Effect of Abscisic Acid (ABA) Combined with Two Different Beekeeping Nutritional Strategies to Confront Overwintering: Studies on Honey Bees’ Population Dynamics and Nosemosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Location Study
2.2. Honey Bees
2.3. Phytochemical
2.4. Treatments
- Control syrup (CS): Five (5) A. mellifera colonies integrated in one (1) Langstroth unit (breeding chamber) were fed with 2 L of sugar syrup 2:1(sugar:water, v:v) weekly, using a Doolittle feeder, until the breeding chamber was full (blocked) of syrup (4 total applications between May 8th to May 29th).
- ABA syrup (AS): Five (5) A. mellifera colonies integrated in one (1) Langstroth unit (breeding chamber) were fed with a supplementary diet of 2 L sugar syrup 2:1 (sugar:water, v:v) + ABA 50µM (dissolved in the syrup) weekly, using a Doolittle feeder, until the breeding chamber was full (blocked) of syrup (4 total applications between May 8th to May 29th).
- Control honey (CH): Five (5) A. mellifera colonies integrated into two (2) Langstroth units, were organized in the upper chamber with 3 combs of honey + 7 empty combs. The honey of the combs corresponded to the one produced by the colonies in the latest season before this experiment. In one of the empty combs that was placed in-between the combs with honey, we applied 0.2 L of sugar syrup 2:1(sugar:water, v:v), two (2) times per week (Monday and Friday), for 4 weeks (8 total applications comprised between May 8th to June 2nd).
- ABA honey (AH): Five (5) A. mellifera colonies integrated into two (2) Langstroth units, were organized in the upper chamber with 3 combs of honey + 7 empty combs. The honey of the combs corresponded to the one produced by the colonies in the latest season before this experiment. In one of the empty combs that was placed in-between the combs with honey, we applied 0.2 L of sugar syrup 2:1(sugar:water, v:v) + ABA 50µM (dissolved in the syrup) two (2) times per week, for 4 weeks (8 total applications comprised between May 8th to June 2nd).
