Semiochemical and Communication Ecology of the Emerald Ash Borer, Agrilus planipennis (Coleoptera: Buprestidae)
Abstract
1. Background
2. Visual Cues
3. Host Kairomones
3.1. Bark Sesquiterpenes
3.2. Foliar Volatiles
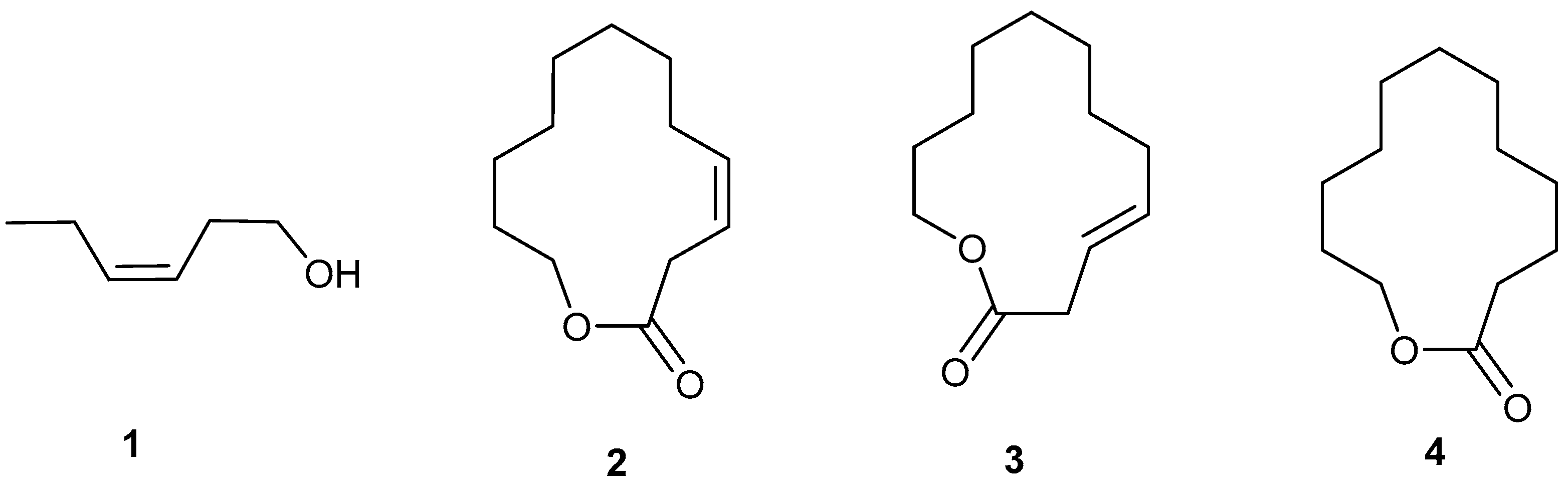
4. The Natural Female-Produced Sex Pheromone
5. Contact Chemoreception
6. Acoustics
7. Progress in Identifying Odor Processing Genes in EAB
8. Semiochemistry of EAB Parasitoids
9. Development of EAB Detection Tools
9.1. Trap Development
9.2. Effective Range of the Trap
9.3. Mechanism of Pheromonal Activity
10. Attractants for Other Agrilus Species
11. Pheromone Analogs
11.1. Macrocycle Isomers
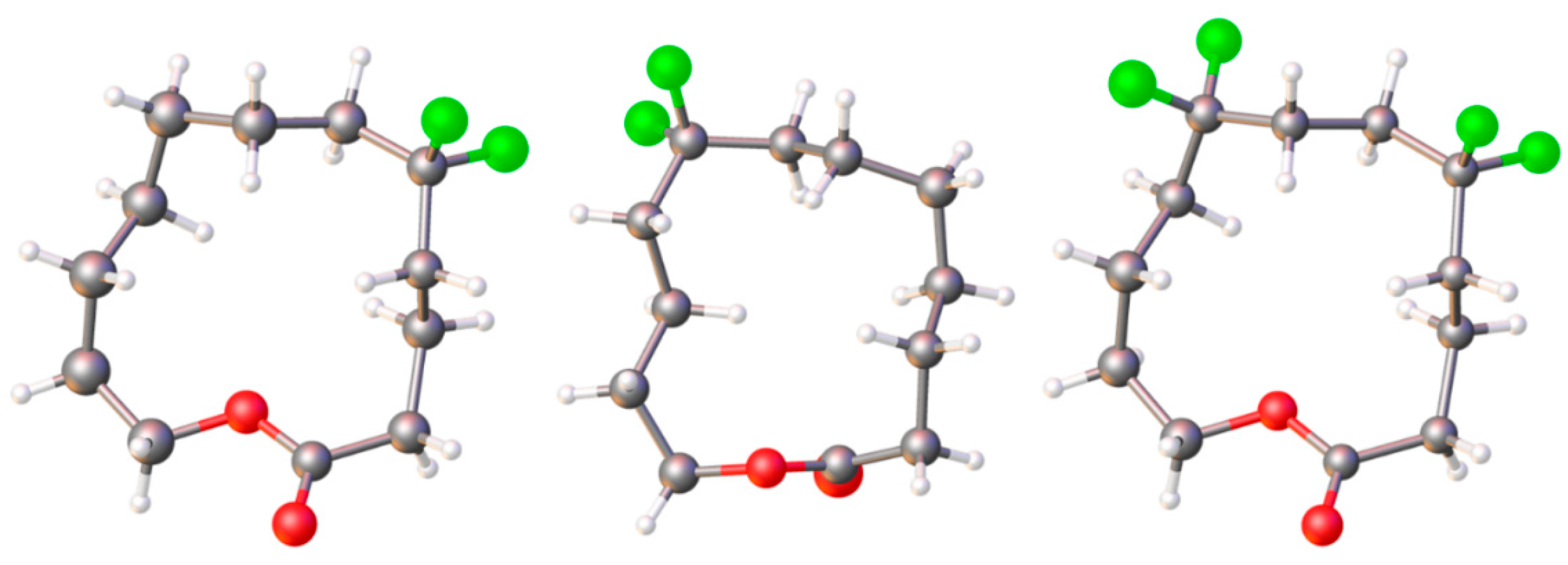
11.2. Macrocyclic Fluoro Analogs
12. Synthetic Chemistry of EAB Macrolides
12.1. Synthesis of (3Z)-12-Dodecenolide 2
- 1. O3, CH2Cl2, −78 °C. 2. PPh3, −78 °C—RT, 93%.
