Abstract
The health of the forestlands of the world is impacted by a number of insect pests and some of them cause significant damage with serious economic and environmental implications. Whether it is damage of the North American cypress aphid in South America and Africa, or the destruction of maple trees in North America by the Asian long horned beetle, invasive forest pests are a major problem in many parts of the world. Several studies explored microbial control opportunities of invasive forest pests with entomopathogenic bacteria, fungi, and viruses, and some are successfully utilized as a part of integrated forest pest management programs around the world. This manuscript discusses some invasive pests and the status of their microbial control around the world with entomopathogenic fungi.
1. Introduction
Globalization of trade and travel directly or indirectly contributed to the spread of several insects to new areas where they have become serious pests. Invasive pests of forests not only cause economic damage, but also have an impact on the ecosystem, regionally or nationally. Use of chemical pesticides has been the primary pest control strategy for the past several decades. Due to the environmental and human health risks from excessive use of chemical pesticides, there are renewed appeals for effective, safe, and economically acceptable alternatives. Integrated pest management (IPM) emerged as an approach to address the safety issue by taking all pest management options into consideration and promoting a balanced strategy that is environmentally sustainable, economically viable, and socially acceptable and applicable various scenarios from crop pests to forest pests []. There are several invasive Coleoptera, Hemiptera, Hymenoptera, and Lepidoptera in forest ecosystems that have been a target of various management practices including microbial control with native, or introduced, entomopathogens. Entomopathogenic fungi are a group of phylogenetically diverse heterotrophic and eukaryotic microorganisms that are pathogens of insects and use them as hosts to develop a part of their life cycle [,]. Today, there are over 700 recognized species of entomopathogenic fungi representing the kingdoms of Chromista and Fungi []. However, a majority of important species belongs to the phylum Ascomycota (order: Hypocreales) and Entomophthoromycota (orders: Entomophthorales and Neozygitales). In general, these fungi are considered excellent candidates for microbial control of various insect pests [,,]. Nonetheless, only a small number of taxa, most notably Beauveria bassiana (Bals. -Criv.) Vuill., B. brongniartii (Sacc.) Petch, Metarhizium anisopliae (Metschn.) Sorokin, Lecanicillium lecanii (Zimm.) Zare and W. Gams and Isaria fumosorosea Wize are in active production, sale, and general use as microbial control agents mainly in crop production systems []. While earlier reviews explained the importance of entomopathogens in controlling forest pests [], the current review focused on various entomopathogenic fungi against invasive forest pests around the world.
2. Invasive Forest Insect Pests
The number of invasions by non-indigenous forest pests is increasing worldwide due to growing travel and trade []. Pest invasions consist of three phases: arrival at a site, establishment at that location, and subsequent spread []. Given the species richness and the wide involvement in ecosystem processes of insects, it is not surprising that they are also prominent as invasive species, both in terms of their number and their impact [,]. Some important examples of invasive forest insect pests, their damage, spread and the current status of microbial control are discussed here.
2.1. Cypress Aphid
Cinara cupressi Buckton, (Hemiptera: Aphididae), native of North America, belonging to a complex of several anatomically similar species [], is currently widespread all over the world. It is exclusively associated with conifers in the Cupressaceae and Pinaceae families. These aphids feed on smaller twigs in the foliated parts of the crown and frequently cause branch die-back, resulting in damage to natural forests and plantations [,,]. The aphid inserts its buccal stylet into the tree until it reaches the phloem and ingests large quantities of phloem sap, which is rich in sugars. A secondary problem caused by aphid feeding is the secretion of copious quantities of honeydew that promotes the growth of sooty mold [,,,]. Aphid feeding decreases photosynthesis and increases respiration, resulting in chlorosis of foliage and stunted growth, especially in young trees [,]. Several studies have pointed out the high economic and environmental impacts of cypress aphid infestations. In Kenya, 12% of the trees were killed over two years, causing significant economic losses []. In the southern and eastern African regions, C. cupressi caused a loss of $27.5 million in 1991 with a continued annual loss of $9.1 million []. Additionally, C. cupressi is also a threat to an endangered Widdringtonia species in Africa []. Cinara cupressi damage has also been reported in South America in the Chilean cedar, Austrocedrus chilensis (D. Don) Pic. Serm. and Bizzarri, and the Patagonian cypress, Fitzroya cupressoides I. M. Johnst. [,,]. To minimize the impact of C. cupressi on A. chilensis, several governmental agencies promote a pest management program with an emphasis on biological control [,].
2.2. Eucalyptus Weevil
Gonipterus platensis Marelli (Coleoptera: Curculionidae), a native of Australia, has been accidentally introduced in other parts of the world where it became a serious pest of eucalyptus [,,]. Based on morphological and molecular data, G. platensis is now recognized as a part of a cryptic species complex known as Gonipterus scutellatus Gyllenhal []. Gonipterus scutellatus species complex invaded countries, including France, Portugal, Italy, and Spain, and is categorized as a quarantine pest listed in Annex IIB of Council Directive 2000/29/EC []. As the major eucalyptus pest, it causes significant damage to eucalyptus trees around the world. The larvae feed on young leaves and defoliate the top parts of the canopy [], while adults feed on the edges of mature leaves, impairing the growth of the tree []. The damaged trees show symptomatic scalloped leaf edges, with a resultant die-back of shoot tips and the development of epicormic shoots [,]. Damage initially appears as a brownish scorched appearance of young foliage and eventually leads to the destruction of young twigs and buds. Severe defoliation gives the trees a stunted and stag-headed appearance. Eucalyptus plantations are the most productive forest stands in Spain with around 500,000 ha of cultivated area where Eucalyptus globulus Labill. is the dominant species in North and North-west Spain []. Since 1991, the high productivity of this eucalyptus species has been threatened by outbreaks of G. scutellatus. It has been estimated that tree growth is sometimes reduced by 30% in Galicia []. Determination of the impact of different levels of defoliation on wood production is difficult because it depends on tree age, tree health status, soil parameters, and orientation of the stands []. Mature and healthy trees could be more tolerant to defoliation: by using an empirical growth model, it has been predicted that for 10-year-old trees the 75% and 100% defoliation would produce wood volume losses of 43% and 86%, respectively []. However, 20% defoliation of 3-year-old E. globulus results in significant reduction of stem growth within just one year after defoliation [].
2.3. Gypsy Moth
Gypsy moth, Lymantria dispar L. (Lepidoptera: Erebidae), is native to Eurasia and North Africa and is one of the most important pests of deciduous trees in Europe spreading from west to east and from north to south [,]. Regular outbreaks are very common, especially in the Balkan Peninsula. Since L. dispar was accidentally introduced from France to the United States near Boston, its distribution has continued to expand due to favorable environmental conditions in the pest’s new home [,]. The extremely broad host range for larval feeding and the non-discriminating oviposition behavior of females has allowed L. dispar to disperse and become established through much of northeastern United States. At present, it is considered one of the most destructive forest insects in the eastern United States [,]. Lymantria dispar is also a global threat to both commercial and native forest systems due to its host range [,,,]. In the United States, the economic impacts of one subspecies, the European gypsy moth (L. dispar dispar L.), is estimated to be in excess of $250 million per year [], and this is likely to increase as this species continues to spread through North America. Two other subspecies, the Asian gypsy moth (L. dispar asiatica Vinkovskij), found in China, the Korean peninsula and far East Russia, and the Japanese gypsy moth (L. dispar japonica Motschulsky), found in Japan, have not yet established outside their native range, but are of significant global concern []. The ecological implications of L. dispar defoliation include changes in forest succession patterns and watershed characteristics, stand patchiness, and sporadic masting, all of which can affect wildlife distribution patterns [,]. In urban landscapes, human health concerns are associated with high populations of mobile caterpillars with urticating hairs, as well as copious frass production [].