2.5. Colony Population Dynamics
2.6. Nosema Quantification
2.7. Statistics
2.7.1. Colony Population Dynamics
2.7.2. Nosema Prevalence and Intensity
3. Results
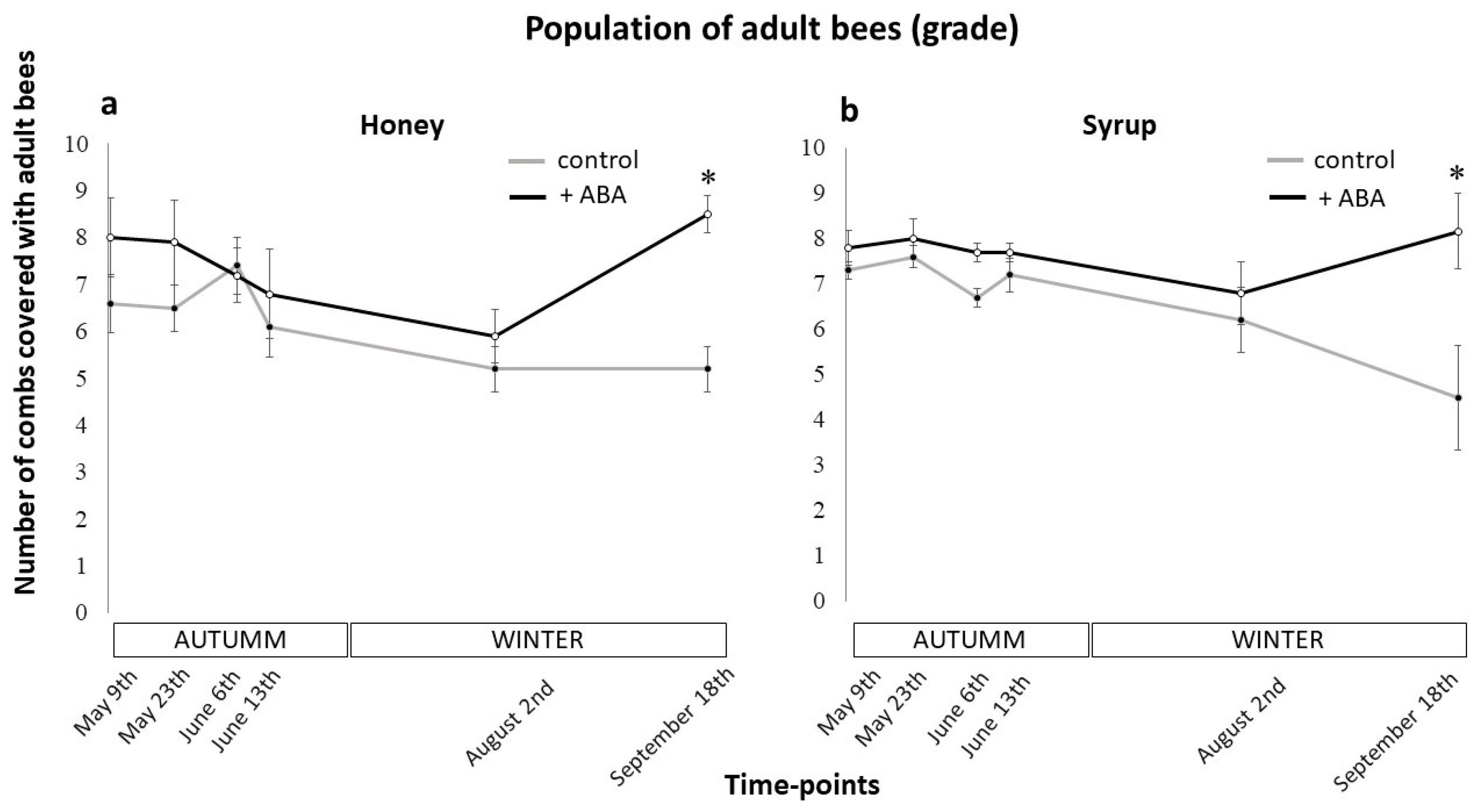
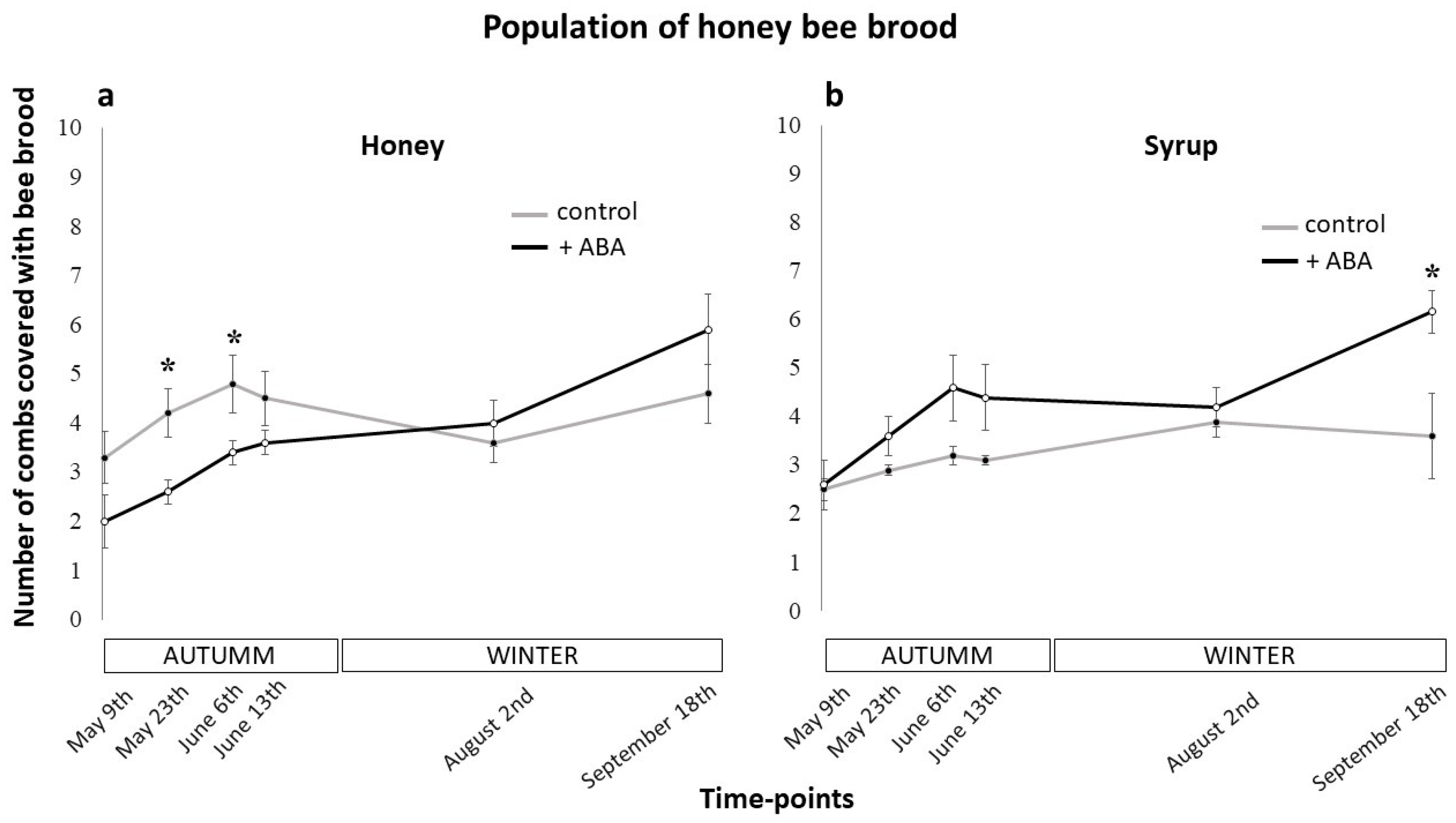
3.1. Effects of ABA Dietary Supplementation on Colony Population Dynamics
3.2. Effects of ABA Dietary Supplementation on Nosema Levels
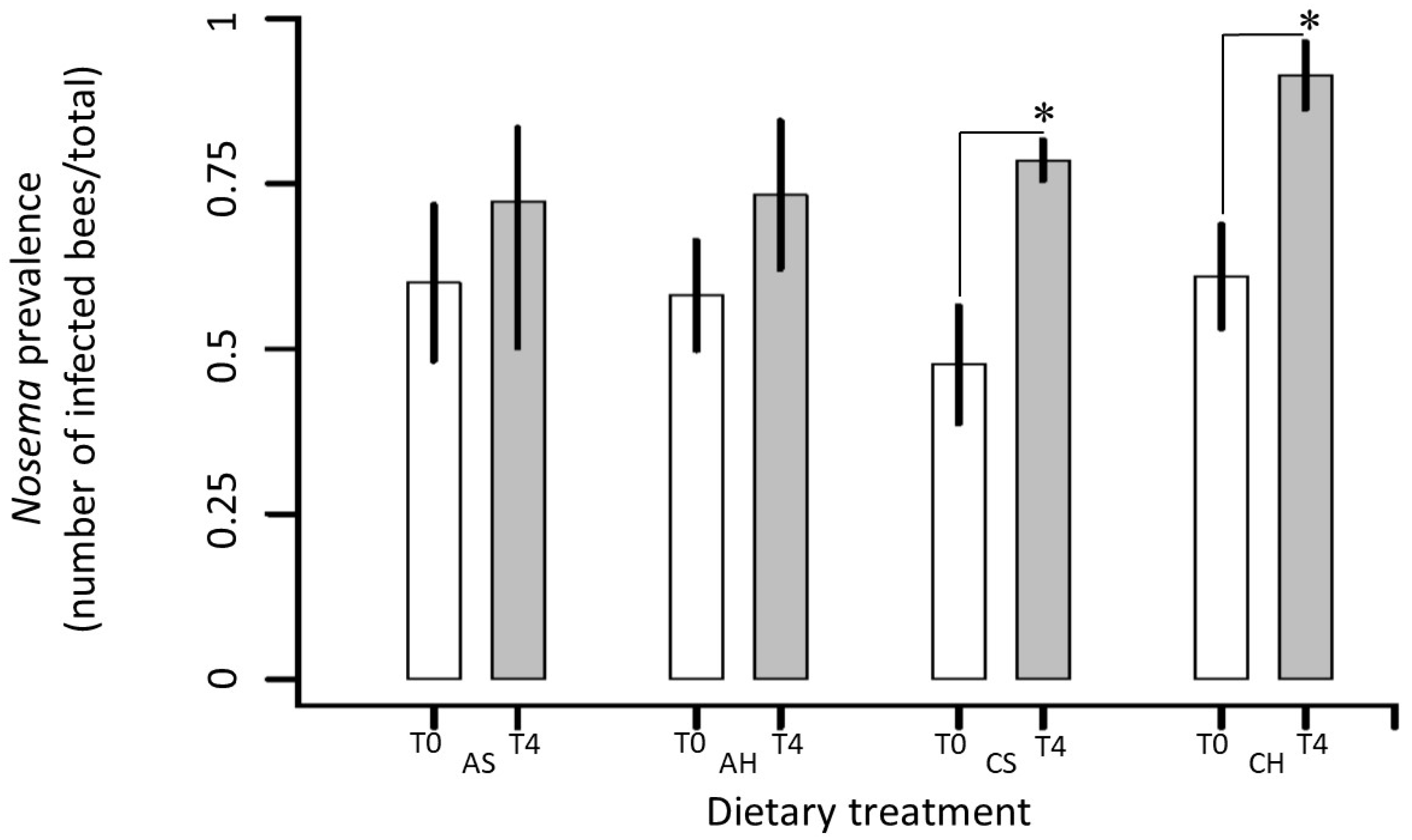
3.2.1. Nosemosis at Colony Level
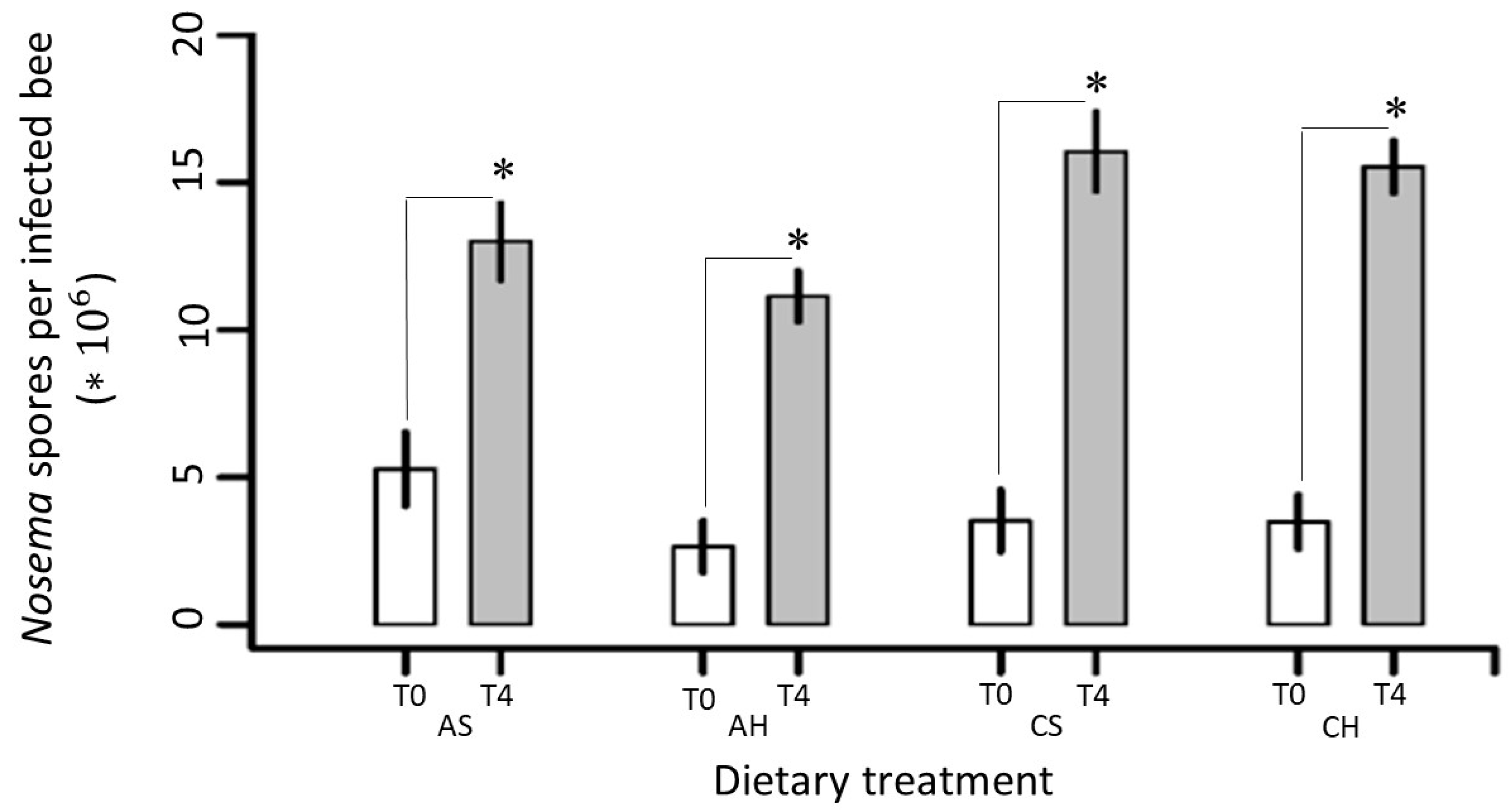
3.2.2. Nosemosis at Individual Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2006, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Eardley, C.; Freitas, B.M.; Kevan, P.G.; Rader, R. Background to Pollinators, Pollination and Food Production. In The Assessment Report on Pollinators, Pollination and Food Production; Potts, S.G., Imperatriz-Fonseca, V.L., Ngo, H.T., Eds.; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; pp. 1–25. [Google Scholar]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, D.; Langeveldeb, F.V. Interacting effects of landscape context and habitat quality on flower visiting insects in agricultural landscapes. Basic Appl. Ecol. 2006, 7, 201–214. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef] [PubMed]
- Van Engelsdorp, D.; Caron, D.; Hayes, J.; Underwood, R.; Henson, M.; Rennich, K.; Spleen, A.; Andree, M.; Snyder, R.; Lee, K.; et al. A national survey of managed honey bee 2010–11 winter colony losses in the USA: Results from the Bee Informed Partnership. J. Apic. Res. 2012, 51, 115–124. [Google Scholar] [CrossRef]
- Maggi, M.; Antúnez, K.; Invernizzi, C.; Aldea, P.; Vargas, M.; Negri, P.; Brasesco, C.; De Jong, D.; Message, D.; Teixeira, E.W. Honeybee health in South America. Apidologie 2016, 47, 835–854. [Google Scholar] [CrossRef]
- Moritz, R.F.A.; Erler, S. Lost colonies found in a data mine: Global honey trade but not pests or pesticides as a major cause of regional honeybee colony declines. Agric. Ecosyst. Environ. 2016, 216, 44–50. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Pettis, J.S. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Vedova, G.D.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef]
