Population Dynamics of Six Major Insect Pests During Multiple Crop Growing Seasons in Northwestern New Mexico
Abstract
1. Introduction
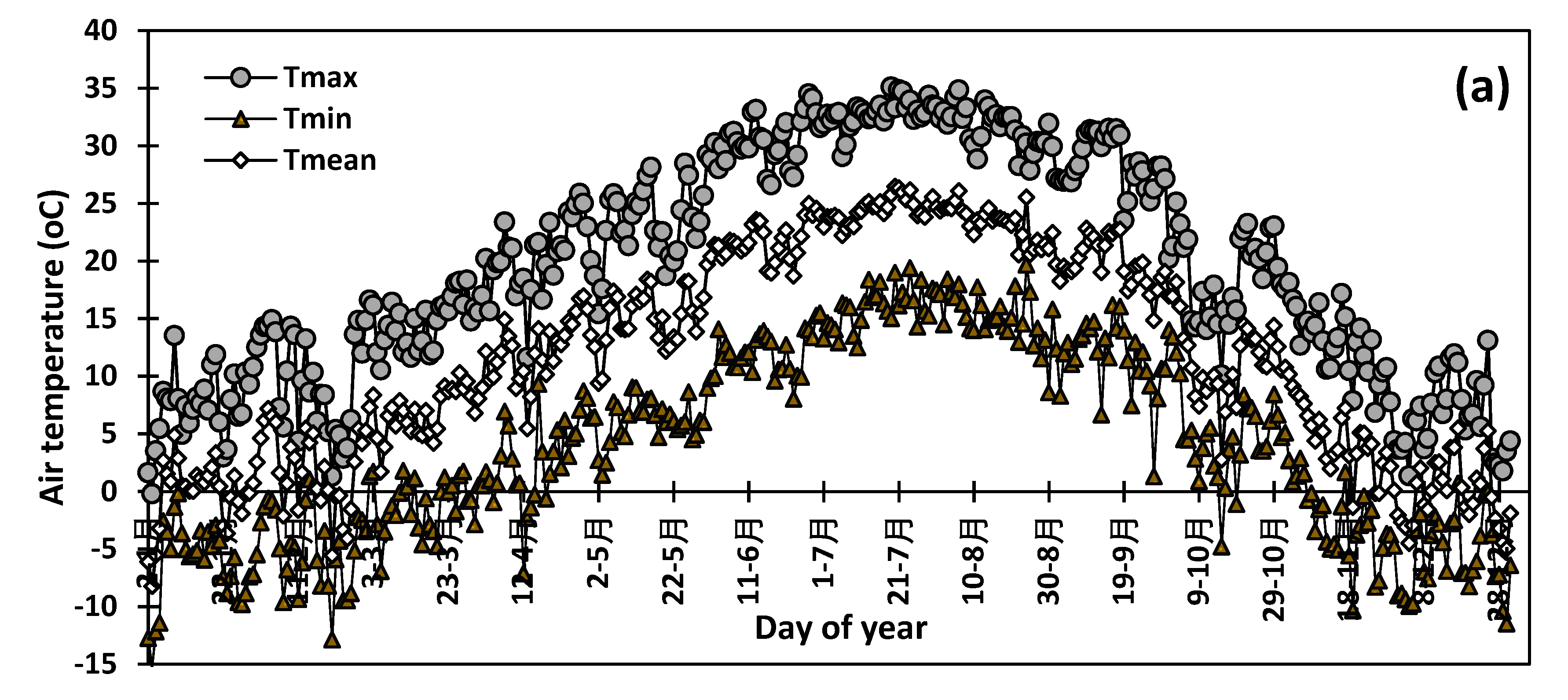
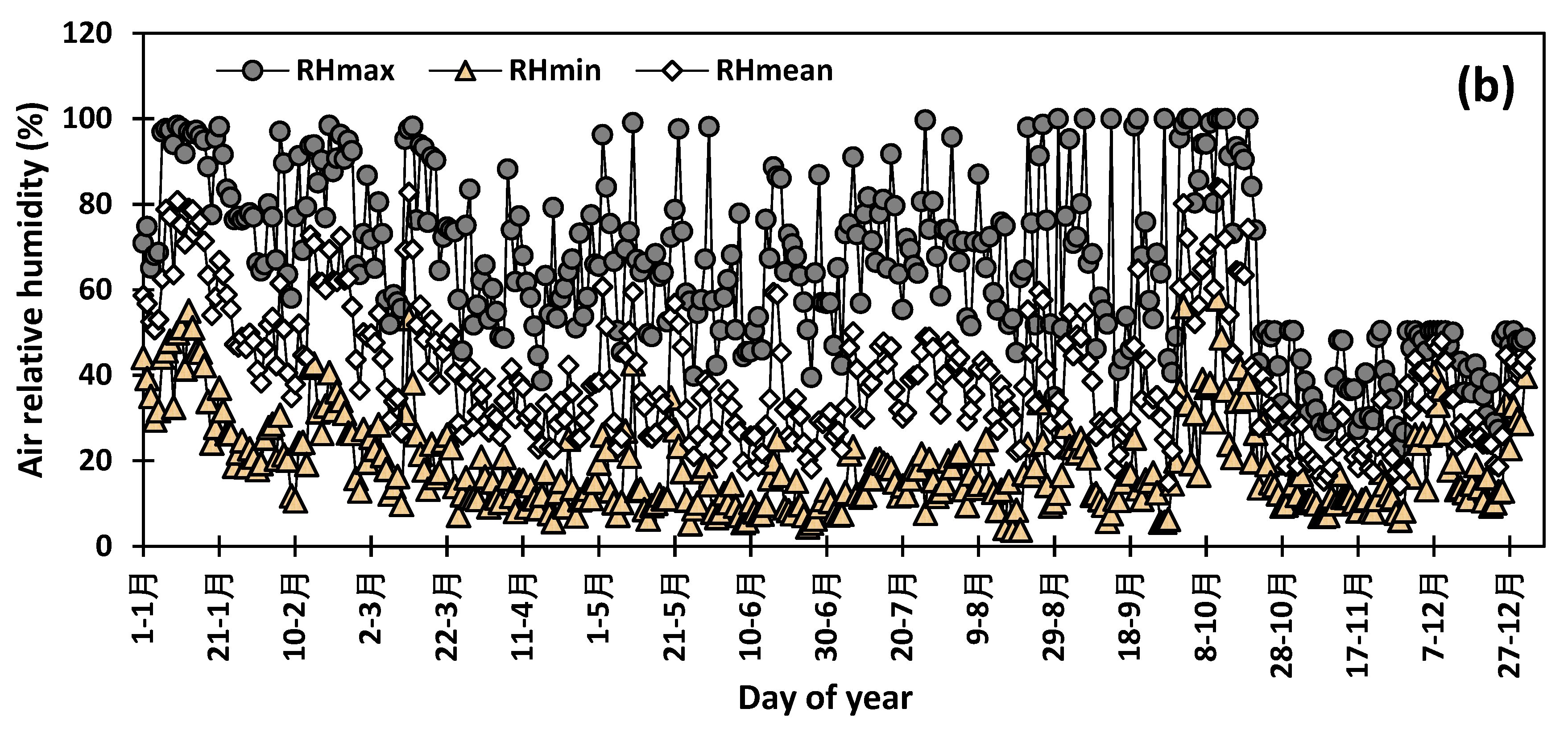
2. Materials and Methods
3. Results
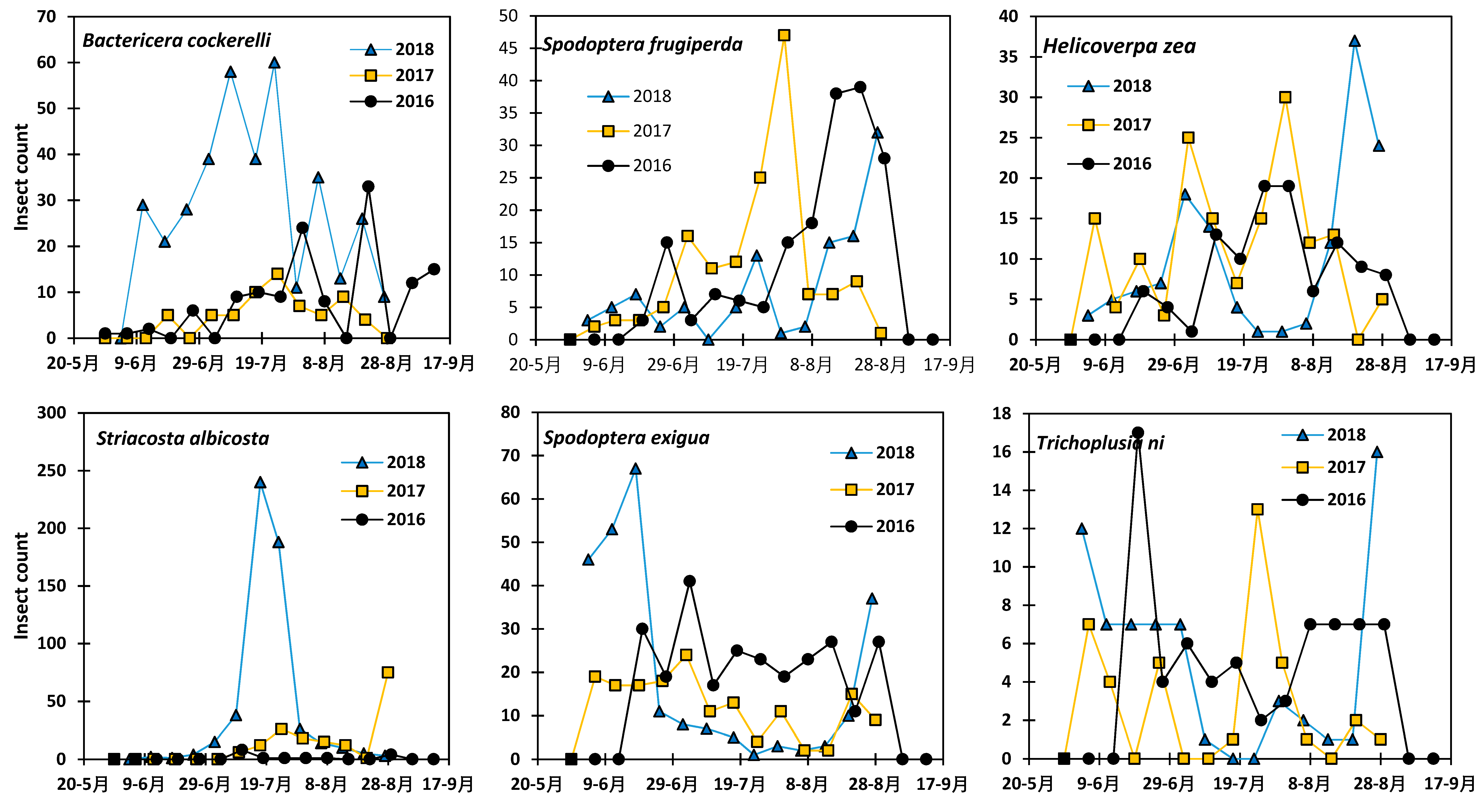
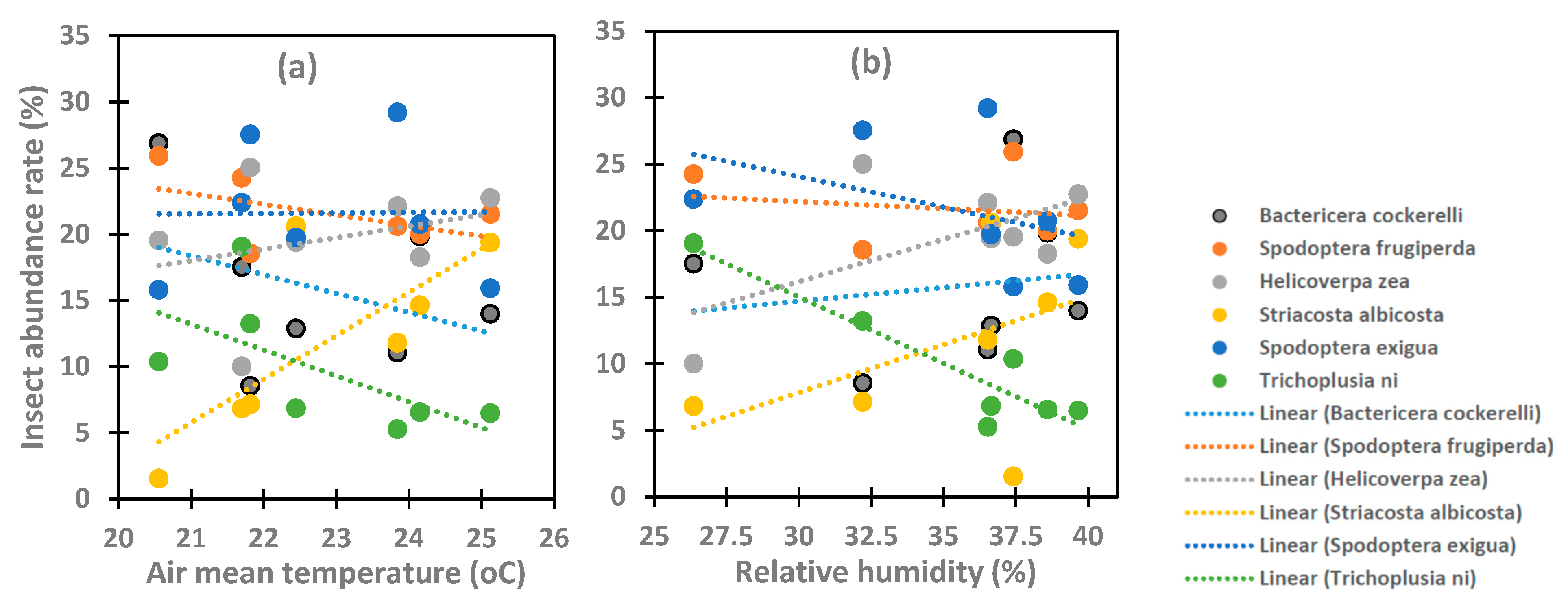
3.1. Variation in the Abundance of the Six Insect Species
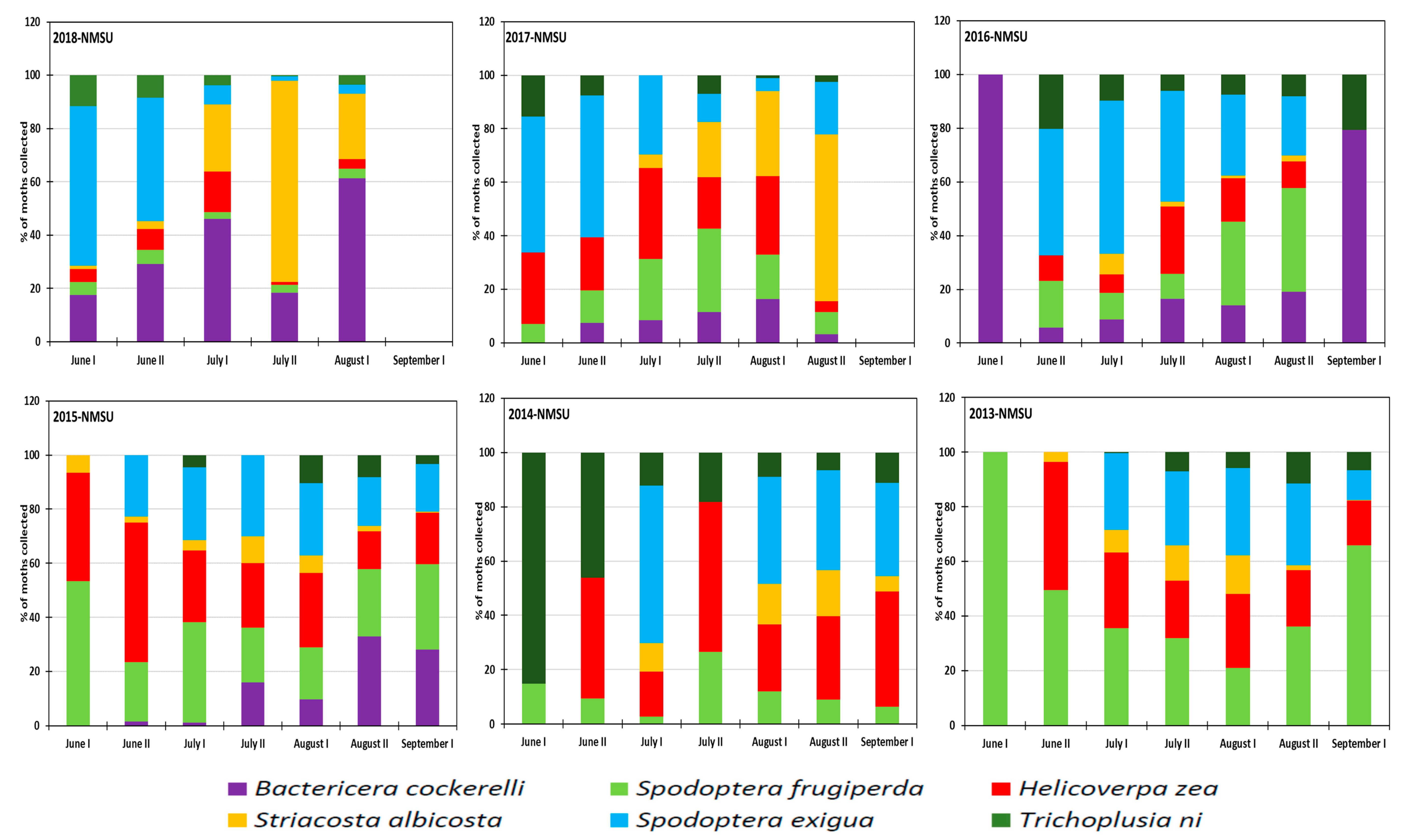
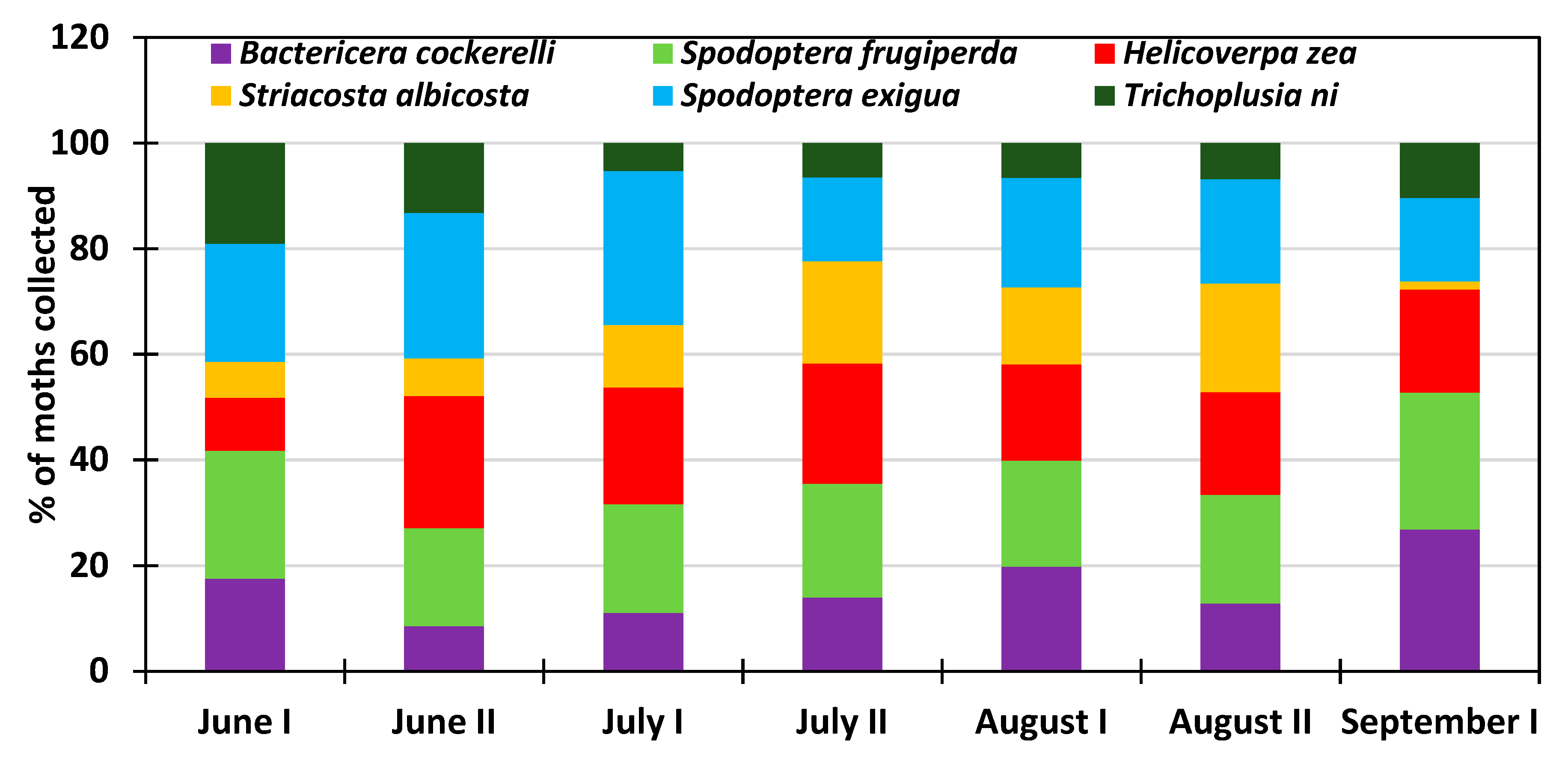
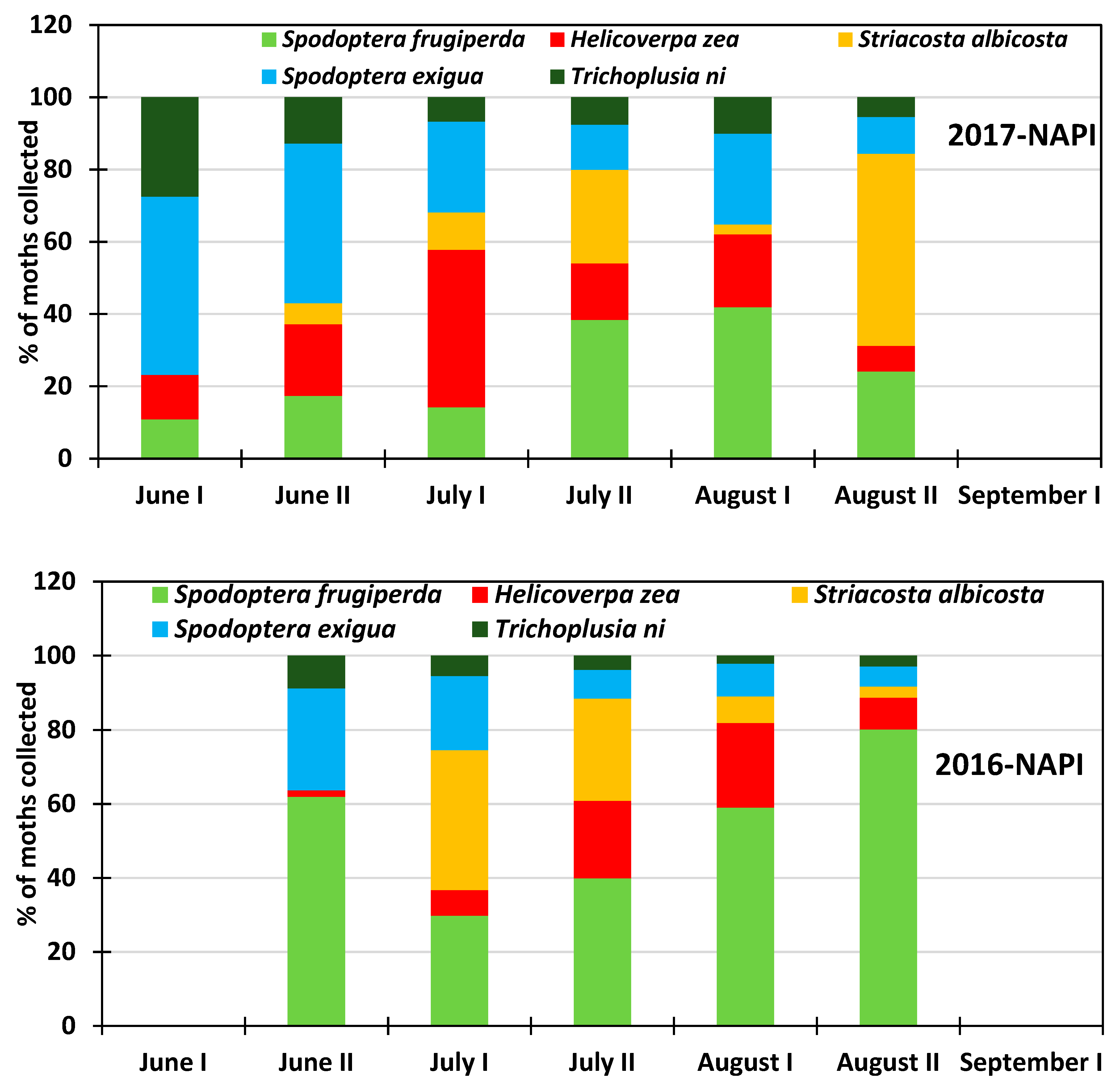
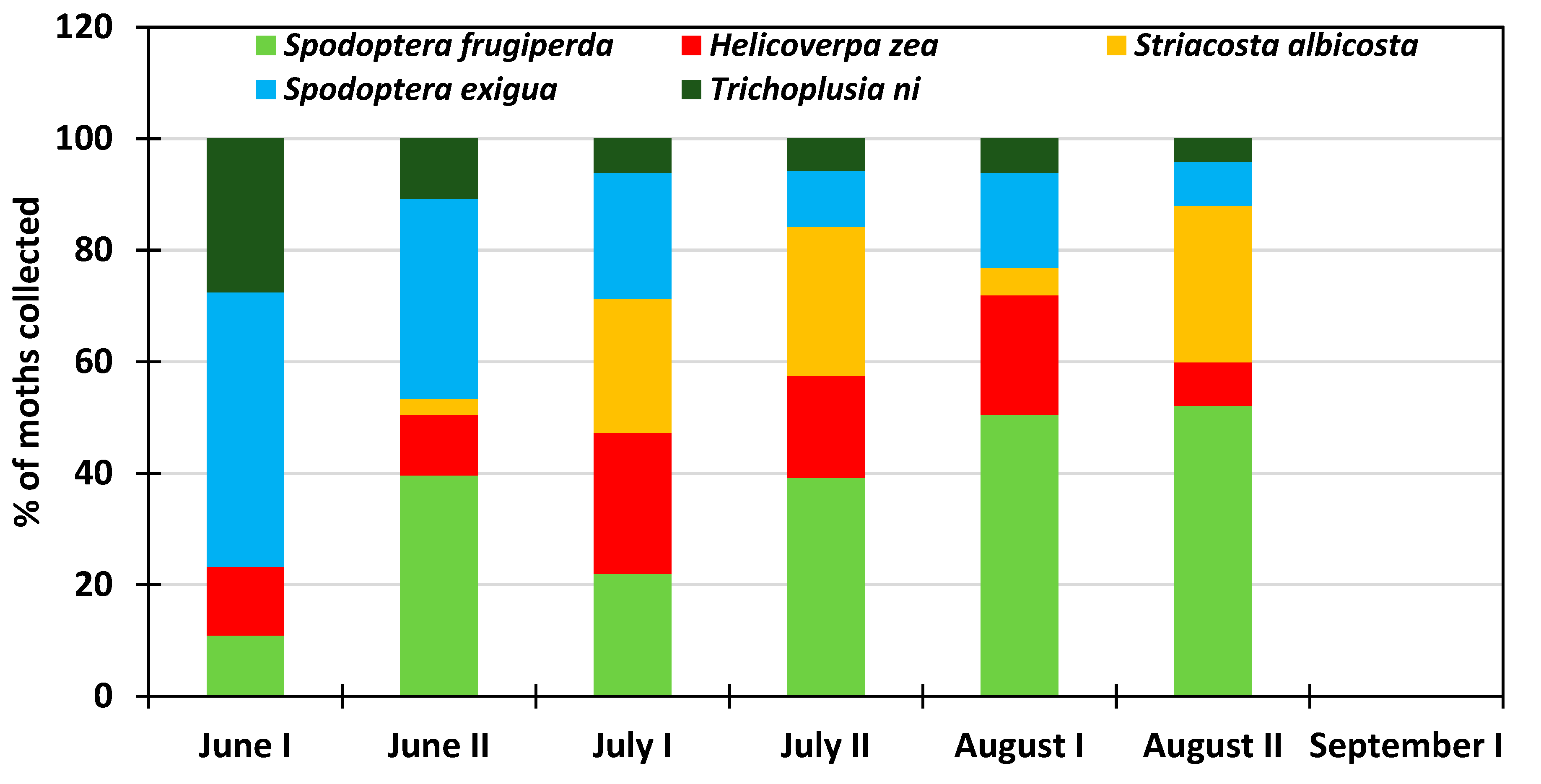
3.2. Monthly Distribution of the Six Insect Species at the Research Station for the 2013–2018 Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations Statistics (FAOSTAT). Food and Agriculture Data. 2019. Available online: http://www.fao.org/faostat/en/?