2.4. Asian Longhorned Beetle
Anoplophora glabripennis Motschulsky (Coleoptera: Cerambycidae) is a destructive polyphagous woodborer that attacks and kills healthy trees native to Asia [,]. It has become a serious forest pest in China since the 1980s, as a result of the planting of vast forest and urban monocultures dominated by non-native Populus and Salix [,]. Between 1980 and 1990, widespread outbreaks of A. glabripennis occurring in Ningxia Province and Inner Mongolia led to the destruction of over 90 million infested trees []. To date, this insect continues to be particularly problematic in landscapes such as agricultural windbreaks, roadside greenways, plantations, and urban street trees []. In the last two decades, as international trade increased between China and western countries, numerous accidental introductions of A. glabripennis occurred in North America and Europe. It was first intercepted in the United States and Canada in 1992, on wood packaging material, and an established population was found in North America in 1996. Maple trees (Acer spp.) are the most commonly infested by A. glabripennis in both these countries. In Europe, the first A. glabripennis infestation was found in north-west Austria in 2001 []. Since then, it has been detected in France, Germany, Finland, Montenegro, Switzerland, the Netherlands and the United Kingdom [,,,,]. Adults feed primarily on the bark and phloem of 2–3-year-old twigs and leaf petiole. Females bore into the cambial region to deposit eggs individually []. Larvae feed solitarily beneath the bark along the phloem–cambium interface in the early instars before boring into and feeding on the heartwood. Larvae move upward through sapwood and heartwood, forming galleries as they develop. Adults chew through the bark and exit the galleries. Due to its economic and long-term ecological damage, A. glabripennis is considered a serious pest.
In Italy, the citrus longhorned beetle, Anoplophora chinensis Forster, a related polyphagous species, was detected in the Milan area in 2000 []. Many ornamental trees in Lombardy region were severely affected by this pest, which was introduced through bonsai plant imported from the Far East. Major monitoring and eradication efforts began in 2004 [,]. Until now, the species has been observed in Italy, France, Croatia, Germany and Switzerland [,,]. Although intercepted in the United States a few times, this pest has not yet been established in the United States or Canada [].
2.5. Emerald Ash Borer
Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) is an invasive tree-boring beetle native to temperate northeastern Asia, including China, Korea, and Russia. It was accidentally introduced in North America in 2002 and has currently spread to a range that now includes 31 states and 3 provinces [,]. It killed millions of ash trees in the United States and Canada, causing extensive ecological and economic damage []. In Europe, it was first recorded in 2003 in Moscow, Russia and spread 460 km south and 250 km west in the past decade [,]. All ash species native to Europe and North America are susceptible to A. planipennis, although to varying levels. While the black, green, and white ash species are the most susceptible, the white ash is less preferred, and the blush ash is the most resistant in North America. Agrilus planipennis causes progressive canopy decline []. Adults feed on foliage and females deposit eggs under the bark or within the cracks in the bark. Larvae cause rapid tree mortality via their feeding in the cambial and phloem tissue, which creates serpentine galleries that sever sap transport between shoots and roots, disrupting the water and nutrient supply [,]. Infested trees usually die within 2–6 years []. Economic consequences associated with this pest are also significant. Following spread of A. planipennis in Canada, the potential cost of treating ca 1.2 million ash trees in urban landscapes was estimated to be $890 million in 2010 []. Cost prediction for treatment following similar spread of A. planipennis in urban centers of 25 states in the eastern United States is $10.7 billion []. In addition to the economic losses to forests, properties, and affiliated industries by the mortality of trees, the pest also caused significant ecological losses by disrupting the species composition, nutrient cycles, and contributing to the spread of unwanted invasive species [,].
2.6. Oak Lace Bug
The Nearctic species Corythucha arcuata (Say) (Hemiptera: Tingidae), native to North America, is among one of the most important pests of oak trees (Quercus spp.) in forest, urban, and rural areas worldwide [,,,]. Corythucha arcuate was first detected in North Italy in 2000 [] and continued to spread in Europe with a current distribution in south to central Europe, Turkey, Russia, and Iran [,,,,]. Adults and nymphs feed on the lower side of the leaves of host trees producing numerous characteristic black spots. Corresponding upper leaf surfaces develop discoloration and whitish blotching or stippling. Due to the leaf damage, photosynthesis and respiration are reduced. Under heavy infestations, premature leaf fall may occur [,,,]. In Turkey, a few years after the first record, the oak lace bug affected an area of about 28,116 km2 []. In Bulgaria, just five years after the first recorded C. arcuata invaded most of the country, about 85% of the leaves displayed discoloration []. Besides oaks, C. arcuata can also occasionally attack hosts from the genera Acer, Castanea, Malus, Pyrus and Rosa [,]. In 2001, C. arctuata was added to the alert list of the European and Mediterranean Plant Protection Organization and subsequently deleted in 2007. The main reason for deletion was that no efficient phytosanitary measures could stop the natural spread of this species.
3. Microbial Control of Invasive Pests with Entomopathogenic Fungi
Biocontrol of pests is the use of living organisms to reduce pest populations and is an important part of IPM []. Entomopathogenic bacteria, fungi, nematodes, and viruses are commonly used microbial control agents, within the framework of biocontrol, for pest management in various cropping systems. While bacteria and viruses are more effective against pests that have chewing mouthparts, entomopathogenic fungi can be effective against a variety of pests. Similar to biocontrol agents, such as parasitoids and predators, entomopathogens can also be released in classical or augmentation approach to control invasive pests []. While the objective of classical biological control is permanent establishment of biocontrol agents for self-sustained long-term control of target pests, the augmentation approach represents periodic release of pathogens as biocontrol agents with the expectation that they will multiply and control pests for an extended period, but not permanently. Both the concepts are suitable for microbial control of invasive forest pests by entomopathogenic fungi. Currently, there are over 700 recognized species of entomopathogenic fungi. A majority of economically important species belongs to the order Hypocreales and the new phylum Entomophthoromycota. In general, these fungi are considered excellent candidates for microbial control of many insect pests [,]. Several characteristics of entomopathogenic fungi make them an ideal alternative or supplement to chemical insecticide usage. Hypocreales are more general pathogens while Entomopathoromycota are relatively host specific, both with minimal effect on non-target beneficial organisms and are compatible with IPM programs. Entomopathogenic fungi and their metabolites pose no obvious risk to mammalians [], however tissue infections and allergies can be very rarely observed in immunocompromised individuals [,,]. A further reason in favor of using microbial biocontrol agents is the increasing emergence of resistance in pests to chemical pesticides [,]. Among entomopathogenic fungi, hypocrealeans such as Beauveria spp., Isaria fumosorosea, and Metarhizium spp. are available as commercial formulations for inoculative and inundative applications. Entomophthoraleans, such as Entomophaga maimaiga Humber, Shimazu, and Soper, are naturally occurring fungi and cause epizootics in pest populations. Microbial control efforts of the invasive forest pests discussed in this article are presented here.
3.1. Cypress Aphid Control
A survey of entomopathogenic fungi of C. cupressi, was carried out in southern Chile (project DID 2011-11) between 2007 and 2013. Several strains of Lecanicillium attenuatum were isolated and a Neozygites species wasreported from naturally infected C. cupressi cadavers (Figure 1) in different localities [,]. Several laboratory studies around the world demonstrated high levels of mortality in C. cupressi with entomopathogenic fungi of the order Hypocreales, especially with Lecanicillium sp. [,,,]. However, field efficacy varies considerably as these fungi are strongly influenced by the environmental conditions such as humidity and temperature. On the other hand, some fungi of the order Neozygitales were cited and collected in different locations in Chile [,,]. For example, Neozygites turbinata (Kenneth) Remaudière and Keller and Neozygites osornensis Montalva and Barta are highly specific to certain aphids and can cause epizootics in pest populations, and they can also be multiplied as protoplasts or hyphal bodies. However, challenges of in vitro and in vivo production of Neozygites spp. limit their use as an augmentation control option []. Releasing live aphids infected with Neozygites spp. could be an option, as seen with Neozygites floridana (Weiser and Muma) Remaudière and S. Keller, for controlling the cassava green mite, Mononychellus tanajoa (Bondar), in West Africa [].
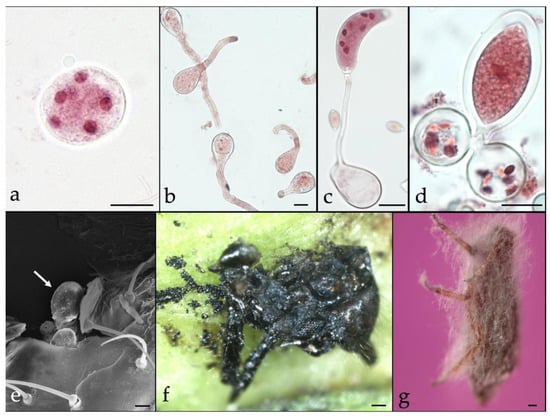
Figure 1.