- De Grandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Blacquière, T. The Darwin cure for apiculture? Natural selection and managed honeybee health. Evol. Appl. 2017, 10, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. R. Soc. B 2017, 284, 20171711. [Google Scholar] [CrossRef] [PubMed]
- Döke, M.A.; Frazier, M.; Grozinger, C.M. Overwintering honey bees: Biology and management. Curr. Opin. Insect Sci. 2015, 10, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, X.; Zhu, X.; Chen, L.; Zhou, S.; Huang, Z.Y.; Zhou, B. Low-temperature stress during capped brood stage increases pupal mortality, misorientation and adult mortality in honey bees. PLoS ONE 2016, 11, e0154547. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, L.; Negri, P.; Sturla, L.; Guida, L.; Vigliarolo, T.; Maggi, M.; Eguaras, M.; Zocchi, E.; Lamattina, L. Abscisic acid enhances cold tolerance in honey bee larvae. Proc. Biol. Sci. 2017, 284, 20162140. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Nozal, M.J.; Bernal, J.L.; Jiménez, J.J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Van Dooremalen, C.; Gerritsen, L.; Cornelissen, B.; van der Steen, J.J.; van Langevelde, F.; Blacquière, T. Winter survival of individual honey bees and honey bee colonies depends on level of Varroa destructor infestation. PLoS ONE 2012, 7, e36285. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Fries, I. Nosema Apis—A Parasite in the Honey Bee Colony. Bee World 1993, 74, 5–19. [Google Scholar] [CrossRef]
- Higes, M.; Martín, R.; Meana, A. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 2006, 92, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Fries, I.; Martín, R.; Meana, A.; García-Palencia, P.; Higes, M. Natural infections of Nosema ceranae in European honeybees. J. Apic. Res. 2006, 45, 230–233. [Google Scholar] [CrossRef]