- 13 + Sodium bis(trimethylsilyl)amide, tetrahydrofuran (THF) and toluene, 0 °C—RT, then 12, −99 °C—RT, 90% yield, 98% Z-selectivity.
- TsOH, wet THF, reflux.
- NaClO2, H2NSO3H, 1-methyl-1-cyclohexene, CH2Cl2, H2O, 0 °C—RT, 70% over 2 steps.
- diisopropylazodicarboxylate (DIAD) or diethylazodicarboxylate (DEAD), PPh3, toluene, RT, 64% with DIAD; overall yield = 37% over 6 steps.
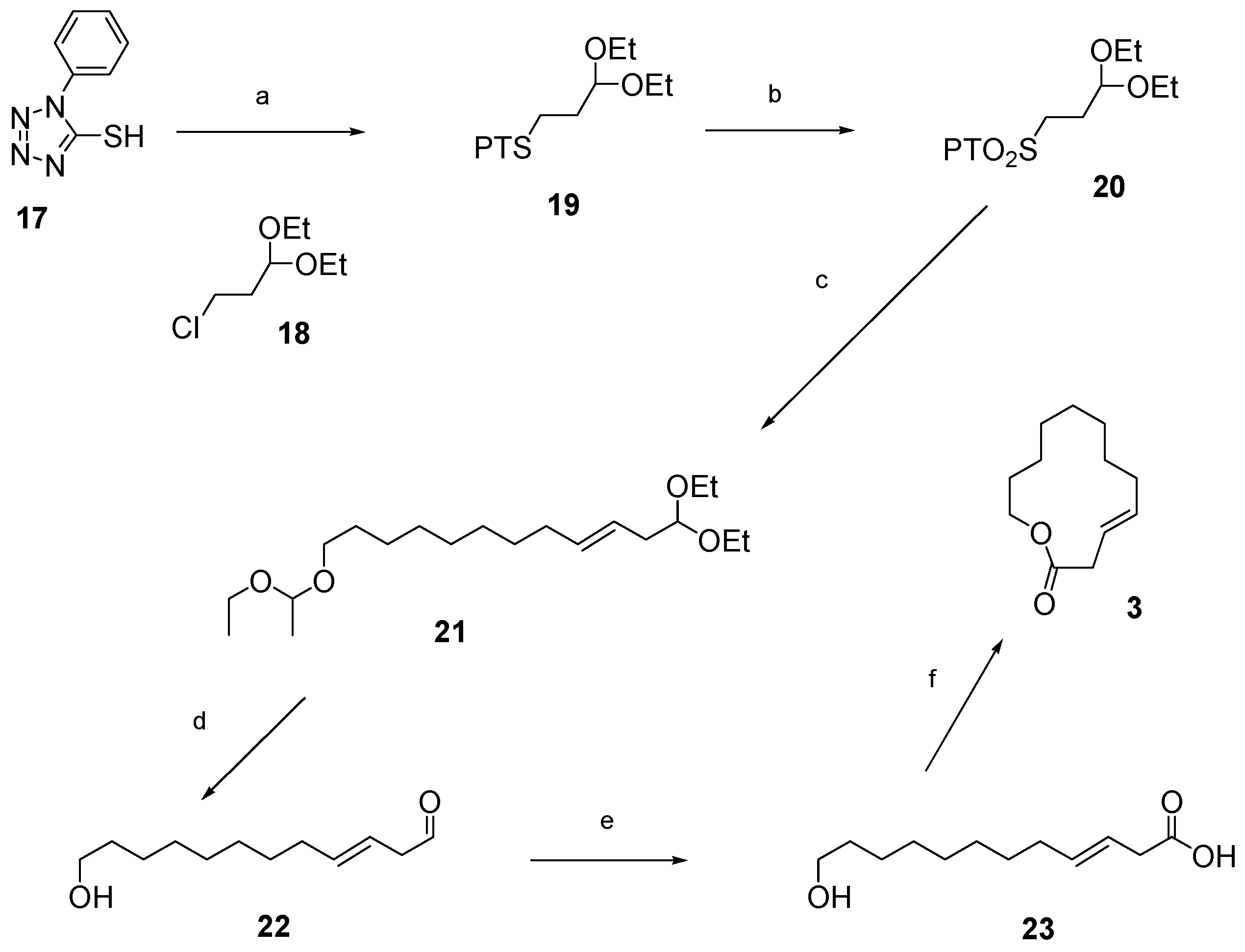
12.2. Synthesis of (3E)-12-Dodecenolide 3
- 1. NaH, DMF, 0 °C–90 °C. 2. 3-chloropropionaldehyde diethylacetal 18, NaI, 90 °C, 76%.
- (NH4)6Mo7O24·4H2O, H2O2, EtOH, RT, 73%.
- 1. Potassium bis(trimethylsilyl)amide, dimethyoxyethane (DME), −56 °C, then 12, −56 °C—RT, 60% yield, 97% E-selectivity.
- TsOH, wet THF, reflux.
- NaClO2, H2NSO3H, 1-methyl-1-cyclohexene, CH2Cl2, H2O, 0 °C—RT, 60% over 2 steps.
- DIAD or DEAD, PPh3, toluene, RT, 82% with DIAD; overall yield = 16% over 6 steps.