Entomopathogenic fungi, Neozygites osornensis (a–f) and Lecanicillum attenuatum (g). on Cinara cupressi. (a). Subspherical hyphal body with visible nuclei (Bar = 10 μm), (b). Primary conidia germinating apically (Bar = 10 μm), (c). Capilliconidium developed from primary conidium on capillary tube (Bar = 10 μm), (d). Zygospore developing by conjugation of two hyphal bodies (Bar = 10 μm), and (e). Fully developed zygospore attached on killed aphid (Bar = 10 μm), (f). Fungus-killed C. cupressi (Bar = 100 μm), and (g). Mycelial growth on fungus-killed C. cupressi (Bar = 100 μm).
3.2. Eucalyptus Weevil Control
A survey with the primary objective of discovering entomopathogenic fungi of G. platensis was carried out in Chile (project Fondecyt de Iniciación Nº 11160555) between 2016 and 2018. Different species of the genera Beauveria, Hirsutella, and Metarhizium (Figure 2) were found from natural infections in adult G. platensis in or when insects were exposed to soil samples containing entomopathogenic fungi (project Fondecyt Regular Nº 1190390). In South Africa, Echeverri and Santolamazza [] evaluated three formulations of B. bassiana and a suspension containing spores of Metarhizium acridum against adults G. scutellatus under laboratory conditions. Beauveria bassiana (strain PPRI 5339) exhibited the highest efficiency, both by contact and ingestion, resulting in 100% adult mortality; thus, appearing to be the most promising strain to promote an IPM program in South Africa.
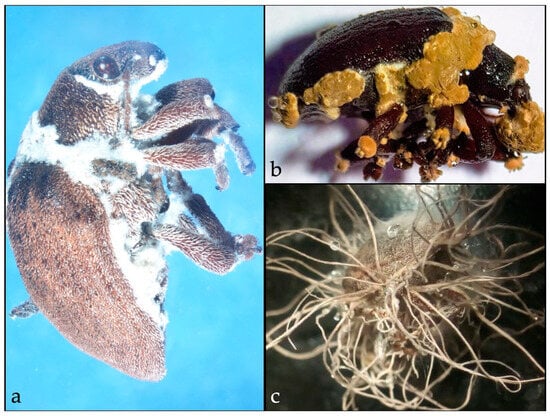
Figure 2.
Entomopathogenic fungi on Gonipterus platensis infected by. (a). Beauveria sp. (b). Hirsutella sp., and (c). Metarhizium sp.
3.3. Gypsy Moth Control
Lymantria dispar probably has more microbial control options than the other invasive pests reviewed in this article. Bacillus thuringiensis Berliner subsp. kurstaki, Lymantria dispar multicapsid nucleopolyhedrovirus (LdMNPV) and E. maimaiga (Entomophthoromycota: Entomophthorales) have been used for controlling L. dispar [,]. The introduction of E. maimaiga from Asia to the United States is a good example of classical microbial control. Following the introduction of this fungus, epizootics by this species predominate in L. dispar populations, although low levels of consistent infections by I. fumosorosea or occasional infections by B. bassiana are also detected []. Currently, E. maimaiga is the most important host-specific fungal pathogen of L. dispar larvae in North America (Figure 3). It was originally described from Japanese gypsy moth L. dispar japonensis []. In 1910–11, the fungus was intentionally introduced into the United States as a classical microbial control agent, using two cadavers containing resting spores. However, it was not recovered in L. dispar populations in subsequent years, and the program was terminated as unsuccessful in 1912 []. In 1989, unexpected high mortality of L. dispar larvae, caused by E. maimaiga, was recorded in the northeastern United States []. Molecular studies and models suggest that the E. maimaiga strain, now active in the United States, was an accidental introduction after 1971 []. At present, the fungus has a major impact on L. dispar populations in the United States and reduces defoliation caused by the pest [,]. The successful introduction of E. maimaiga into North American populations of L. dispar inspired its introduction into Bulgaria in 1999 []. Surveys conducted in subsequent years confirmed that the pathogen was successfully established and the first epizootics of E. maimaiga in L. dispar populations were observed in 2005 [,,]. Since 2011, the fungus has been recovered in several countries of central and southeastern Europe [,,,,,,].

Figure 3.
Entomopathogenic fungi, Entomophaga maimaiga (a–e) and Beauveria bassiana (f) on Lymantria dispar. (a). Primary conidia attached on setae of killed larva (Bar = 200 μm) (b). Primary conidium attached on larval seta (Bar = 5 μm), (c). Larval cadaver with emerged conidia, (d). Infected-live larvae, (e). Fungus-killed larvae collected from a forest, and (f). Sporulating cadaver.
3.4. Asian Longhorned Beetle Control
Multiple studies demonstrated the potential of B. brongniartii, M. anisopliae, and M. brunneum Petch in controlling A. glabripennis [,,]. Adult longevity and female oviposition of A. glabripennis was significantly affected when exposed to non-woven fiber bands impregnated with commercial and native isolates of B. asiatica Rehner and Humber and B. brongniartii []. In a study conducted with M. brunneum (strain F52), fungal bands based on agar and two oil formulations and agar-based bands resulted in improved conidial acquisition by beetles and rapid mortality []. Similarly, conidial viability and virulence of M. brunneum strain F52 was maintained for at least 112 days under field conditions in studies conducted against A. glabripennis []. Two M. anisopliae isolates significantly reduced female longevity and fecundity of A. glabripennis and also reduced the eclosion of larvae from eggs deposited by infected females []. It appeared that starvation had a similar impact on the survival of M. brunneum inoculated beetles compared to imidacloprid exposure []. The synergy, however, was not completely due to starvation, as imidacloprid reduced the beetles’ melanotic encapsulation response and capsule area, while starvation did not significantly reduce these immune responses. Their results suggest that multiple interacting mechanisms are involved in the synergy between M. brunneum and imidacloprid. Furthermore, it appeared that mature and old females of A. glabripennis were more susceptible to M. brunneum than males of equal ages, and more females had detectable fungal blastospores in their hemolymph compared to mature and old males []. Also, laboratory conditions demonstrated that M. brunneum-infected A. glabripennis does not exhibit behavioral fever (elevating body temperature by exposing to a heat source to ward off fungal infections []. Bioassays conducted in the United States showed that the Japanese commercial strain of B. asiatica and the commercial strain F52 of M. brunneum were more virulent than two North American B. brongniartii isolates against A. glabripennis []. In Japan, basic experiments were performed in order to develop biorational control of cerambycid beetle, including A. malasaica (=chinensis). Local strains of B. brongniartii incorporated in non-woven pulp fabric sheets, or polyurethane sheets, were applied around branches and tree trunks against cerambycid adults emerging from trees [,]. Between 46% and 100% adult mortality was observed when adults were delivered on polyurethane sheets wrapped around the lower portion of the trunk [].
3.5. Emerald Ash Borer Control
Agrilus planipennis is attacked by several species of entomopathogenic fungi. However, B. bassiana appeared to be a potential microbial control agent based on multiple studies. Spray applications of the commercial formulation of B. bassiana strain GHA to the trunks of ash trees, especially before the adult emergence in summer, reduced adult longevity and female fecundity and delayed larval development (Figure 4) []. Castillo et al. [] also found that conidial sprays of B. bassiana, on the ash bark before adult emergence, remain viable enough to be a significant mortality factor. A study conducted in Canada demonstrated the potential of using insect traps equipped with B. bassiana conidia as an attract-and-kill strategy for A. planipennis []. Two additional genera demonstrated pathogenicity in laboratory conditions. Isaria farinosa (Holmsk.) Fr. and Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel-Jones and Samson infected adults with high mortality rates (75% and 51%, respectively) under laboratory conditions [] (Figure 5). Lyons et al. [] evaluated the use of fluorescent dyes as a cost-effective method of tracking dispersal by A. planipennis of B. bassiana isolates that were introduced by using an autocontamination device. Neither of the two fluorescent dyes tested (Arc Yellow and Aurora Pink, DayGlow Color Corp) interfered with fungal germination or growth, nor did they affect survival of beetles in the laboratory or affect virulence of the fungus in bioassays. The dyes persisted outdoor exposure for at least 10 days on dead beetles in sticky band traps, and for at least 14 days on pouches inside autocontamination traps. Duplicate field trials in late June–early July 2012, using autocontamination traps containing powder-dusted fungal pouches in ash-borer infested plantations in southwestern Ontario showed fluorescent dyes in 8.0% of the 4010 beetles captured in nearby green prism and sticky-band traps. However, only half (46.2%–57.8%) of the beetles with dyes carried viable fungal conidia, as determined by plating of beetle rinses, possibly as a result of patchy growth of fungal isolates and reduced conidia production on pouch surfaces during the 16-day trapping experiment.