- Medici, S.K.; Sarlo, E.G.; Porrini, M.P.; Braunstein, M.; Eguaras, M.J. Genetic variation and widespread dispersal of Nosema ceranae in Apis mellifera apiaries from Argentina. Parasitol. Res. 2012, 110, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Sarlo, E.G.; Medici, S.K.; Porrini, M.P.; Garrido, P.M.; Floris, I.; Eguaras, M.J. Comparison between different fumagillin dosage and evaluation method in the apiary control of Nosemosis type C. Redia 2012, 94, 39–44. [Google Scholar]
- Williams, G.R.; Sampson, M.A.; Shutler, D.; Rogers, R.E. Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J. Invertebr. Pathol. 2008, 99, 342–344. [Google Scholar] [CrossRef]
- Lopez, M.I.; Pettis, J.S.; Smith, I.B.; Chu, P.S. Multiclass determination and confirmation of antibiotic residues in honey using LC-MS/MS. J. Agric. Food Chem. 2008, 56, 1553–1559. [Google Scholar] [CrossRef]
- van den Heever, J.P.; Thompson, T.S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Fumagillin: An overview of recent scientific advances and their significance for apiculture. J. Agric. Food Chem. 2014, 62, 2728–2737. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Cruz, M.S.; Benítez-Ahrendts, M.R.; Audisio, M.C. Beneficial effects of Bacillus subtilis subsp. subtilis Mori2, a honey-associated strain, on honeybee colony performance. Probiotics Antimicrob. Prot. 2012, 4, 39–46. [Google Scholar]
- Maggi, M.; Negri, P.; Plischuk, S.; Szawarski, N.; De Piano, F.; De Feudis, L.; Eguaras, M.; Audisio, C. Effects of the organic acids produced by a lactic acid bacterium in Apis mellifera colony development, Nosema ceranae control and fumagillin efficiency. Vet. Microbiol. 2013, 167, 474–483. [Google Scholar] [CrossRef]
- De Piano, F.G.; Maggi, M.; Pellegrini, M.C.; Cugnata, N.M.; Szawarski, N.; Buffa, F.; Negri, P.; Fuselli, S.R.; Audisio, A.M.; Ruffinengo, S.R. Effects of lactobacillus johnsonii AJ5 metabolites on nutrition, Nosema ceranae development and performance of Apis Mellifera, L. J. Apic. Sci. 2017, 61, 93–104. [Google Scholar] [CrossRef][Green Version]