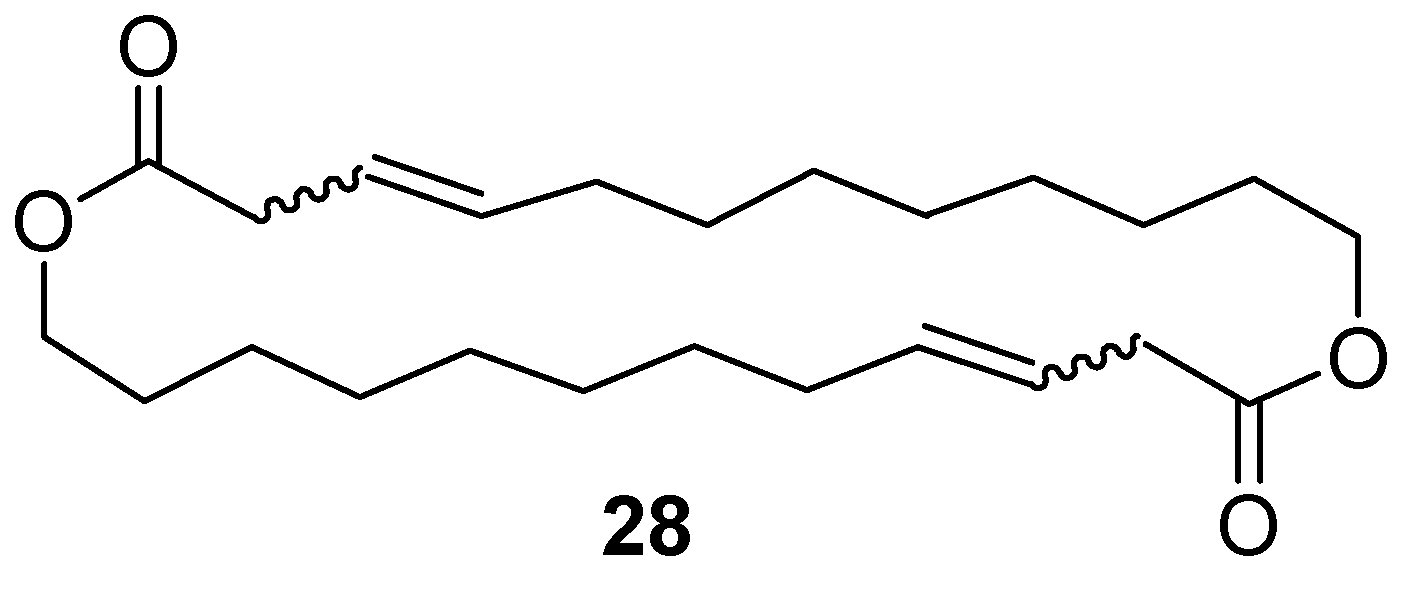
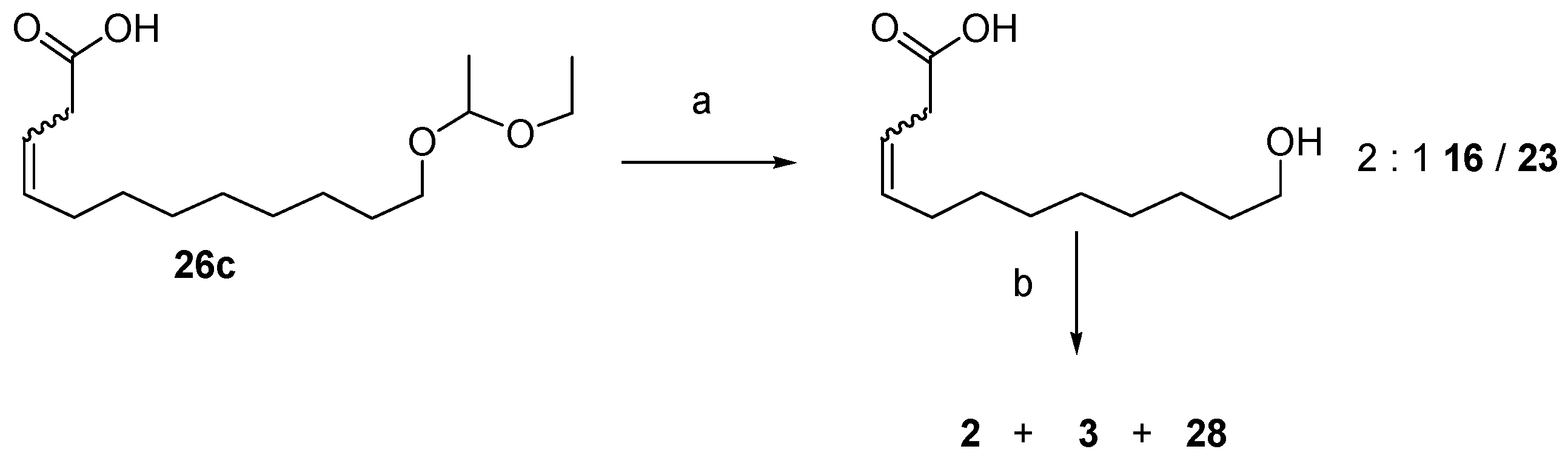
12.3. Synthesis of (3Z)- and (3E)-12-Dodecenolide Mixture (2 and 3)
- 2 equiv. sodium bis(trimethylsilyl)amide, THF, 0 °C—RT, then 25a, 25b or 12, −78 °C—RT, 70%, 67% and 61% yields, respectively and 2:1 Z/E in all 3 cases.
- For 25a and 25b, K2CO3, KI (only for 25a), acetone, reflux, 82% and 72% yields, respectively, 2:1 2:3 (Z:E) for both 26a and 26b.
- 1. O3, CH2Cl2, −78 °C. 2. PPh3, −78 °C—RT, 100% yield for 25a and 93% for 25b.
- HCl, H2O, THF, RT, 100%.
- DIAD, PPh3, toluene, RT, 84% combined yield of 2 and 3, ~5% 28. 2:3 = 2:1.
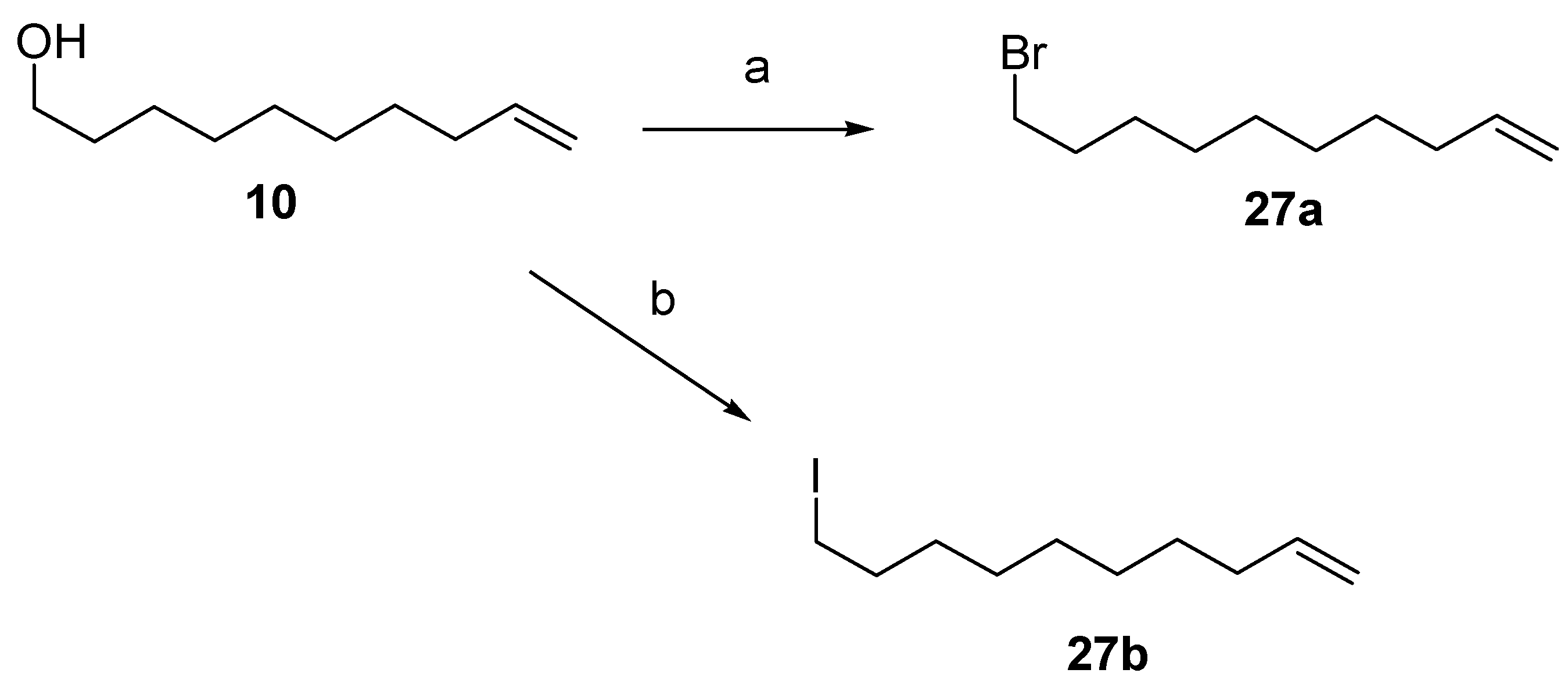
- CBr4, PPh3, CH2Cl2, 0 °C, 100%.