Figure 4.
Entomopathogenic fungi on Agrilus planipennis. (a). Infected adult and a culture of Beauveria sp., (b). Infected adult and a culture of Lecanicillium sp., (c). Infected adult and a culture of Metarhizium sp., and (d). Infected adult and culture of Paecilomyces sp.

Figure 5.
Adult of Agrilus planipennis infected with Beauveria bassiana as a result of pre-emergence field cage trials.
3.6. Oak Lace Bug Control
Effective control methods for C. arcuata are limited and include mostly application of oils or contact insecticides on infested ornamental oaks []. To date, no biocontrol programs were used to regulate C. arcuata infestations. However, a pilot study has been initiated in Turkey recently to test ten entomopathogenic fungi against both nymphs and adults of C. arcuata under laboratory conditions []. Entomopathogenic fungi of the genera Metarhizium, Beauveria, Isaria, and Myriodontium were included in this study. All fungal strains were able to infect the pest after application of 1 × 107 mL−1 conidial concentration, but B. bassiana strain was very pathogenic to both nymphs and adults, with 80% and 90% mortality within 14 days of exposure, respectively.
4. Concluding Remarks
Although a variety of IPM tactics are used for controlling forest pests, chemical pesticide use is still one of the primary choices around the world. Considering the environmental impacts and the risk of resistance, alternative control options are always important. While application of biopesticide formulations can be expensive, they play a critical role in pest suppression, especially in areas close to urban dwellings, waterbodies, and other such sensitive locations. Natural epizootics by fungi and viruses can help with forest pest control a great deal and understanding the disease dynamics will be useful in delaying pesticide applications or developing integrated strategies. Since several forest pests are invasive and continue to spread in their new homes, or to other areas, the knowledge of microbial control potential with entomopathogenic fungi will contribute to their sustainable management.
Author Contributions
Writing—original draft preparation and writing—review and editing, S.K.D., C.M., and M.B.; supervision and funding acquisition, S.K.D.
Funding
This research received no external funding.
Acknowledgments
We are grateful to George Kyei-Poku, Research Scientist of the Canadian Forest Service and Houping Liu, Michigan State University in providing picture material of entomopathogenic fungi on Agrilus planipennis to be used in this review. This study was supported by FONDECYT (Fondo Nacional de Desarrollo Científico y Tecnológico, Chile) project 1190390.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to Section 3.2 and the Acknowledgments. This change does not affect the scientific content of the article.
References
- Dara, S.K. The new integrated pest management paradigm for the modern age. J. IPM 2019, 10, 12. [Google Scholar] [CrossRef]
- Wraight, S.; Inglis, G.; Goettel, M. Fungi. In Field Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L., Kaya, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 223–248. [Google Scholar]
- Samson, R.; Evans, H.; Latgé, J.P. Atlas of Entomopathogenic Fungi, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1988; p. 187. [Google Scholar]
- Goettel, M.; Inglis, G.; Wraight, S. Fungi. In Field Manual of Techniques in Invertebrate Pathology, 1st ed.; Lacey, L., Kaya, H., Eds.; Kluwer Academic Publishers: London, UK, 2000; pp. 255–282. [Google Scholar]
- Hajek, A. Ecology of terrestrial fungal entomopathogens. In Advances in Microbial Ecology, 1st ed.; Jones, G.J., Ed.; Springer: New York, NY, USA, 1997; Volume 15, pp. 193–249. [Google Scholar]
- Charnley, A.; Collins, S. Entomopathogenic fungi and their role in pest control. In The Mycota IV, Environmental and Microbial Relationships, 2nd ed.; Kubicek, C.P., Druzhinina, I.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 159–187. [Google Scholar]
- Roy, H.; Brodie, E.; Chandler, D.; Goettel, M.; Pell, J.; Wajnberg, E.; Vega, F. Deep space and hidden depths: Understanding the evolution and ecology of fungal entomopathogens. BioControl 2010, 55, 1–6. [Google Scholar] [CrossRef]
- Humber, R. Seeking stability for research and applied uses of entomopathogenic fungi as biological control agents. J. Asia Pac. Entomol. 2016, 19, 1019–1025. [Google Scholar] [CrossRef]
- Augustyniuk-Kram, A.; Kram, K.J. Entomopathogenic fungi as an important natural regulator of insect outbreaks in forests (Review). In Forest Ecosystems-More than Just Trees; Blanco, J.A., Lo, Y.H., Eds.; InTech: Rijeka, Croatia, 2012; pp. 265–294. [Google Scholar] [CrossRef]
- Liebhold, A. Forest pest management in a changing world. Int. J. Pest Manag. 2012, 58, 289–295. [Google Scholar] [CrossRef]
- Liebhold, A.; Tobin, P. Population ecology of insect invasions and their management. Annu. Rev. Entomol. 2008, 53, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Auger-Rozenberg, M.; Roques, A.; Timms, L.; Péré, C.; Cock, M.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Brockerhoff, E.; Barratt, B.; Beggs, J.; Fagan, L.; Malcolm, K.; Phillips, C.; Vink, C. Impacts of exotic invertebrates on New Zealand’s indigenous species and ecosystems. N. Z. J. Ecol. 2010, 34, 158–174. [Google Scholar]
- Watson, G.; Voegtlin, D.; Murphy, S.; Fottit, R. Biogeography of the Cinara cupressi complex (Hemiptera: Aphididae) on Cupressaceae, with description of a pest species introduced into Africa. Bull. Entomol. Res. 1999, 89, 271–283. [Google Scholar] [CrossRef]
- Blackman, R.; Eastop, V. Aphids on the World’s Trees: An Identification and Information Guide, 1st ed.; CAB International: England, UK, 1994; p. 986. [Google Scholar]
- FAO. Global Review of Forest Pests and Diseases, FAO Forestry Paper 156; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; p. 222. [Google Scholar]
- Montalva, C.; Rojas, E.; Ruiz, C.; Lanfranco, D. El pulgón del ciprés en Chile: Una revisión de la situación actual y antecedentes del control biológico. Bosque 2010, 31, 81–88. [Google Scholar] [CrossRef]
- Carter, C.; Maslen, N. Conifer Lachnids in Britain (Forestry Commission Bulletin); Her Majesty’s Stationery Office: Richmond, UK, 1982; Volume 58, 75p. [Google Scholar]
- Chilima, C. Cinara Cupressi: A Pest of Mulanje cedar and Cypress Trees in Malawi; FRIM Report Nº 89009; Forestry Research Institute of Malawi: Zomba, Malawi, 1989; p. 8. [Google Scholar]
- Ciesla, W.M. The Cypress aphid, Cinara cupressi (Buckton) in Africa. In FAO Exotic Aphid Pests of Conifers: A Crisis in African Forestry, Workshop proceedings, Muguga, Kenya, 3–6 June 1991; Ciesla, W.M., Ed.; Kenya Forestry Research Institute: Muguga, Kenya, 1991; p. 160. [Google Scholar]
- Eskiviski, E.; Agostini, J.; Toloza, R.; Coll, O. Daños Producidos por el Pulgón del Pino Cinara Atlantica W. (Hemiptera: Aphididae) en Plantas Jóvenes de Pinus Taeda L; XI Jornadas Técnicas Forestales y Ambientales; INTA Montecarlo: Montecarlo, Argentina, 2005; p. 6.