- Couvillon, M.J.; Al Toufailia, H.; Butterfield, T.M.; Schrell, F.; Ratnieks, F.L.; Schürch, R. Caffeinated forage tricks honeybees into increasing foraging and recruitment behaviors. Curr. Biol. 2015, 25, 2815–2818. [Google Scholar] [CrossRef] [PubMed]
- Erler, S.; Moritz, R.F. Pharmacophagy and pharmacophory: Mechanisms of self-medication and disease prevention in the honey bee colony (Apis mellifera). Apidologie 2016, 47, 389–411. [Google Scholar] [CrossRef]
- Palmer-Young, E.C.; Sadd, B.M.; Irwin, R.E.; Adler, L.S. Synergistic effects of floral phytochemicals against a bumble bee parasite. Ecol. Evol. 2017, 7, 1836–1849. [Google Scholar] [CrossRef]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2013, 110, 8842–8846. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.L.; Adler, L.S.; Leonard, A.S.; Andicoechea, J.; Regan, K.H.; Anthony, W.E.; Manson, J.S.; Irwin, R.E. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. B 2015, 282, 2014–2471. [Google Scholar] [CrossRef]
- Baracchi, D.; Brown, M.J.; Chittka, L. Behavioural evidence for self-medication in bumblebees? F1000Research 2015, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Palmer-Young, E.C.; Tozkar, C.Ö.; Schwarz, R.S.; Chen, Y.; Irwin, R.E.; Adler, L.S.; Evans, J.D. Nectar and pollen phytochemicals stimulate honey bee (Hymenoptera: Apidae) immunity to viral infection. J. Econ. Entom. 2017, 110, 1959–1972. [Google Scholar] [CrossRef]
- Lipp, J. Nachweis und herkunft von abscisinsäure und prolin in honig. Apidologie 1990, 21, 249–259. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Bifulco, E.; Caboni, P.; Cottiglia, F.; Cabras, P.; Floris, I. Floral markers of strawberry tree (Arbutus unedo L.) honey. J. Agric. Food Chem. 2010, 58, 384–389. [Google Scholar] [CrossRef]
