, A.; Whitmore, M. Induction of cold hardiness in an invasive herbivore: The case of hemlock woolly adelgid (Hemiptera: Adelgidae). Environ. Entomol. 2016, 46, 118–124. [Google Scholar] [CrossRef]
| Population | City | Location | Elevation | Number Collected | Plant Hardiness Zone * |
|---|---|---|---|---|---|
| Northern | Nagano | N 36.68213, E 138.50032 | 1720 m | 85 | 7b |
| Northern | Gunma | N 36.81287, E 139.34099 | 1550 m | 248 | 8a |
| Northern | Tochigi | N 36.80325, E 139.42031 | 1485 m | 134 | 7b |
| Southern | Nara | N 34.41465, E 135.51145 | 290 m | 2 | 8b |
| Southern | Kyoto | N 34.04233, E 135.45733 | 150 m | 14 | 9b |
| Southern | Kobe | N 34.44392, E 135.10750 | 440 m | 5 | 9b |
| Southern | West of Kyoto | N 34.57570, E 135.36691 | 375 m | 67 | 9b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toland, A.A.; Wantuch, H.A.; Mullins, D.E.; Kuhar, T.P.; Salom, S.M. Seasonal Assessment of Supercooling Points for Two Introduced and One Native Laricobius spp. (Coleoptera: Derodontidae), Predators of Adelgidae. Insects 2019, 10, 426. https://doi.org/10.3390/insects10120426
Toland AA, Wantuch HA, Mullins DE, Kuhar TP, Salom SM. Seasonal Assessment of Supercooling Points for Two Introduced and One Native Laricobius spp. (Coleoptera: Derodontidae), Predators of Adelgidae. Insects. 2019; 10(12):426. https://doi.org/10.3390/insects10120426
Chicago/Turabian StyleToland, Ashley A., Holly A. Wantuch, Donald E. Mullins, Thomas P. Kuhar, and Scott M. Salom. 2019. "Seasonal Assessment of Supercooling Points for Two Introduced and One Native Laricobius spp. (Coleoptera: Derodontidae), Predators of Adelgidae" Insects 10, no. 12: 426. https://doi.org/10.3390/insects10120426
APA StyleToland, A. A., Wantuch, H. A., Mullins, D. E., Kuhar, T. P., & Salom, S. M. (2019). Seasonal Assessment of Supercooling Points for Two Introduced and One Native Laricobius spp. (Coleoptera: Derodontidae), Predators of Adelgidae. Insects, 10(12), 426. https://doi.org/10.3390/insects10120426