- I2, PPh3, imidazole, 1:3 CH3CN/Et2O, RT, 100%.
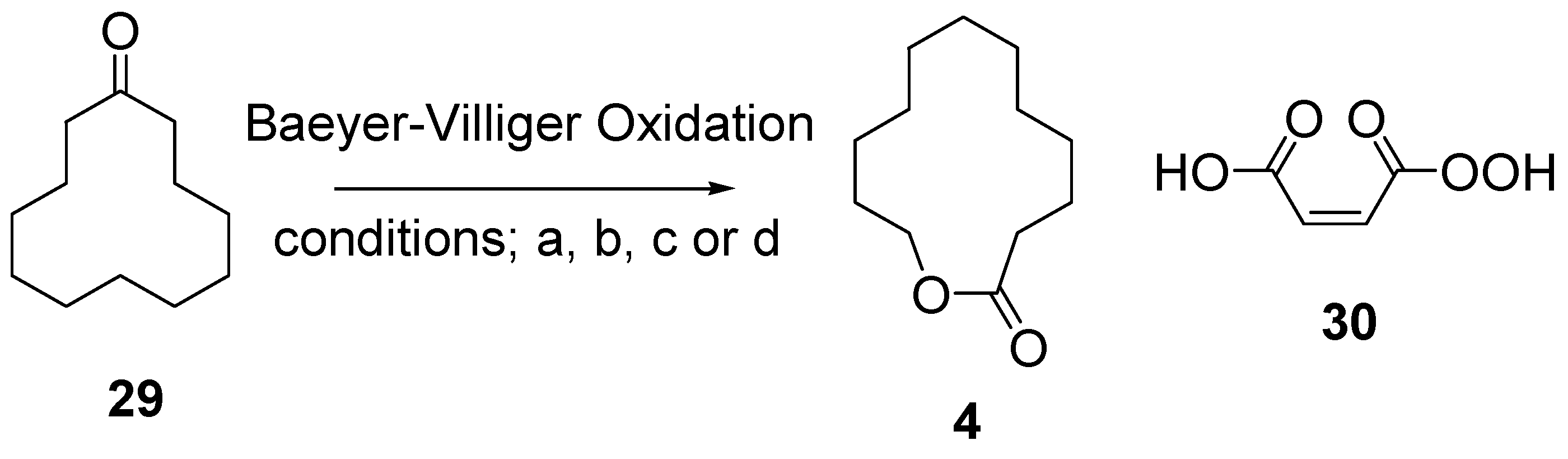
12.4. Synthesis of Saturated Lactone Analog, 12-Dodecanolide 4
- 2.1 equiv. mCPBA, 0.04 equiv. TsOH·H2O, CH2Cl2, RT, 87%.
- 10 equiv. mCPBA, 2 equiv. NaHCO3, CH2Cl2, RT, 59%.
- 10 equiv. H2O2 (35 wt% aqueous), 31 equiv. (CF3CO)2, 1 equiv. Na2HPO4·7H2O, CH2Cl2, RT, 72%.
- Permaleic acid 30 (generated in situ from maleic anhydride, 35 wt% aqueous H2O2 and acetic anhydride), CH2Cl2, RT, 75%.
13. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haack, R.A.; Jendek, E.; Liu, H.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The emerald ash borer: A new exotic pest in North America. Newsl. Mich. Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- Gwynne, D.T.; Rentz, D.C.F. Beetles on the bottle: Male buprestids mistake stubbies for females (Coleoptera). Aust. J. Entomol. 1983, 22, 79–80. [Google Scholar] [CrossRef]
- Dunn, J.P.; Potter, D.A. Evidence for sexual attraction by the twolined chestnut borer, Agrilus bilineatus (Weber) (Coleoptera: Buprestidae). Can. Entomol. 1988, 120, 1037–1039. [Google Scholar] [CrossRef]
- Carlson, R.W.; Knight, F.B. Biology, taxonomy, and evolution of sympatric Agrilus beetles (Coleoptera: Buprestidae). Contr. Am. Entomol. Inst. 1969, 3, 100–105. [Google Scholar]
- Akers, R.C.; Nielsen, D.G. Mating behavior of the bronze birch borer (Coleoptera: Buprestidae). J. Entomol. Sci. 1992, 27, 44–49. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Poland, T.M.; Miller, J.R.; Stelinski, L.L.; Grant, G.G.; de Groot, P.; Buchan, L.; MacDonald, L. Behavioral and electrophysiological responses of the emerald ash borer, Agrilus planipennis, to induced volatiles of Manchurian ash, Fraxinus mandshurica. Chemoecology 2006, 16, 75–86. [Google Scholar] [CrossRef]
- Lelito, J.P.; Fraser, I.; Mastro, V.C.; Tumlinson, J.H.; Böröczky, K.; Baker, T.C. Visually mediated ‘paratrooper copulations’ in the mating behavior of Agrilus planipennis (Coleoptera: Buprestidae), a highly destructive invasive pest of North American ash trees. J. Insect Behav. 2007, 20, 537–552. [Google Scholar] [CrossRef]
- Silk, P.J.; Ryall, K.; Lyons, B.; Sweeney, J.D.; Wu, J. A contact sex pheromone component of the emerald ash borer Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). Naturwissenschaften 2009, 96, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, R.; Cossé, A.A.; Zilkowski, B.W.; Fraser, I. Antennally active macrolide from the emerald ash borer Agrilus planipennis emitted predominantly by females. J. Chem. Ecol. 2007, 33, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Pureswaran, D.S.; Poland, T.M. The role of olfactory cues in short-range mate finding by the emerald ash borer, Agrilus planipennis, Fairmaire (Coleoptera: Cerambycidae). J. Insect Behav. 2009, 22, 205–216. [Google Scholar] [CrossRef]
- Francese, J.A.; Crook, D.J.; Fraser, I.; Lance, D.R.; Sawyer, A.J.; Mastro, V.C. Optimization of trap color for the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). J. Econ. Entomol. 2010, 103, 1235–1241. [Google Scholar] [CrossRef]
- Crook, D.J.; Khrimian, A.; Francese, J.A.; Fraser, I.; Poland, T.M.; Sawyer, A.J.; Mastro, V.C. Development of a host-based semiochemical 12lure for trapping emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Environ. Entomol. 2008, 37, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Silk, P.J.; Ryall, K.; Mayo, P.; Lemay, M.; Grant, G.; Crook, D.; Cossé, A.; Fraser, I.; Sweeney, J.D.; Lyons, D.B.; et al. Evidence for a volatile pheromone in Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) that increases attraction to a host foliar volatile. Environ. Entomol. 2011, 40, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Silk, P.J.; Ryall, K.L. Semiochemistry and chemical ecology of the emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Can. Entomol. 2015, 147, 277–289. [Google Scholar] [CrossRef]
- Lelito, J.P.; Böröczky, K.; Jones, T.H.; Frazer, I.; Mastro, V.C.; Tumlinson, J.H.; Baker, T.C. Behavioral evidence for a contact pheromone component of the emerald ash borer, Agrilus planipennis Fairmaire. J. Chem. Ecol. 2009, 35, 104–110. [Google Scholar] [CrossRef]
- Silk, P.J.; Price, J.; Brophy, M.; Roscoe, L.; Ryall, K.L. Influence of light on sound production behaviors in the Emerald Ash Borer, Agrilus planipennis Fairmaire. Entomol. Exp. Appl. 2018, 166, 844–853. [Google Scholar] [CrossRef]
- Crook, D.J.; Mastro, V.C. Chemical ecology of the emerald ash borer, Agrilus planipennis. J. Chem. Ecol. 2010, 36, 101–112. [Google Scholar] [CrossRef]
- Domingue, M.J.; Baker, T.C. A multidisciplinary approach for developing tools to monitor invasive buprestid beetle species. In Invasive Species: Threats, Ecological Impact and Control Methods; Blanco, J.J., Fernandes, A.T., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 77–100. [Google Scholar]
- Rodriguez-Saona, C.R.; Miller, J.R.; Poland, T.M.; Kuhn, T.M.; Otis, G.W.; Turk, T.; Ward, D.L. Behaviors of adult Agrilus planipennis (Coleoptera: Buprestidae). Great Lakes Entomol. 2007, 40, 1–16. [Google Scholar]
- Domingue, M.J.; Lelito, J.P.; Fraser, I.; Mastro, V.C.; Tumlinson, J.H.; Baker, T.C. Visual and chemical cues affecting the detection rate of the emerald ash borer in sticky traps. J. Appl. Entomol. 2011, 137, 77–87. [Google Scholar] [CrossRef]
- Domingue, M.; Andreadis, S.S.; Silk, P.J.; Ryall, K.; Baker, T.C. Interaction of visual and chemical cues in promoting attraction of Agrilus planipennis (Coleoptera:Buprestidae). J. Chem. Ecol. 2016, 42, 490–496. [Google Scholar] [CrossRef]
- Lelito, J.P.; Domingue, M.J.; Fraser, I.; Mastro, V.C.; Tumlinson, J.H.; Baker, T.C. Field investigation of mating behaviour of Agrilus cyanescens and Agrilus subcinctus. Can. Entomol. 2011, 143, 370–379. [Google Scholar] [CrossRef]
- Domingue, M.J.; Csóka, G.; Tóth, M.; Vétek, G.; Pénzes, B.; Mastro, V.C.; Baker, T.C. Field observations of visual attraction of three European oak buprestid beetles towards conspecific and heterospecific models. Entomol. Exp. Appl. 2011, 140, 112–121. [Google Scholar] [CrossRef]
- Domingue, M.J.; Imrei, Z.; Lelito, J.P.; József, M.; Janik, G.; Csóka, G.; Mastro, V.C.; Baker, T. Trapping of European buprestid beetles in oak forests using visual and olfactory cues. Entomol. Exp. Appl. 2013, 148, 116–129. [Google Scholar] [CrossRef]
- Lelito, J.P.; Fraser, I.; Mastro, V.C.; Tumlinson, J.H.; Baker, T.C. Novel visual-cue-based sticky traps for monitoring of emerald ash borers, Agrilus planipennis (Col., Buprestidae). J. Appl. Entomol. 2008, 132, 668–674. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts and management. Ann. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.G.; Ryall, K.L.; Lyons, D.B.; Abou-Zaid, M.M. Differential response of male and female emerald ash borers (Col., Buprestidae) to (Z)-3-hexenol and Manuka oil. J. Appl. Entomol. 2010, 134, 26–33. [Google Scholar] [CrossRef]
- Krimian, A.; Cossé, A.A.; Crook, D.J. Absolute configuration of 7-epi-sesquithujene. J. Nat. Prod. 2011, 74, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Crook, D.J.; Khrimian, A.; Cossé, A.; Fraser, I.; Mastro, V.C. Influence of trap color and host volatiles on capture of the emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2012, 105, 429–437. [Google Scholar] [CrossRef]
- Dunn, J.P.; Kimmerer, T.W.; Nordin, G.L. The role of host tree condition in attack of white oaks by the twolined chestnut borer, Agrilus bilineatus (Weber) (Coleoptera: Buprestidae). Oecologia 1986, 70, 596–600. [Google Scholar] [CrossRef]