- Negri, P.; Maggi, M.D.; Ramirez, L.; De Feudis, L.; Szawarski, N.; Quintana, S.; Eguaras, M.; Lamattina, L. Abscisic acid enhances the immune response in Apis mellifera and contributes to the colony fitness. Apidologie 2015, 46, 542–557. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.K. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Abrahamovich, A.; Atela, O.; De la Rúa, P.; Galián, J. Assessment of the mitochondrial origin of honey bees from Argentina. J. Apic. Res. 2007, 46, 191–194. [Google Scholar] [CrossRef]
- Delaplane, K.; Steen, J.; Guzman-Novoa, E. Standard methods for estimating strength parameters of Apis mellifera colonies. J. Apic. Res. 2013, 52, 1–12. [Google Scholar] [CrossRef]
- Meana, A.; Martin-Hernandez, R.; Higes, M. The reliability of spore counts to diagnose Nosema ceranae infections in honey bees. J. Apic. Res. 2010, 49, 212–214. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D. Mixed-effects models in S and S-PLUS, 1st ed.; Springer Science & Business Media: New York, NY, USA, 2000; 528p. [Google Scholar]
- Gelman, A.; Hill, J. Data analysis Using Regression and Multilevel/Hierarchical Models, 1st.; Cambridge University Press: Cambridge, UK, 2006; 648p. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P. Package ‘lme4′: Linear Mixed-Effects Models using ‘Eigen’ and S4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Negri, P.; Ramirez, L.; Quintana, S.; Szawarski, N.; Maggi, M.; Le Conte, Y.; Lamattina, L.; Eguaras, M. Dietary Supplementation of Honey Bee Larvae with Arginine and Abscisic Acid Enhances Nitric Oxide and Granulocyte Immune Responses after Trauma. Insects 2017, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.; Zaat, S.A. Antibacterial components of honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Brudzynski, K.; Sjaarda, C. Honey glycoproteins containing antimicrobial peptides, jelleins of the major royal jelly protein 1, are responsible for the cell wall lytic and bactericidal activities of honey. PLoS ONE 2015, 10, e0120238. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, W.J.; Yu, L.; Ding, J.; Feng, Y.Q. Comprehensive Profiling of Phytohormones in Honey by Sequential Liquid–Liquid Extraction Coupled with Liquid Chromatography–Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Ricigliano, V.A.; Mott, B.M.; Maes, P.W.; Floyd, A.S.; Fitz, W.; Copeland, D.C.; Meikle, W.G.; Anderson, K.E. Honey bee colony performance and health are enhanced by apiary proximity to US Conservation Reserve Program (CRP) lands. Sci. Rep. 2019, 9, 4894. [Google Scholar] [CrossRef]