- Penteado, S.; Trentini, R.; Tadeo, E.; Reis, W. Ocorrencia, distribucicao, dano e controle de Pulgoes do genero Cinara em Pinus spp. no Brasil. Floresta 2000, 30, 55–64. [Google Scholar] [CrossRef]
- Orondo, S.; Day, R. Cypress aphid (Cinara cupressi) damage to a cypress (Cupressus lusitanica) stand in Kenya. Int. J. Pest Manag. 1994, 40, 141–144. [Google Scholar] [CrossRef]
- Murphy, S.; Nair, K.; Sharma, J. Status and impact of invasive conifer aphid pests in Africa. In Impact of Diseases and Insect Pests in Tropical Forests, Proceedings of the IUFRO Symposium, Peechi, India, 23–26 November 1993; Nair, K., Sharma, J., Varma, R., Eds.; Kerala Forest Research Institute and Forestry Research Support Programme for Asia and the Pacific: Bangkok, Thailand, 1996; pp. 289–297. [Google Scholar]
- Estay, S. Invasive insects in the Mediterranean forests of Chile. In Insects and Diseases of Mediterranean Forest Systems; Paine, T.D., Lieutier, F., Eds.; Springer: Cham, Switzerland, 2016; pp. 379–396. [Google Scholar] [CrossRef]
- Hechenleitner, P.; Gardner, M.; Thonas, T.; Echeverría, C.; Escobar, B.; Brownless, P.; Martínez, C. Plantas Amenazadas del centro-sur de Chile. Distribución, Conservación y Propagación, 1st ed.; Universidad Austral de Chile y Real Jardín Botánico de Edimburgo: Valdivia, Chile, 2005; p. 188. [Google Scholar]
- Jeger, M.; Bragard, C.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Jaques-Miret, J.A.; MacLeod, A.; Navajas-Navarro, M.; et al. Pest categorisation of the Gonipterus scutellatus species complex. EFSA J. 2018, 16, 5107. [Google Scholar] [CrossRef]
- Mansilla-Vázquez, J.P. Presencia sobre Eucalyptus globulus Labill de Gonipterus scutellatus Gyll. (Col.Curculionidae) en Galicia. Bol. San. Veg. Plagas 1992, 18, 547–554. [Google Scholar]
- Mally, C.W. The Eucalyptus Snout-beetle. (Gonipterus scutellatus, Gyll.). J. Dep. Agric. S. Afr. 1924, 9, 415–442. [Google Scholar]
- Tooke, F.G.C. The Eucalyptus Snout beetle, Gonipterus scutellatus Gyll. A Study of its Ecology and Control by biological means. In Entomology Memoirs. Department of Agriculture and Forestry, Union of South Africa Union of South Africa; Department of Agricultural Technical Services: Pretoria, South Africa, 1955; p. 282. [Google Scholar]
- Álvarez, M.F.; Lorenzo, H.; Rodriguez, J.R.; Picos, J. Workflow to Improve the Forest Management of Eucalyptus Globulus Stands Affected by Gonipterus Scutellatus in Galicia (Spain) Using Remote Sensing and GIS. 11th SPIE International Symposium on Remote Sensing; SPIE: Maspalomas, Spain, 2004; Volume 5574, pp. 372–383. [Google Scholar] [CrossRef]
- Reis, A.R.; Ferreira, L.; Tomé, M.; Araujo, C.; Branco, M. Efficiency of biological control of Gonipterus platensis (Coleoptera: Curculionidae) by Anaphes nitens (Hymenoptera: Mymaridae) in cold areas of the Iberian Peninsula: Implications for defoliation and wood production in Eucalyptus globulus. For. Ecol. Manag. 2012, 270, 216–222. [Google Scholar] [CrossRef]
- Pinkard, E.A.; Baillie, C.; Patel, V.; Mohammed, C.L. Effects of fertilizing with nitrogen and phosphorus on growth and crown condition of Eucalyptus globulus Labill. Experiencing insect defoliation. For. Ecol. Manag. 2006, 231, 131–137. [Google Scholar] [CrossRef]
- McNamara, D.G. EPPO’s perspective on the gypsy moth in Europe. In Proceedings of the 1995 Annual Gypsy Moth Review, Traverse City, MI, USA, 5–8 November 1995; Department of Agriculture: Lansing, MI, USA, 1996; pp. 60–65. [Google Scholar]
- McManus, M.; Csóka, G. History and impact of gypsy moth in North America and comparison to recent outbreaks in Europe. Acta Silv. Lign. Hung. 2007, 3, 47–64. [Google Scholar]
- Tobin, P.C.; Blackburn, L.M. Slow the Spread: A National Program to Manage the Gypsy Moth; USDA Forest Service General Technical Report NRS-6; USDA Forest Service, Northern Research Station: Newtown Square, PA, USA, 2007; p. 109. [Google Scholar]
- Leuschner, W.A.; Young, J.A.; Walden, S.A.; Ravlin, F.W. Potential benefits of slowing the gypsy moth’s spread. South J. Appl. For. 1996, 20, 65–73. [Google Scholar] [CrossRef][Green Version]
- Doane, C.C.; McManus, M.L. The Gypsy Moth: Research toward Integrated Pest Management; USDA Forest Service Technical Bulletin 1584; Government Printing Office: Washington DC, USA, 1981; p. 514.
- Elkinton, J.S.; Liebhold, A.M. Population dynamics of gypsy moth in North America. Annu. Rev. Entomol. 1990, 35, 571–596. [Google Scholar] [CrossRef]
- Peterson, T.; Williams, R.; Chen, G. Modeled global invasive potential of Asian gypsy moths, Lymantria dispar. Entomol. Exp. Appl. 2007, 125, 39–44. [Google Scholar] [CrossRef]
- Paini, D.R.; Mwebaze, P.; Kuhnert, P.M.; Kriticos, D.J. Global establishment threat from a major forest pest via international shipping: Lymantria dispar. Sci. Rep. 2018, 8, 13723. [Google Scholar] [CrossRef] [PubMed]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef] [PubMed]
- Twery, M.J. Effects of defoliation by gypsy moth. In Proceedings of USDA Interagency Gypsy Moth Research Review 1990; Gottschalk, K.W., Twery, M.J., Smith, S.I., Eds.; General Technical Report NE-146; USDA Forest Service, Northeastern Forest Experiment Station: East Windsor, CT, USA, 1990; pp. 27–39. [Google Scholar]
- Fajvan, M.; Wood, J.M. Stand structure and development after gypsy moth defoliation in the Appalachian Plateau. For. Ecol. Manag. 1996, 89, 79–88. [Google Scholar] [CrossRef]
- Haack, R.A.; Hérard, F.; Sun, J.; Turgeon, J.J. Managing invasive populations of Asian longhorned beetle and citrus longhorned beetle: A worldwide perspective. Annu. Rev. Entomol. 1981, 55, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.S.; Hoover, K.; Keena, M.A. Asian longhorned beetle (Coleoptera: Cerambycidae), an introduced pest of maple and other hardwood trees in North America and Europe. J. Integr. Pest Manag. 2015, 6, 4. [Google Scholar] [CrossRef]
- Hsiao, K.J. Forest entomology in China: A general review. Crop Prot. 1982, 1, 359–367. [Google Scholar] [CrossRef]
- Williams, D.W.; Lee, H.P.; Kim, I.K. Distribution and abundance of Anoplophora glabripennis (Coleoptera: Cerambycidae) in natural Acer stands in South Korea. Environ. Entomol. 2004, 33, 540–545. [Google Scholar] [CrossRef]
- Pan, H.Y. Review of the Asian Longhorned Beetle: Research, Biology, Distribution and Management in China; For. Dep. Work Pap. FBS/6E; FAO: Rome, Italy, 2005. [Google Scholar]
- Smith, M.T.; Turgeon, J.J.; de Groot, P.; Gasman, B. Asian longhorned beetle, Anoplophora glabripennis (Motschulsky): Lessons learned and opportunities to improve the process of eradication and management. Am. Entomol. 2008, 55, 21–25. [Google Scholar] [CrossRef]
- Tomiczek, C.; Krehan, H.; Menschhorn, P. Dangerous Asian Longhorn Beetle found in Austria: A new threat for our trees. Allg. Forst Z. Waldwirtsch. Umw. 2002, 57, 52–54. [Google Scholar]
- Benker, U.; Bogel, C.; Blaschke, M. Black longicorn required tree felling: Asian longhorned beetle was found in the rural district of Passau. Allg. Forst Z. Waldwirtsch. Umweltvorsorge 2004, 59, 1112. [Google Scholar]
- Cocquempot, C.; Prost, M.; Carmignac, D. Interceptions et introductions en France de Longicornes asiatiques: Cas des Anoplophora glabripennis (Motschulsky) et chinensis (Förster) (Coleoptera Cerambycidae). Bull. Mens. Soc. Linnéenne Lyon 2003, 72, 273–278. [Google Scholar] [CrossRef]
- Morall, L. Insect pests on trees and shrubs in forests and rural areas in 2010. Vakbl. Nat. Bos Landsch. 2011, 8, 23–27. [Google Scholar]
- Forster, B.; Wermelinger, B. First records and reproductions of the Asian longhorned beetle Anoplophora glabripennis (Motschulsky) (Coleoptera, Cerambycidae) in Switzerland. Mitt. Schweiz. Entomol. Ges. 2012, 85, 267–275. [Google Scholar]
- Straw, N.A.; Fielding, N.J.; Tilbury, C.; Williams, D.T.; Inward, D. Host plant selection and resource utilisation by Asian longhorn beetle Anoplophora glabripennis (Coleoptera: Cerambycidae) in southern England. Forestry 2014, 88, 84–95. [Google Scholar] [CrossRef]
- Haack, R.A.; Law, K.R.; Mastro, V.C.; Ossenbruggen, H.S.; Raimo, B.J. New York’s battle with the Asian long-horned beetle. J. For. 1997, 95, 11–15. [Google Scholar]
- Colombo, M.; Limonta, L. Anoplophora malasiaca Thomson (Coleoptera Cerambycidae Lamiinae Lamiini) in Europe. Boll. Zool. Agrar. E Bachic. 2001, 33, 65–68. [Google Scholar]
- Hérard, F.; Ciampitti, M.; Maspero, M.; Krehan, H.; Benker, U.; Boegel, C.; Schrage, R.; Bouhot-Delduc, L.; Bialooki, P. Anoplophora spp. in Europe: Infestations and management process. EPPO Bull. 2006, 36, 470–474. [Google Scholar] [CrossRef]
- Hérard, F.; Maspero, M.; Ramualde, N.; Jucker, C.; Colombo, M.; Ciampitti, M.; Cavagna, B. Anoplophora glabripennis infestation (col.: Cerambycidae) in Italy. EPPO Bull. 2009, 39, 146–152. [Google Scholar] [CrossRef]
- Wermelinger, B. First report of the Citrus longhorned beetle in Switzerland. Der Gart. 2006, 46, 2–4. [Google Scholar]
- Vukadin, A.; Hrašovec, B. Anoplophora chinensis (Forster) in Croatia. Forstsch. Aktuell 2008, 44, 23–24. [Google Scholar]
- Lingafelter, S.W.; Hoebeke, E.R. Revision of the Genus Anoplophora (Coleoptera: Cerambycidae); The Entomological Society of Washington: Washington, DC, USA, 2002; p. 236. [Google Scholar]
- Haack, R.A.; Jendek, E.; Liu, H.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The emerald ash borer: A new exotic pest in North America. Newsl. Mich. Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- USDA Forest Service. Available online: http://www.emeraldashborer.info/index.php (accessed on 13 February 2018).