- McCullough, D.G.; Poland, T.M.; Anulewicz, A.C.; Cappaert, D. Emerald ash borer (Coleoptera: Buprestidae) attraction to stressed or baited ash trees. Environ. Entomol. 2009, 38, 1668–1679. [Google Scholar] [CrossRef]
- McCullough, D.G.; Poland, T.M.; Cappaert, D. Attraction of the emerald ash borer to ash trees stressed by girdling, herbicide treatment or wounding. Can. J. For. Res. 2009, 38, 1331–1345. [Google Scholar] [CrossRef]
- Tluczek, A.R.; McCullough, D.G.; Poland, T.M. Influence of host stress on emerald ash borer (Coleoptera: Buprestidae) adult density, development, and distribution in Fraxinus pennsylvanica trees. Environ. Entomol. 2011, 40, 357–366. [Google Scholar] [CrossRef]
- Cossé, A.A.; Bartelt, R.J.; Zikowski, B.W.; Fraser, I. Identification and antennal electrophysiology of ash bark volatiles for emerald ash borer. In Proceedings of the Emerald Ash Borer and Asian Longhorned Beetle Research and Technology Development Meeting, Pittsburgh, Pennsylvania, 23–24 October 2007; Mastro, V., Lance, D., Reardon, R., Parra, G., Eds.; United States Department of Agriculture Forest Service Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2018; pp. 81–82. [Google Scholar]
- Grant, G.G.; Poland, T.M.; Ciaramitaro, T.; Lyons, D.B.; Jones, G.C. Comparison of male and female emerald ash borer (Coleoptera: Buprestidae) responses to Phoebe oil and (Z)-3-hexenol lures in light green prism traps. J. Econ. Entomol. 2011, 104, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.M.; Storer, A.J.; Fraser, I.; Mastro, V.C. Efficacy of trap and lure types for detection of Agrilus planipennis (Col, Buprestidae) at low density. J. Appl. Entomol. 2010, 134, 296–302. [Google Scholar] [CrossRef]
- DeGroot, P.; Grant, G.G.; Poland, T.M.; Scharbach, R.; Buchan, L.; Nott, R.W.; Macdonald, L.; Pitt, D. Electrophysiological response and attraction of emerald ash borer to green leaf volatiles (GLVs) emitted by host foliage. J. Chem. Ecol. 2008, 34, 1170–1179. [Google Scholar] [CrossRef]
- Ryall, K.; Silk, P.J.; Mayo, P.; Crook, D.; Khrimian, A.; Cossé, A.A.; Sweeney, J.; Scarr, T. Attraction of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) to a volatile pheromone: Effects of release rate, host volatile and trap placement. Environ. Entomol. 2012, 41, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Ryall, K.; Fidgen, J.G.; Silk, P.J.; Scarr, T. Efficacy of (3Z)-lactone and/or (3Z)-hexenol at detecting early infestation of Agrilus planipennis (Col., Buprestidae). Entomol. Exp. Appl. 2013, 147, 126–131. [Google Scholar] [CrossRef]
- Ryall, K.L.; Dutkiewicz, D.; Silk, P.J.; Antunes, P.M.; Ochoa, I. Ovarian development of Agrilus planipennis: Effects of age and mating status and influence on attraction to host volatiles. Entomol. Exp. Appl. 2013, 149, 77–84. [Google Scholar] [CrossRef]
- Millar, J.G.; Pierce, H.D.; Pierce, A.M.; Oehlschlager, A.C.; Borden, J.H.; Barak, A.V. Aggregation pheromones of the flat grain beetle Cryptolestes pusillus (Coleoptera: Cucujidae). J. Chem. Ecol. 1985, 11, 1053–1070. [Google Scholar] [CrossRef]
- Ryall, K.; Silk, P.J.; Fidgen, J.; Mayo, P.; Lavallee, R.; Guertin, C.; Scarr, T. Effects of pheromone release rate and trap placement on trapping of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in Canada. Environ. Entomol. 2015, 44, 734–745. [Google Scholar] [CrossRef]
- Otis, G.W.; Young, M.E.; Umphrey, G. Effects of colored objects and purple background on emerald ash borer trapping. In Emerald Ash Borer Research and Technology Development Meeting; Mastro, V., Reardon, R., Eds.; FHTET-2004–15; United States Department of Agriculture Forest Service Forest: Morgantown, WV, USA, 2005; pp. 31–32. [Google Scholar]
- Göpfert, M.C.; Hennig, R.M. Hearing in Insects. Ann. Rev. Entomol. 2016, 61, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Mamidala, P.; Wijeratne, A.J.; Wijeratne, S.; Poland, T.; Qazi, S.S.; Doucet, D.; Cusson, M.; Beliveau, C.; Mittapalli, O. Identification of Odor-Processing Genes in the Emerald Ash Borer, Agrilus planipennis. PLoS ONE 2013, 8, e56555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Teschers, C.; Cellejo, R.; Yang, M.; Wang, M.; Silk, P.J.; Ryall, K.; Roscoe, L.; Cordes, D.; Slawin, A.M.Z.; et al. Synthesis of fluorinated 12-dodecanolides as Emerald Ash Borer pheromone mimetics. Tetrahedron 2019. [Google Scholar] [CrossRef]
- Bauer, L.S.; Liu, H.; Miller, D.; Gould, J. Developing a classical biological control program for Agrilus planipennis (Coleoptera: Buprestidae), an invasive ash pest in North America. Newsl. Mich. Entomol. Soc. 2008, 53, 38–39. [Google Scholar]
- Duan, J.; Bauer, L.; van Driesche, R.; Gould, J. Progress and challenges of protecting North American ash trees from the emerald ash borer using biological control. Forests 2018, 9, 142. [Google Scholar] [CrossRef]
- Abell, K.J.; Bauer, L.S.; Duan, J.J.; Van Driesche, R. Long-term monitoring of the introduced emerald ash borer (Coleoptera: Buprestidae) egg parasitoid, Oobius agrili (Hymenoptera: Encyrtidae), in Michigan, USA and evaluation of a newly developed monitoring technique. Biol. Control 2014, 79, 36–42. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Lelito, J.P.; Van Driesche, R. Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: Potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2013, 106, 1145–1154. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Ulyshen, M.D.; Van Driesche, R.G. Population dynamics of an invasive forest insect and associated natural enemies in the aftermath of invasion: Implications for biological control. J. Appl. Ecol. 2015, 52, 1246–1254. [Google Scholar] [CrossRef]
- Davidson, W.; Rieske, L.K. Establishment of classical biological control targeting emerald ash borer is facilitated by use of insecticides, with little effect on native arthropod communities. Biol. Control 2016, 101, 78–86. [Google Scholar] [CrossRef]
- Duan, J.; Bauer, L.; Abell, K.; van Driesche, R. Population responses of hymenopteran parasitoids to the emerald ash borer (Coleoptera: Buprestidae) in recently invaded areas in north central United States. Biocontrol 2012, 57, 199–209. [Google Scholar] [CrossRef]
- Johnson, T.D.; Lelito, J.P.; Raffa, K.F. Responses of two parasitoids, the exotic Spathius agrili Yang and the native Spathius floridanus Ashmead, to volatile cues associated with the emerald ash borer, Agrilus planipennis Fairmaire. Biol. Control 2014, 79, 110–117. [Google Scholar] [CrossRef]
- Cossé, A.A.; Petroski, R.J.; Zilkowski, B.W.; Vermillion, K.; Lelito, J.P.; Cooperband, M.F.; Gould, J.R. Male-produced pheromone of Spathius agrili, a parasitoid introduced for the biological control of the invasive emerald ash borer, Agrilus planipennis. J. Chem. Ecol. 2012, 38, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.R.; Bauer, L.S.; Duan, J.J.; Petrice, T. Emerald ash borer, Agrilus planipennis (Fairmaire), biological control release and recovery guidelines. GAO-06-353 Riverdale USDA-APHIS-ARS-FS; 2019. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/EAB-FieldRelease-Guidelines.pdf (accessed on 14 September 2019).