- Meana, A.; Picher, M.L.; Euba, A.; Bernal, J.L.; Bernal, J.; Chao, M.G.; Dagnac, T.; Castro-Hermida, J.; González-Porto, A.; Higes, M.; et al. Risk factors associated with honey bee colony loss in apiaries in Galicia, NW Spain. Span. J. Agric. Res. 2017, 15, e0501. [Google Scholar] [CrossRef]
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.P.; van Engelsdorp, D. Drivers of colony losses. Curr. Opin. Insect Sci. 2018, 26, 142–148. [Google Scholar] [CrossRef]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet. Res. 2013, 44, 25. [Google Scholar]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef] [PubMed]
- Little, C.M.; Shutler, D.; Williams, G.R. Associations among Nosema spp. fungi, Varroa destructor mites, and chemical treatments in honey bees, Apis mellifera. J. Apic. Res. 2015, 54, 378–385. [Google Scholar] [CrossRef]
- Retschnig, G.; Williams, G.; Schneeberger, A.; Neumann, P. Cold ambient temperature promotes Nosema spp. intensity in honey bees (Apis mellifera). Insects 2017, 8, 20. [Google Scholar] [CrossRef]
- Jack, C.J.; Uppala, S.S.; Lucas, H.M.; Sagili, R.R. Effects of pollen dilution on infection of Nosema ceranae in honey bees. J. Insect Physiol. 2016, 87, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Porrini, M.P.; Sarlo, E.G.; Medici, S.K.; Garrido, P.M.; Porrini, D.P.; Damiani, N.; Eguaras, M.J. Nosema ceranae development in Apis mellifera: Influence of diet and infective inoculum. J. Apic. Res. 2011, 50, 35–41. [Google Scholar] [CrossRef]