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Ann. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Orlova-Bienkowskaja, M.J.; Volkovitsh, M.G. Range expansion of Agrilus convexicollis in European Russia expedited by the invasion of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Biol. Invasions 2015, 17, 537–544. [Google Scholar] [CrossRef]
- Valenta, V.; Moser, D.; Kapeller, S.; Essl, F. A new forest pest in Europe: A review of Emerald ash borer (Agrilus planipennis) invasion. J. Appl. Entomol. 2017, 141, 507–526. [Google Scholar] [CrossRef]
- Smitley, D.; Davis, T.; Rebek, E. Progression of ash canopy thinning and dieback outward from the initial infestation of emerald ash borer (Coleoptera: Buprestidae) in southeastern Michigan. J. Econ. Entomol. 2008, 101, 1643–1650. [Google Scholar] [CrossRef]
- Cappaert, D.; Mccullough, D.G.; Poland, T.M.; Siegert, N.W. Emerald ash borer in North America a research and regulatory challenge. Am. Entomol. 2005, 51, 152–165. [Google Scholar] [CrossRef]
- Flower, C.E.; Knight, K.S.; Rebbeck, J.; Gonzalez-Meler, M.A. The relationship between the emerald ash borer (Agrilus planipennis) and ash (Fraxinus spp.) tree decline: Using visual canopy condition assessments and leaf isotope measurements to assess pest damage. For. Ecol. Manag. 2013, 303, 143–147. [Google Scholar] [CrossRef]
- Knight, K.S.; Brown, J.P.; Long, R.P. Factors affecting the survival of ash trees (Fraxinus spp.) infested by emerald ash borer (Agrilus planipennis). Biol. Invasions 2013, 15, 371–383. [Google Scholar] [CrossRef]
- McKenney, D.W.; Pedlar, J.; Yemshanov, D.; Lyons, D.B.; Campbell, K.L.; Lawerence, K. Estimates of the potential cost of emerald ash borer (Agrilus planipennis Fairmaire) in Canadian municipalities. Arboric. Urban For. 2012, 38, 81–91. [Google Scholar]
- Kovacs, K.F.; Haight, R.G.; McCullough, D.G.; Mercader, R.J.; Siegert, N.W.; Liebhold, A.M. Cost of potential emerlad ash borer damage in US communities, 2009–2019. Ecol. Econ. 2010, 69, 569–578. [Google Scholar] [CrossRef]
- Gandhi, J.K.J.; Herms, D.A. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol. Invasions 2010, 12, 389–405. [Google Scholar] [CrossRef]
- Mutun, S.; Ceyhan, Z.; Sözen, C. Invasion by the oak lace bug, Corythucha arcuata (Say) (Heteroptera: Tingidae), in Turkey. Turk. J. Zool. 2009, 33, 263–268. [Google Scholar] [CrossRef]
- Dobreva, M.; Simov, N.; Georgiev, G.; Mirchev, P.; Georgieva, M. First record of Corythucha arcuata (Say) (Heterotera: Tingidae) on the Balkan Peninsula. Acta Zool. Bulg. 2013, 65, 409–412. [Google Scholar]
- Hrašovec, B.; Posarić, D.; Lukić, I.; Pernek, M. First record of oak lace bug (Corythucha arcuata) in Croatia. Šumarski List 137 2013, 9–10, 499–503. [Google Scholar]
- Pap, P.; Drekić, M.; Poljaković-Pajnik, L.; Marković, M.; Vasić, V. Forest health monitoring in Vojvodina in 2015. Topola 2015, 195–196, 117–133. [Google Scholar]
- Bernardinelli, I.; Zandigiacomo, P. First record of the oak lace bug Corythucha arcuata (Say) (Heteroptera, Tingidae) in Europe. Inf. Fitopatol. 2000, 12, 47–49. [Google Scholar]
- Bernardinelli, I. Distribution of the oak lace bug Corythucha arcuata (Say) in northern Italy (Heteroptera, Tingidae). Redia 2000, 83, 157–162. [Google Scholar]
- Mutun, S. First report of the oak lace bug, Corythucha arcuata (Say, 1832) (Heteroptera: Tingidae) from Bolu, Turkey. Isr. J. Zool. 2003, 49, 323–324. [Google Scholar]
- Forster, B.; Giacalone, I.; Moretti, M.; Dioli, P.; Wermelinger, B. Die amerikanische Eichennetzwanze Corythucha arcuata (Say) (Heteroptera, Tingidae) hat die Südschweitz erreicht. Mitt. Schweiz. Entomol. Ges. 2005, 78, 317–323. [Google Scholar]
- Chireceanu, C.; Teodoru, A.; Chiriloaie, A. New records of the oak lace bug Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) in Southern Romania. Acta Zool. Bulg. 2017, 9, 297–299. [Google Scholar]
- Connell, W.A.; Beacher, J.H. Life History and Control of the Oak Lace Bug; Delaware Agricultural Experiment Station Bulletin, No. 265; University of Delaware Agricultural Experiment Station: Newark, DE, USA, 1947; p. 28. [Google Scholar]
- Bernardielli, I. Potential host plants of Corythucha arcuata (Het., Tingidae) in Europe: A laboratory study. J. Appl. Entomol. 2006, 130, 480–484. [Google Scholar] [CrossRef]
- Mutun, S. Corythucha ciliata, a new Platanus pest in Turkey. Phytoparasitica 2009, 37, 65–66. [Google Scholar] [CrossRef]
- Simov, N.; Grozeva, S.; Langourov, M.; Georgieva, M.; Mirchev, P.; Georgiev, G. Rapid expansion of the Oak lace bug Corythucha arcuata (Say, 1832) (Hemiptera: Tingidae) in Bulgaria. Hist. Nat. Bulg. 2018, 27, 51–55. [Google Scholar]
- Drake, C.J.; Ruhoff, F.A. Lace Bugs of the World: A Catalog (Hemiptera: Tingidae); United States National Museum Bull. No. 243; Smithsonian Institution: Washington DC, USA, 1965; p. 634. [Google Scholar]
- Drew, W.A.; Arnold, D.C. Tingoidea of Oklahoma (Hemiptera). Proc. Okla. Acad. Sci. 1977, 57, 29–31. [Google Scholar]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for unifying the terminology in biological control. BioControl 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Pell, J.K.; Eilenberg, J.; Hajek, A.E.; Steinkraus, D.C. Biology, ecology and pest management potential of Entomophthorales. In Fungi as Biocontrol Agents; Butt, T.M., Jackson, C., Magan, N., Eds.; CABI Publishing: Wallingfort, UK, 2001; pp. 71–153. [Google Scholar]
- Shah, P.; Pell, J. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Humber, R.A. Entomophthoromycota: A new phylum and reclassification for entomophthoroid fungi. Mycotaxon 2012, 120, 477–492. [Google Scholar] [CrossRef]
- Strasser, H.; Vey, A.; Butt, T.M. Are there any risks in using entomopathogenic fungi for pest control, with particular reference to the bioactive metabolites of Metarhizium, Tolypocladium and Beauveria species? Biocontrol Sci. Technol. 2000, 10, 717–735. [Google Scholar] [CrossRef]
- Tucker, D.L.; Beresford, C.H.; Sigler, L.; Rogers, K. Disseminated Beauveria bassiana infection in a patient with acute lymphoblastic leukemia. J. Clin. Microbiol. 2004, 42, 5412–5414. [Google Scholar] [CrossRef] [PubMed]
- Westwood, G.S.; Huang, S.W.; Keyhani, N.O. Allergens of the entomopathogenic fungus Beauveria bassiana. Clin. Mol. Allergy 2005, 3, 1–8. [Google Scholar] [CrossRef]