- Bauer, L.S.; Liu, H.; Gao, R.; Zhao, T. Egg and larval parasitoids of emerald ash borer from China: Potential for biocontrol in North America. In Proceedings of the 2005 Emerald Ash Borer Research and Technology Development Meeting, Pittsburgh, PA, USA, 26–27 September 2005; FHTET-2005-16; Mastro, V., Reardon, R., Parra, G., Eds.; U.S. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2016; pp. 48–49. [Google Scholar]
- Roscoe, L.E.; Lyons, D.B.; Ryall, K.L.; Smith, S.M. Courtship sequence and evidence of volatile pheromones in Phasgonophora sulcata (Hymenoptera: Chalcididae), a North American parasitoid of the invasive Agrilus planipennis (Coleoptera: Buprestidae). Can. Entomol. 2016, 48, 151–162. [Google Scholar] [CrossRef]
- Roscoe, L.E. Phasgonophora sulcata Westwood (Hymenoptera: Chalcididae): A Potential Augmentative Biological Control Agent for the Invasive Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in Canada. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2014. [Google Scholar]
- Silk, P.J.; Ryall, K.; Roscoe, L. Emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), detection and monitoring in Canada. For. Int. J. For. Res. 2019. [Google Scholar] [CrossRef]
- Ryall, K.L.; Fidgen, J.G.; Turgeon, J.J. Detection of Emerald Ash Borer in Urban Environments Using Branch Sampling; Frontline Technical Note 111; Natural Resources Canada, Canadian Forest Service, Great Lakes Forestry Centre: Sault Ste. Marie, ON, Canada, 2011; 3p.
- Francese, J.A.; Rietz, M.L.; Mastro, V.C. Optimization of Multifunnel Traps for Emerald Ash Borer (Coleoptera: Buprestidae): Influence of Size, Trap Coating, and Color. J. Econ. Entomol. 2013, 106, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Cardé, R.T.; Baker, T.C. Sexual communication with pheromones. In Chemical Ecology of Insects; Bell, W.J., Cardé, R.T., Eds.; Springer: Boston, MA, USA, 1984. [Google Scholar] [CrossRef]
- Blain, K. (Forestry Commission England—Tree Health, Bristol, UK); Ryall, K. (Natural Resources Canada, Canadian Forest Service—Great Lakes Forestry Center, Sault Ste. Marie, ON, Canada); Aukema, B.H. (University of Minnesota, St. Paul, MN, USA); Silk, P.J. (Natural Resources Canada, Canadian Forest Service—Atlantic Forestry Center, Fredericton, NB, Canada). Personal communication, 2019.
- Vuts, J.; Woodcock, C.M.; Sumner, M.E.; Caulfield, J.C.; Reed, K.; Inward, D.J.; Leather, S.R.; Pickett, J.A.; Birkett, M.A.; Denman, S. Responses of the two-spotted oak buprestid, Agrilus biguttatus (Coleoptera: Buprestidae), to host tree volatiles. Pest Manag. Sci. 2016, 72, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Silk, P.J.; Ryall, K.; Grant, G.G.; Roscoe, L.E.; Mayo, P.; Williams, M.; LeClair, G.; Kimoto, T.; Williams, D.; Rutledge, C. Tree girdling and host tree volatiles provides a useful trap for bronze birch borer Agrilus anxius Gory (Coleoptera: Buprestidae). For. Int. J. For. Res. 2019. [Google Scholar] [CrossRef]
- Rutledge, C.E. Preliminary studies on using emerald ash borer (Coleoptera: Buprestidae) monitoring tools for bronze birch borer (Coleoptera: Buprestidae) detection and management. For. Int. J. For. Res. 2019. [CrossRef]
- Byers, J.A.; Zhang, Q.-H.; Schlyter, F. Volatiles from Nonhost Birch Trees Inhibit Pheromone Response in Spruce Bark Beetles. Naturwissenschaften 1998, 85, 557–561. [Google Scholar] [CrossRef]
- Zou, Y.; Rutledge, C.E.; Nakamuta, K.; Maier, C.T.; Hanks, L.M.; Richards, A.B.; Lacey, E.S.; Millar, J.G. Identification of a pheromone component and a critical synergist for the invasive beetle Callidiellum rufipenne (Coleoptera: Cerambycidae). Environ. Entomol. 2015, 45, 216–222. [Google Scholar] [CrossRef]
- Silk, P.J.; Sweeney, J.D.; Wu, J.; Price, J.; Gutowski, J.; Kettela, E.G. Evidence for a male-produced pheromone in Tetropium fuscum (F.) and Tetropium cinnamopterum (Kirby) (Coleoptera: Cerambycidae). Naturwissenschaften 2007, 94, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, J.D.; Silk, P.J.; Gutowski, J.M.; Wu, J.; Lemay, M.A.; Mayo, P.D.; Magee, D.I. Effect of chirality, release rate, and host volatiles on response of Tetropium fuscum (F.), Tetropium cinnamopterum Kirby, and Tetropium castaneum (L.) to the aggregation pheromone, fuscumol. J. Chem. Ecol. 2010, 36, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Muilenburg, V.L.; Herms, D.A. A review of bronze Birch Borer (Coleoptera: Buprestidae) life history, ecology and management. Environ. Entomol. 2013, 41, 1372–1385. [Google Scholar] [CrossRef] [PubMed]
- Silk, P.J.; Ryall, K.; Mayo, P.; MaGee, D.I.; Leclair, G.; Fidgen, J.; Lavallee, R.; Price, J.; McConaghy, J. A biologically active analog of the sex pheromone of the emerald ash borer, Agrilus planipennis Fairmaire. J. Chem. Ecol. 2015, 41, 294–302. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308. [Google Scholar] [CrossRef] [PubMed]