#home (accessed on 20 August 2019).
- United States Department of Agriculture (USDA). United States Summary and State Data; USDA, National Agricultural Statistics Service: Washington, DC, USA, 2019; p. 820.
- Webster, J.P.G.; Bowles, R.G.; Williams, N.T. Estimating the Economic Benefits of Alternative Pesticide Usage Scenarios: Wheat Production in the United Kingdom. Crop Prod. 1999, 18, 83. [Google Scholar] [CrossRef]
- Karl, I.; Stoks, R.; De Block, M.; Janowitz, S.A.; Fischer, K. Temperature extremes and butterfly fitness: Conflicting evidence from life history and immune function. Glob. Chang. Biol. 2011, 17, 676–687. [Google Scholar] [CrossRef]
- Nyamukondiwa, C.; Weldon, C.W.; Chown, S.L.; le Roux, P.C.; Terblanche, J.S. Thermal biology, population fluctuations and implications of temperature extremes for the management of two globally significant insect pests. J. Insect Physiol. 2013, 59, 1199–1211. [Google Scholar] [CrossRef]
- Danks, H.V. Life cycles in polar arthropods-flexible or programmed? Eur. J. Ent. 1999, 96, 83–102. [Google Scholar]
- Régnière, J.; St-Amant, R.; Duval, P. Predicting insect distributions under climate change from physiological responses: Spruce budworm as an example. Biol. Invasions. 2012, 14, 1571–1586. [Google Scholar] [CrossRef]