- Henke, M.O.; de Hoog, G.S.; Gross, U.; Zimmermann, G.; Kraemer, D.; Weig, M. Human deep tissue infection with an entomopathogenic Beauveria species. J. Clin. Microbiol. 2002, 40, 2698–2702. [Google Scholar] [CrossRef] [PubMed]
- Patil, S. Development of Entomopathogenic Fungi Based Biopesticide Technology; Lambert Academic Publishing: Latvia, European Union, 2011; p. 188. [Google Scholar]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Montalva, C.; Barta, M.; Rojas, E.; Valenzuela, E. Neozygites osornensis sp. nov., a new fungal species causing mortality to the cypress aphid Cinara cupressi in Chile. Mycologia 2013, 105, 661–669. [Google Scholar] [CrossRef]
- Loureiro, E.; Oliveira, N.; Wilcken, C.; Filho, A. Patogenicidade de Verticillium lecanii ao pulgão do pinus. Rev. Árvore 2004, 28, 765–770. [Google Scholar] [CrossRef]
- Pereira, M.; Chiarello, S. Seleção de isolados de Verticillium lecanii para o controle de Cinara atlantica. Pesqui Agropecuária Bras. 2005, 40, 1141–1144. [Google Scholar] [CrossRef]
- Montalva, C.; Valenzuela, E.; Barta, M.; Rojas, E.; Arismendi, N.; Rodrigues, J.; Humber, R. Lecanicillium attenuatum isolates affecting the invasive cypress aphid (Cinara cupressi) in Chile. BioControl 2017, 62, 625–637. [Google Scholar] [CrossRef]
- Barta, M.; Cagáň, Ľ. Aphid-pathogenic Entomophthorales (their taxonomy, biology and ecology). Biologia 2006, 61, 543–616. [Google Scholar] [CrossRef]
- Montalva, C.; Barta, M.; Rojas, E.; Gutiérrez, M.; Valenzuela, E. Neozygites species associated with aphids in Chile: Current status and new reports. Mycotaxon 2014, 129, 233–245. [Google Scholar] [CrossRef]
- Montalva, C.; Luz, C.; Humber, R. Neozygites osornensis (Neozygitales: Neozygitaceae) Infecting Cinara sp. (Hemiptera: Aphididae) in Brazil. Neotrop. Entomol. 2016, 45, 227–230. [Google Scholar] [CrossRef]
- Hountondji, F.C.C.; Lomer, C.J.; Hanna, R.; Cherry, A.J.; Dara, S.K. Field evaluation of Brazilian isolates of Neozygites floridana (Entomophthorales: Neozygitaceae) for the microbial control of cassava green mite in Benin, West Africa. Biocontrol Sci. Technol. 2002, 12, 361–370. [Google Scholar] [CrossRef]
- Echeverri-Molina, D.; Santolamazza-Carbone, S. Toxicity of synthetic and biological insecticides against adults of the Eucalyptus snout-beetle Gonipterus scutellatus Gyllenhal (Coleoptera: Curculionidae). J. Pest Sci. 2010, 83, 297–305. [Google Scholar] [CrossRef]
- Hajek, A.E.; van Frankenhuyzen, K. Use of entomopathogens against forest pests. In Microbial Control of Insect and Mite Pests: From Theory to Practice; Lacey, L.A., Ed.; Elsevier: Cambridge, MA, USA, 2017; pp. 313–330. [Google Scholar]
- Gencer, D.; Bayramoglu, Z.; Nalcacioglu, R.; Kleespies, R.G.; Demirbag, Z.; Demir, I. Characterisation of three Alphabaculovirus isolates from the gypsy moth, Lymantria dispar dispar (Lepidoptera: Erebidae), in Turkey. Biocontrol Sci. Technol. 2018, 28, 107–121. [Google Scholar] [CrossRef]
- Blackburn, L.M.; Hajek, A.E. Gypsy Moth Larval Necropsy Guide; General Technical Report NRS-179; USDA Forest Service, Northern Research Station: Newtown Square, PA, USA, 2018; p. 30. [Google Scholar] [CrossRef]
- Soper, R.S.; Shimazu, M.; Humber, R.A.; Ramos, M.E.; Hajek, A.E. Isolation and characterization of Entomophaga maimaiga sp. nov., a fungal pathogen of gypsy moth, Lymantria dispar, from Japan. J. Invertebr. Pathol. 1988, 51, 229–241. [Google Scholar] [CrossRef]
- Speare, A.T.; Colley, R.H. The Artificial Use of the Brown-Tail Fungus in Massachusetts, with Practical Suggestions for Private Experiment, and a Brief Note on a Fungous Disease of the Gypsy Caterpillar; Wright and Potter Printing Co.: Boston, MA, USA, 1912. [Google Scholar]
- Andreadis, T.G.; Weseloh, R.M. Discovery of Entomophaga maimaiga in North American gypsy moth, Lymantria dispar. Proc. Natl. Acad. Sci. USA 1990, 87, 2461–2465. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Milgroom, M.G.; Hajek, A.E. Genetic diversity in the gypsy moth fungal pathogen Entomophaga maimaiga from founder populations in North America and source populations in Asia. Mycol. Res. 2005, 109, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E. Introduction of the entomopathogenic fungus Entomophaga maimaiga into North America. In Biological Control: A Global Perspective; Vincent, C., Goettel, M.S., Lazarovits, G., Eds.; CABI Publishing: Wallingford, UK, 2007; pp. 53–62. [Google Scholar]
- Tobin, P.C.; Hajek, A.E. Release, establishment, and initial spread of the fungal pathogen Entomophaga maimaiga in island populations of Lymantria dispar. Biol. Control 2012, 63, 31–39. [Google Scholar] [CrossRef]
- Pilarska, D.; McManus, M.; Hajek, A.; Herard, F.; Vega, F.; Pilarski, P.; Markova, G. Introduction of the entomopathogenic fungus Entomophaga maimaiga Hum., Shim. & Sop. (Zygomycetes: Entomophtorales) to a Lymantria dispar (L.) (Lepidoptera: Lymantriidae) population in Bulgaria. J. Pest Sci. 2000, 73, 125–126. [Google Scholar]
- Pilarska, D.; McManus, M.; Pilarski, P.; Georgiev, G.; Mirchev, P.; Linde, A. Monitoring the establishment and prevalence of the fungal entomopathogen Entomophaga maimaiga in two Lymantria dispar L. populations in Bulgaria. J. Pest Sci. 2006, 79, 63–67. [Google Scholar] [CrossRef]
- Pilarska, D.; Todorov, M.; Pilarski, P.; Djorova, V.; Solter, L.; Georgiev, G. Bioassays for detection of the entomopathogenic fungus Entomophaga maimaiga (Entomophthorales: Entomophthoraceae) in soil from different sites in Bulgaria. Acta Zool. Bulg. 2013, 65, 173–177. [Google Scholar]
- Georgiev, G.; Mirchev, P.; Rossnev, B.; Petkov, P.; Georgieva, M.; Pilarska, D.; Golemansky, V.; Pilarski, P.; Hubenov, Z. Potential of Entomophaga maimaiga for suppressing Lymantria dispar outbreaks in Bulgaria. Cr. Acad. Bulg. Sci. 2013, 66, 1025–1032. [Google Scholar]
- Kereselidze, M.; Pilarska, D.; Hajek, A.; Jensen, A.B.; Linde, A. First record of Entomophaga maimaiga Humber, Shimazu & Soper (Entomophthorales: Entomophthoraceae) in Georgia. Biocontrol Sci. Technol. 2011, 21, 1375–1380. [Google Scholar] [CrossRef]
- Georgiev, G.; Mirchev, P.; Georgieva, M.; Rossnev, B.; Petkov, P.; Matova, M.; Kitanova, S. First record of entomopathogenic fungus Entomophaga maimaiga Humber, Shimazu and Soper (Entomophthorales: Entomophthoraceae) in Lymantria dispar (Linnaeus) (Lepidoptera: Lymantriidae) in Turkey. Acta Zool. Bulg. 2012, 64, 123–127. [Google Scholar]