- Skibinski, M.; Wang, Y.; Slawin, A.M.Z.; Lebl, T.; Kirsch, P.; O’Hagan, D. Alicyclic Ring Structure: Conformational Influence of the CF2 Group in Cyclododecanes. Angew. Chem. Int. Ed. 2011, 50, 10581–10584. [Google Scholar] [CrossRef]
- O’Hagan, D.; Yi Wang, Y.; Skibinski, M.; Slawin, A.M.Z. Influence of the difluoromethylene group (CF2) on the conformation and properties of selected organic compounds. Pure Appl. Chem. 2012, 84, 1587–1595. [Google Scholar] [CrossRef]
- Boden, C.D.J.; Chambers, J.; Stevens, I.D.R. A concise, efficient and flexible strategy for the synthesis of the pheromones of Oryzaephilus and Cryptolestes grain beetles. Synthesis 1993, 411–420. [Google Scholar] [CrossRef]
- Mayo, P.D.; Silk, P.J.; MaGee, D.I.; McConaghy, J. Concise synthesis of (3Z)-dodecen-12-olide, pheromone component of the emerald ash borer. Synth. Commun. 2014, 44, 1957–1969. [Google Scholar] [CrossRef]
- Blakemore, P.R.; Cole, W.J.; Kocienski, P.J.; Morley, A. A stereoselective synthesis of trans-1,2-disubstituted alkenes based on the condensation of aldehydes with metallated 1-phenyl-1H-tetrazol-5-yl sulfones. Synlett 1998, 26–28. [Google Scholar] [CrossRef]
- Justus, K.; Steglich, W. First synthesis of a strained 14-membered biaryl ether lactone by macrolactonization. Tetrahedron Lett. 1991, 32, 5781–5784. [Google Scholar] [CrossRef]
- MaGee, D.I.; Mayo, P.D.; Silk, P.J.; Beattie, B. Synthesis of (3E)-dodecen-12-olide, a potential pheromone component of the Emerald Ash Borer. Synth. Commun. 2013, 43, 1368–1377. [Google Scholar] [CrossRef]
- Schlosser, M.; Christmann, K.F. Trans-selective olefin syntheses. Angew. Chem. Int. Ed. 1966, 5, 126. [Google Scholar] [CrossRef]
- Van der Mee, L.; Helmich, F.; de Bruijn, R.; Vekemans, J.A.J.M.; Palmans, A.R.A.; Meijer, E.W. Investigation of lipase-catalyzed ring-opening polymerizations of lactones with various ring sizes: Kinetic evaluation. Macromolecules 2006, 39, 5021–5027. [Google Scholar] [CrossRef]
- Suginome, H.; Yamada, S. Photoinduced transformations. 77. A four-step substitution of a carbonyl group of steroidal ketones by an oxygen atom. A new method for the synthesis of cyclic ethers. J. Org. Chem. 1985, 50, 2489–2494. [Google Scholar] [CrossRef]
- Fãrcasiu, D.; Jähme, J.; Rüchardt, C. Relative reactivity of bridgehead adamantyl and homoadamantyl substrates from solvolysis with heptafluorobutyrate as a highly reactive carboxylate leaving group. Absence of SN2 character of solvolysis of tert-butyl derivatives. J. Am. Chem. Soc. 1985, 107, 5717–5722. [Google Scholar] [CrossRef]
- Mino, T.; Masuda, S.; Nishio, M.; Yamashita, M. Synthesis of lactones by Baeyer-Villiger oxidation with magnesiuim monoperphthalate hexahydrate. J. Org. Chem. 1997, 62, 2633–2635. [Google Scholar] [CrossRef]
- Taber, D.F.; Qiu, J. Permaleic acid: Baeyer-Villiger oxidation of cyclododecanone. J. Chem. Ed. 2013, 90, 1103–1104. [Google Scholar] [CrossRef]
- Bellamy, C.L.; Nelson, G.H. Buprestidae Leach 1815. In American Beetles. Vol. 2: Polyphaga: Scarabaeoidea through Curculionoidea; Arnett, R.H., Thomas, M.C., Skelley, P.E., Frank, J.H., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 98–112. [Google Scholar]
- Schulz, S.; Hötling, S. The use of the lactone motif in chemical communication. Nat. Prod. Rep. 2015, 32, 1042–1066. [Google Scholar] [CrossRef]
- Jennings, D.E.; Taylor, P.B.; Duan, J.J. The mating and oviposition behavior of the invasive emerald ash borer, with references to influences of host tree condition. J. Pest Sci. 2014, 87, 71–78. [Google Scholar] [CrossRef]
- Singh, V.; Murphy, N.R.; Vijay Balasubramanian, V.; Mainland, J.D. Competitive binding predicts nonlinear responses of olfactory receptors to complex mixtures. Proc. Natl. Acad. Sci. USA 2019, 116, 9598–9603. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Reram, P.S.; Menke, M.; Hotling, S.; Ropke, R.; Melnik, K.; Poth, D.; Mann, F.; Henrichsen, S.; Dreyer, K. Mass Spectrometry of Aliphatic Macrolides, Important Semiochemicals or Pheromones. J. Nat. Prod. 2017, 80, 2572–2582. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silk, P.; Mayo, P.; Ryall, K.; Roscoe, L. Semiochemical and Communication Ecology of the Emerald Ash Borer, Agrilus planipennis (Coleoptera: Buprestidae). Insects 2019, 10, 323. https://doi.org/10.3390/insects10100323
Silk P, Mayo P, Ryall K, Roscoe L. Semiochemical and Communication Ecology of the Emerald Ash Borer, Agrilus planipennis (Coleoptera: Buprestidae). Insects. 2019; 10(10):323. https://doi.org/10.3390/insects10100323
Chicago/Turabian StyleSilk, Peter, Peter Mayo, Krista Ryall, and Lucas Roscoe. 2019. "Semiochemical and Communication Ecology of the Emerald Ash Borer, Agrilus planipennis (Coleoptera: Buprestidae)" Insects 10, no. 10: 323. https://doi.org/10.3390/insects10100323
APA StyleSilk, P., Mayo, P., Ryall, K., & Roscoe, L. (2019). Semiochemical and Communication Ecology of the Emerald Ash Borer, Agrilus planipennis (Coleoptera: Buprestidae). Insects, 10(10), 323. https://doi.org/10.3390/insects10100323