- Shatz, A.J.; Rogan, J.; Sangermano, F.; Ogneva-Himmelberger, Y.; Chen, H. Characterizing the potential distribution of the invasive Asian longhorned beetle (Anoplophora glabripennis) in Worcester County, Massachusetts. Appl. Geogr. 2013, 45, 259–268. [Google Scholar] [CrossRef]
- Finlay-Doney, M.; Walter, G.H. The conceptual and practical implications of interpreting diet breadth mechanistically in generalist predatory insects. Biol. J. Linnean Soc. 2012, 107, 737–763. [Google Scholar] [CrossRef]
- Danks, H.V. The elements of seasonal adaptations in insects. Can. Entomol. 2007, 139, 1–44. [Google Scholar] [CrossRef]
- Konopka, J.K.; Hanyu, K.; Macfie, S.M.; McNeil, J.N. Does the response of insect herbivores to cadmium depend on their feeding strategy? J. Chem. Ecol. 2013, 39, 546–554. [Google Scholar] [CrossRef]
- Tetsuo, T.; Nakajo, M.; Furuki, T.; Umamoto, N.; Moku, M.; Sekimoto, T.; Katagiri, C. Seasonal Change in Distribution and Heat Coma Temperature of Oceanic Skaters, Halobates (Insecta, Heteroptera: Gerridae). Insects 2018, 9, 133. [Google Scholar] [CrossRef]
- Barretto, J.W.; Cultid-Medina, C.A.; Escobar, F. Annual Abundance and Population Structure of Two Dung Beetle Species in a Human-Modified Landscape. Insects 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, I.; Esser, A.D.; Crowder, D.W. Seasonal population dynamics of wireworms in wheat crops in the Pacific Northwestern United States. J. Pest. Sci. 2016, 90, 77–86. [Google Scholar] [CrossRef]
- O’Neill, M.K.; Smeal, D.; West, M.M.; Allen, S.C.; Djaman, K. Forty-Eight Years (1969–2016) of Climatological Data: NMSU Agricultural Science Center–Farmington, New Mexico; NMSU Extension Bulletin 809; College of Agricultural, Consumer and Environmental Sciences: Urbana, IL, USA, 2018; p. 10. [Google Scholar]
- Franklin, M.T.; Ritland, C.E.; Myers, J.H. Genetic analysis of cabbage loopers, Trichoplusia ni (Lepidoptera: Noctuidae), a seasonal migrant in western North America. Evol. Appl. 2011, 4, 89–99. [Google Scholar] [CrossRef] [PubMed]
- McGrath, D. VegNet Newletter. Available online: http://extension.oregonstate.edu/linn (accessed on 15 August 2019).