- Basualdo, M.; Barragán, S.; Antúnez, K. Bee bread increases honeybee haemolymph protein and promote better survival despite of causing higher Nosema ceranae abundance in honeybees. Environ. Microbiol. Rep. 2014, 6, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Panek, J.; El Alaoui, H.; Mone, A.; Urbach, S.; Demettre, E.; Texier, C.; Brun, C.; Zanzoni, A.; Peyretaillade, E.; Parisot, N.; et al. Hijacking of the host cellular functions by an intracellular parasite, the microsporidian Anncaliia algerae. PLoS ONE 2014, 9, e100791. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szawarski, N.; Saez, A.; Domínguez, E.; Dickson, R.; De Matteis, Á.; Eciolaza, C.; Justel, M.; Aliano, A.; Solar, P.; Bergara, I.; et al. Effect of Abscisic Acid (ABA) Combined with Two Different Beekeeping Nutritional Strategies to Confront Overwintering: Studies on Honey Bees’ Population Dynamics and Nosemosis. Insects 2019, 10, 329. https://doi.org/10.3390/insects10100329
Szawarski N, Saez A, Domínguez E, Dickson R, De Matteis Á, Eciolaza C, Justel M, Aliano A, Solar P, Bergara I, et al. Effect of Abscisic Acid (ABA) Combined with Two Different Beekeeping Nutritional Strategies to Confront Overwintering: Studies on Honey Bees’ Population Dynamics and Nosemosis. Insects. 2019; 10(10):329. https://doi.org/10.3390/insects10100329
Chicago/Turabian StyleSzawarski, Nicolás, Agustín Saez, Enzo Domínguez, Rachel Dickson, Ángela De Matteis, Carlos Eciolaza, Marcelino Justel, Alfredo Aliano, Pedro Solar, Ignacio Bergara, and et al. 2019. "Effect of Abscisic Acid (ABA) Combined with Two Different Beekeeping Nutritional Strategies to Confront Overwintering: Studies on Honey Bees’ Population Dynamics and Nosemosis" Insects 10, no. 10: 329. https://doi.org/10.3390/insects10100329
APA StyleSzawarski, N., Saez, A., Domínguez, E., Dickson, R., De Matteis, Á., Eciolaza, C., Justel, M., Aliano, A., Solar, P., Bergara, I., Pons, C., Bolognesi, A., Carna, G., Garcia, W., Garcia, O., Eguaras, M., Lamattina, L., Maggi, M., & Negri, P. (2019). Effect of Abscisic Acid (ABA) Combined with Two Different Beekeeping Nutritional Strategies to Confront Overwintering: Studies on Honey Bees’ Population Dynamics and Nosemosis. Insects, 10(10), 329. https://doi.org/10.3390/insects10100329