- Tabaković-Tošić, M.; Georgiev, G.; Mirchev, P.; Tošić, D.; Golubović-Ćurguz, V. Entomophaga maimaiga–new entomopathogenic fungus in the Republic of Serbia. Afr. J. Biotechnol. 2012, 34, 8571–8577. [Google Scholar] [CrossRef]
- Hrašovec, B.; Pernek, M.; Lukić, I.; Milotić, M.; Diminić, D.; Franjević, M.; Hajek, A.; Linde, A.; Pilarska, D. First record of the pathogenic fungus Entomophaga maimaiga Humber, Shimazu, and Soper (Entomophthorales: Entomophthoraceae) within an outbreak populations of Lymantria dispar (Lepidoptera: Erebidae) in Croatia. Period. Biol. 2013, 115, 379–383. [Google Scholar]
- Csóka, G.Y.; Hirka, A.; Szőcs, L.; Hajek, A.E. First occurrence of the entomopathogenic fungus, Entomophaga maimaiga Humber, Shimazu and Soper (Entomophthorales: Entomophthoraceae) in Hungarian gypsy moth (Lymantria dispar) populations. Növényvédelem 2014, 50, 257–262. [Google Scholar]
- Zúbrik, M.; Barta, M.; Pilarska, D.; Goertz, D.; Úradník, M.; Galko, J.; Vakula, J.; Gubka, A.; Rell, S.; Kunca, A. First record of Entomophaga maimaiga (Entomophthorales: Entomophthoraceae) in Slovakia. Biocontrol. Sci. Technol. 2014, 24, 710–714. [Google Scholar] [CrossRef]
- Zúbrik, M.; Hajek, A.; Pilarska, D.; Spilda, I.; Georgiev, G.; Hrasovec, B.; Hirka, A.; Goertz, D.; Hoch, G.; Barta, M.; et al. The potential for Entomophaga maimaiga to regulate gypsy moth Lymantria dispar (L.) (Lepidoptera: Erebidae) in Europe. J. Appl. Entomol. 2016, 140, 565–579. [Google Scholar] [CrossRef]
- Dubois, T.; Li, Z.; Jaifu, H.; Hajek, A.E. Efficacy of fiber bands impregnated with Beauveria brongniartii cultures against the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Biol. Control 2004, 31, 320–328. [Google Scholar] [CrossRef]
- Hajek, A.E.; Lund, J.; Smith, M.T. Reduction in fitness of female Asian longhorned beetle (Anoplophora glabripennis) infected with Metarhizium anisopliae. J. Invertebr. Pathol. 2008, 98, 198–205. [Google Scholar] [CrossRef]
- Ugine, T.A.; Jenkins, N.E.; Gardescu, S.; Hajek, A.E. Comparing fungal band formulations for Asian longhorned beetle biological control. J. Invertebr. Pathol. 2013, 113, 240–246. [Google Scholar] [CrossRef]
- Shanley, R.P.; Keena, M.; Wheeler, M.M.; Leland, J.; Hajek, A.E. Evaluating the virulence and longevity of non-woven fiber bands impregnated with Metarhizium anisopliae against Asian longhorned beetle, Anoplophora glabripennis (Coleoptera: Cerambycidae). Biol. Conserv. 2009, 50, 94–102. [Google Scholar] [CrossRef]
- Fisher, J.J.; Castrillo, L.A.; Donzelli, B.G.; Hajek, A.E. Starvation and imidacloprid exposure influence immune response by Anoplophora glabripennis (Coleoptera: Cerambycidae) to a fungal pathogen. J. Econ. Entomol. 2017, 110, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.J.; Hajek, A.E. Influence of mating and age on susceptibility of the beetle Anoplophora glabripennis to the fungal pathogen Metarhizium brunneum. J. Invertebr. Pathol. 2016, 136, 142–148. [Google Scholar] [CrossRef]
- Fisher, J.J.; Hajek, A.E. Thermoregulatory behavior and fungal infection of Anoplophora glabripennis (Coleoptera: Cerambycidae). Environ. Entomol. 2014, 43, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Goble, T.A.; Rehner, S.A.; Long, S.J.; Gardescu, S.; Hajek, A.E. Comparing virulence of North American Beauveria brongniartii and commercial pathogenic fungi against Asian longhorned beetles. Biol. Conserv. 2014, 72, 91–97. [Google Scholar] [CrossRef]
- Kashio, T.; Ujiye, T. Evaluation of the use of entomogenous fungus, Beauveria tenella, isolated from the yellow spotted longicorn beetle, Psacothea hilaris for the biological control of white spotted longicorn beetle Anoplophora malasiaca. Proc. Assoc. Plant Prot. Kyushu 1988, 34, 190–193. [Google Scholar] [CrossRef]
- Higuchi, T.; Saika, T.; Senda, S.; Mizobata, T.; Kawata, Y.; Nagai, J. Development of biorational pest control formulation against longicorn beetles using a fungus, Beauveria brongniartii (Sacc.). Petch. J. Biosci. Bioeng. 1997, 84, 236–243. [Google Scholar] [CrossRef]
- Liu, H.; Bauer, L.S. Microbial control of emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae) with Beauveria bassiana strain GHA: Greenhouse and field trials. Biol. Conserv. 2008, 45, 124–132. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Griggs, M.H.; Liu, H.; Bauer, L.S.; Vandenberg, J.D. Assessing deposition and persistence of Beauveria bassiana GHA (Ascomycota: Hypocreales) applied for control of the emeralad ash borer, Agrilus planipennis (Coleoptera: Buprestidae) in a commercial tree nursery. Biol. Conserv. 2010, 54, 61–67. [Google Scholar] [CrossRef]
- Lyons, D.B.; Lavallée, R.; Keyi-Poku, G.; Van Frankenhuyzen, K.; Johny, S.; Guertin, C.; Francese, J.A.; Jones, G.C.; Blais, M. Towards the development of an autocontamination trap system to manage populations of emerald ash borer (Coleoptera: Buprestidae) with the native entomopathogenic fungus, Beauveria bassiana. J. Econ. Entomol. 2012, 105, 1929–1939. [Google Scholar] [CrossRef]
- Johny, S.; Kyei-Poku, G.; Gauthier, D.; van Frankenhuyzen, K. Isolation and characterisation of Isaria farinosa and Purpureocillium lilacinum associated with emerald ash borer, Agrilus planipennis in Canada. Biocontrol Sci. Technol. 2012, 22, 723–732. [Google Scholar] [CrossRef]
- Lyons, B.; van Frankenhuyzen, K.; Kyei-Pokua, G.; Johny, S.; Guertin, C.; Lavallée, R.; Jones, G.C.; Blais, M. The use of fluorescent powders to track autocontamination of emerald ash borer (Coleoptera: Buprestidae) by the entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Biocontrol Sci. Technol. 2016, 26, 1113–1128. [Google Scholar] [CrossRef]
- Sparks, B.; Braman, S.K.; Nair, S. Control of Lace Bugs on Ornamental Plants; The University of Georgia, UGA Extension, Bulletin No. 1102; The University of Georgia: Athens, GA, USA, 2015; p. 2. [Google Scholar]
- Sönmez, E.; Demirbağ, Z.; Demir, I. Pathogenicity of selected entomopathogenic fungal isolates against the oak lace bug, Corythucha arcuata Say (Hemiptera: Tingidae), under controlled conditions. Turk. J. Agric. For. 2016, 40, 715–722. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).