- Cahn, M.; Brittan, K.; Pickel, C. Pest Management Grants Final Report. 2001. Available online: http://www.cdpr.ca.gov/docs/pestmgt/grants/00-01/.../00-0192S.pdf (accessed on 10 July 2019).
- Lafontaine, J.D.; Poole, R.W. Noctuidae (part)–Plusiinae. Fascicle 25.1. In The moths of America North of Mexico; The Wedge Entomological Research Foundation: Washington, DC, USA, 1991. [Google Scholar]
- Parajulee, M.N.; Rummel, D.R.; Arnold, M.D.; Carroll, S.C. Long-Term Seasonal Abundance Patterns of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) in the Texas High Plains. J. Econ. Entomol. 2004, 97, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.A.; Zehnder, G.W.; Speese, J.; Powell, J.E. Parasitization and timing of dia-pause in Virginia Microplitis croceipes (Hymenop–tera: Braconidae): Implications for biocontrol of Heli-coverpa zea (Lepidoptera: Noctuidae) in soybean. Environ. Entomol. 1993, 22, 693–698. [Google Scholar] [CrossRef]
- Tipping, P.W.; Holko, C.A.; Bean, R.A. Helicoverpa zea (Lepidoptera: Noctuidae) Dynamics and Parasitism in Maryland Soybeans. Fla. Entomol. 2005, 88, 55–60. [Google Scholar] [CrossRef]
- Smith, J.L.; Difonzo, C.D.; Baute, T.S.; Michel, P.A.; Krupke, C.H. Ecology and Management of the Western Bean Cutworm (Lepidoptera: Noctuidae) in Corn and Dry Beans—Revision with Focus on the Great Lakes Region. J. Integr. Pest. Manag. 2019, 10, 27. [Google Scholar] [CrossRef]
- Dorhout, D.L.; Rice, M.E. An evaluation of western bean cutworm pheromone trapping techniques (Lepidoptera: Noctuidae) in a corn and soybean agroecosystem. J. Econ. Entomol. 2008, 101, 404–408. [Google Scholar] [CrossRef][Green Version]
- Djaman, K.; O’Neill, M.; Owen, C.K.; Koudahe, K.; West, M.; Smeal, D.; Allen, S.; Lombard, K.; Irmak, S. Crop evapotranspiration, irrigation water requirement and water productivity of maize from meteorological data under semiarid climate. Water 2018, 10, 405. [Google Scholar] [CrossRef]
- Smith, J.L.; Baute, T.S.; Sebright, M.M.; Schaafsma, A.W.; DiFonzo, C.D. Establishment of Striacosta albicosta (Lepidoptera: Noctuidae) as a primary pest of corn in the Great Lakes region. J. Econ. Entomol. 2018, 111, 1732–1744. [Google Scholar] [CrossRef]
- Vajgand, D.; Forgić, G.; Tošev, M. Sovica Spodoptera exigua (Hübner, 1808) (Lep., Noctuidae) i podaci o dinamici leta leptira na području Sombora. Plant. Dr. 2004, 32, 27–31. [Google Scholar]
- Debolt, J.W.; Wolf, W.W.; Henneberry, T.J.; Vail, P.V. Evaluation of light traps and sex pheromone for control of cabbage looper and other lepidopterous insect pests of lettuce. USDA Technic. Bull. 1979, 1606, 39. [Google Scholar]
- Mitchell, E.R.; Chalfant, R.B. Biology, behaviour and dispersal of adults. In Supression and Management of Cabbage Looper Populations; Lingren, P.D., Green, G.L., Eds.; USDA Technical bulletin/Agricultural Research Service: Beltsville, MD, USA, 1984; pp. 14–18. [Google Scholar]
- Caron, V.; Myers, J.H. Positive association between resistance to Bacillus thuringiensis and overwintering survival of cabbage loopers, Trichoplusia ni (Lepidoptera: Noctuidae). Bull. Entomol. Res. 2008, 98, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.K.; Luttrell, R.G. Temporal and Spatial Distribution of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) Moths in Pheromone Traps across Agricultural Landscapes in Arkansas. J. Entomol. Sci. 2011, 46, 269–283. [Google Scholar] [CrossRef]
- Adamczyk, J.J.; Williams, M.R.; Reed, J.T.; Hubbard, D.W.; Hardee, D.D. Spatial and temporal occurrence of beet armyworm (Lepidoptera: Noctuidae) moths in Mississippi. Fla. Entomol. 2002, 86, 229–232. [Google Scholar] [CrossRef]
- Hendricks, D.E.; Hubbard, D.W.; Hardee, D.D. Occurrence of beet armyworm moths in the lower Mississippi River delta as indicated by numbers caught in traps in 1994. Southwest. Entomol. 1995, 20, 157–164. [Google Scholar]
- DiFonzo, C.D.; Hammond, R. Range expansion of western bean cutworm, Striacosta albicosta (Noctuidae), into Michigan and Ohio. Crop. Manag. 2008, 7. [Google Scholar] [CrossRef]
- Munyaneza, J.E.; Crosslin, J.M.; Buchman, J.L. Seasonal occurrence and abundance of the Potato Psyllid, Bactericera cockerelli, in South Central Washington. Am. J. Potato Res. 2009, 86, 513–518. [Google Scholar] [CrossRef]
- Antolínez, C.A.; Moreno, A.; Ontiveros, I.; Pla, S.; Plaza, M.; Sanjuan, S.; Palomo, J.L.; Sjolund, M.J.; Sumner-Kalkun, J.; Arnsdorf, Y.; et al. Seasonal abundance of Psyllid species associated with carrots and potato fields in Spain. Insects 2019, 10, 287. [Google Scholar]
- Teresani, G.; Hernández, E.; Bertolini, E.; Siverio, F.; Marroquín, C.; Molina, J.; de Mendoza, A.H.; Cambra, M. Search for potential vectors of ‘Candidatus Liberibacter solanacearum’: Population dynamics in host crops. Span. J. Agric. Res. 2015, 13, 1002. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Adamczyk, J.J.; Crosslin, J.M.; Troxclair, N.N.; Anciso, J.R.; Bester, G.G.; Bradshaw, J.D.; Bynum, E.D.; Carpio, L.A.; Henne, D.C.; et al. Seasonal Population Dynamics of the Potato Psyllid (Hemiptera: Triozidae) and Its Associated Pathogen Candidatus Liberibacter Solanacearum in Potatoes in the Southern Great Plains of North America. J. Econ. Entomol. 2012, 105, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.N.; Boiteau, G. Management of insect pests. In Potato Health Management; Rowe, R.C., Ed.; American Phytopathological Society Press: St. Paul, MN, USA, 1993; pp. 103–115. [Google Scholar]
- Cranshaw, W.S. Diseases caused by insect toxin: Psyllid yellows. In Compendium of Potato Diseases, 2nd ed.; Stevenson, W.R., Loria, R., Franc, G.D., Weingartner, D.P., Eds.; APS Press: St. Paul, MN, USA, 2001; pp. 73–74. [Google Scholar]
- Capinera, J. Handbook of Vegetable Pests; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Bächli, G.; Flückiger, P.F.; Obrist, M.K.; Duelli, P. On the microdistribution of species of Drosophilidae and some other Diptera across a forest edge. Mitt. Schweiz. Entomol. Ges. 2006, 79, 117–126. [Google Scholar]
- Nguyen, H.Q.; Andersen, D.K.; Kim, Y.; Jang, Y. Urban heat island effect on cicada densities in metropolitan Seoul. PeerJ 2018, 6, e4238. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Tkaczuk, C.; Dinu, M.M.; Fiedler, Z.; Vidal, S.; Zchori-Fein, E.; Messelink, G.J. New opportunities for the integration of microorganisms into biological pest control systems in greenhouse crops. J. Pest. Sci. 2016, 89, 295–311. [Google Scholar] [CrossRef]
- Walker, G.P.; MacDonald, F.H.; Wright, P.J.; Puketapu, A.J.; Gardner-Gee, R.; Connolly, P.G.; Anderson, J.A. Development of Action Thresholds for Management of Bactericera cockerelli and Zebra Chip Disease in Potatoes at Pukekohe, New Zealand. Am. J. Potato Res. 2015, 92, 266. [Google Scholar] [CrossRef]
- Seymour, R.C.; Hein, G.L.; Wright, R.J. Western bean cutworm in corn and dry beans. Univ. Neb. Linc. Ext. Bull. 2010, G2013, 4. [Google Scholar]


| Source | df | Type III SS | MS | F | P | Significance |
|---|---|---|---|---|---|---|
| Year | 5 | 8642.37 | 1728.47 | 1.569 | 0.1721 | ns |
| Species | 5 | 11723.69 | 2344.74 | 2.129 | 0.0650 | ns |
| Date | 6 | 14055.86 | 2342.64 | 2.127 | 0.0534 | ns |
| Year * Species | 25 | 48535.17 | 1941.41 | 1.763 | 0.0204 | * |
| Year * Date | 30 | 59535.60 | 1984.52 | 1.802 | 0.0116 | * |
| Species * Date | 30 | 34129.07 | 1137.64 | 1.033 | 0.4296 | ns |
| Error | 150 | 165207.04 | 1101.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djaman, K.; Higgins, C.; O’Neill, M.; Begay, S.; Koudahe, K.; Allen, S. Population Dynamics of Six Major Insect Pests During Multiple Crop Growing Seasons in Northwestern New Mexico. Insects 2019, 10, 369. https://doi.org/10.3390/insects10110369
Djaman K, Higgins C, O’Neill M, Begay S, Koudahe K, Allen S. Population Dynamics of Six Major Insect Pests During Multiple Crop Growing Seasons in Northwestern New Mexico. Insects. 2019; 10(11):369. https://doi.org/10.3390/insects10110369
Chicago/Turabian StyleDjaman, Koffi, Charles Higgins, Michael O’Neill, Shantel Begay, Komlan Koudahe, and Samuel Allen. 2019. "Population Dynamics of Six Major Insect Pests During Multiple Crop Growing Seasons in Northwestern New Mexico" Insects 10, no. 11: 369. https://doi.org/10.3390/insects10110369
APA StyleDjaman, K., Higgins, C., O’Neill, M., Begay, S., Koudahe, K., & Allen, S. (2019). Population Dynamics of Six Major Insect Pests During Multiple Crop Growing Seasons in Northwestern New Mexico. Insects, 10(11), 369. https://doi.org/10.3390/insects10110369






