Structuring of Dragonfly Communities (Insecta: Odonata) in Eastern Amazon: Effects of Environmental and Spatial Factors in Preserved and Altered Streams
Abstract
:1. Introduction
2. Materials and Methods
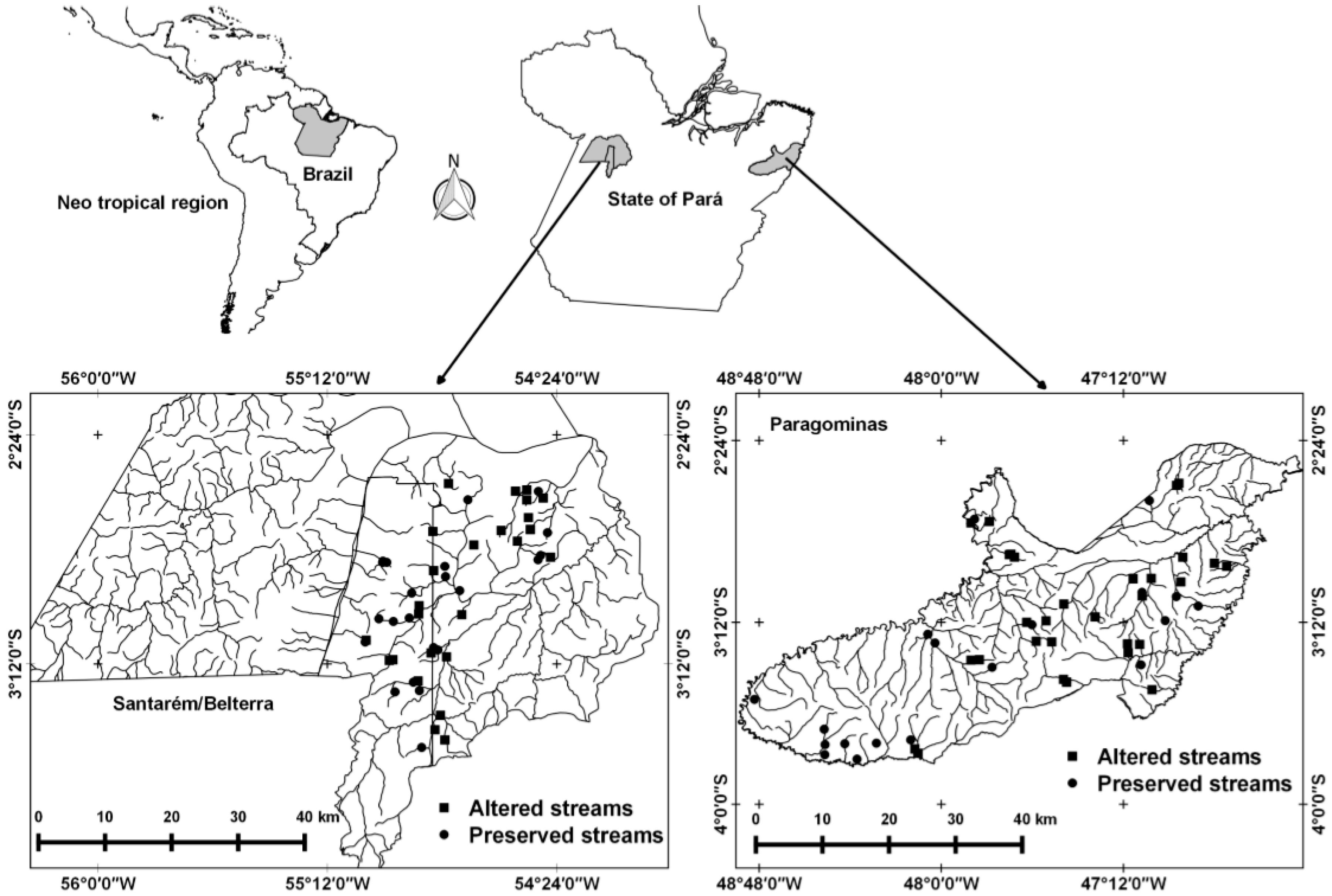
2.1. Study Areas
2.2. Data Collection
2.2.1. Biological Samples
2.2.2. Environmental Predictors
2.2.3. Spatial Predictors
2.3. Data Analysis
3. Results
3.1. Description of the Odonata Communities
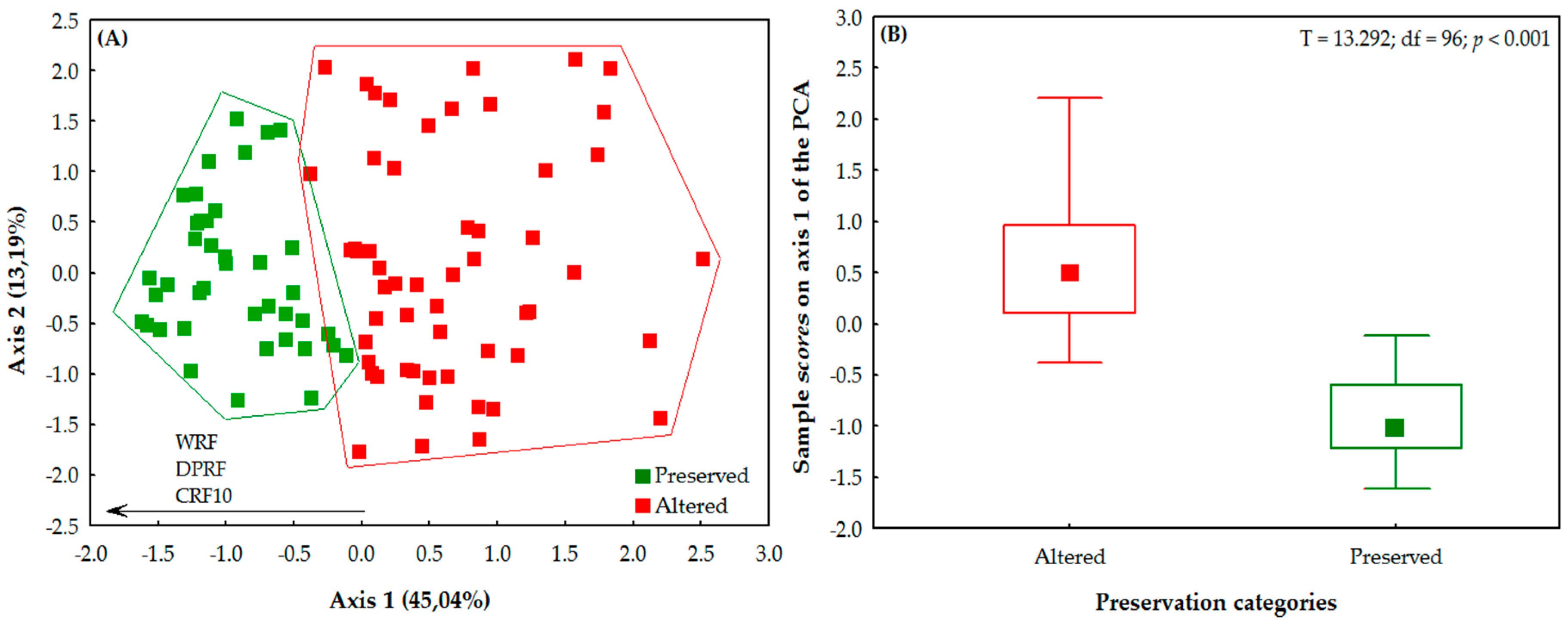
3.2. Abiotic Characteristics of the Streams
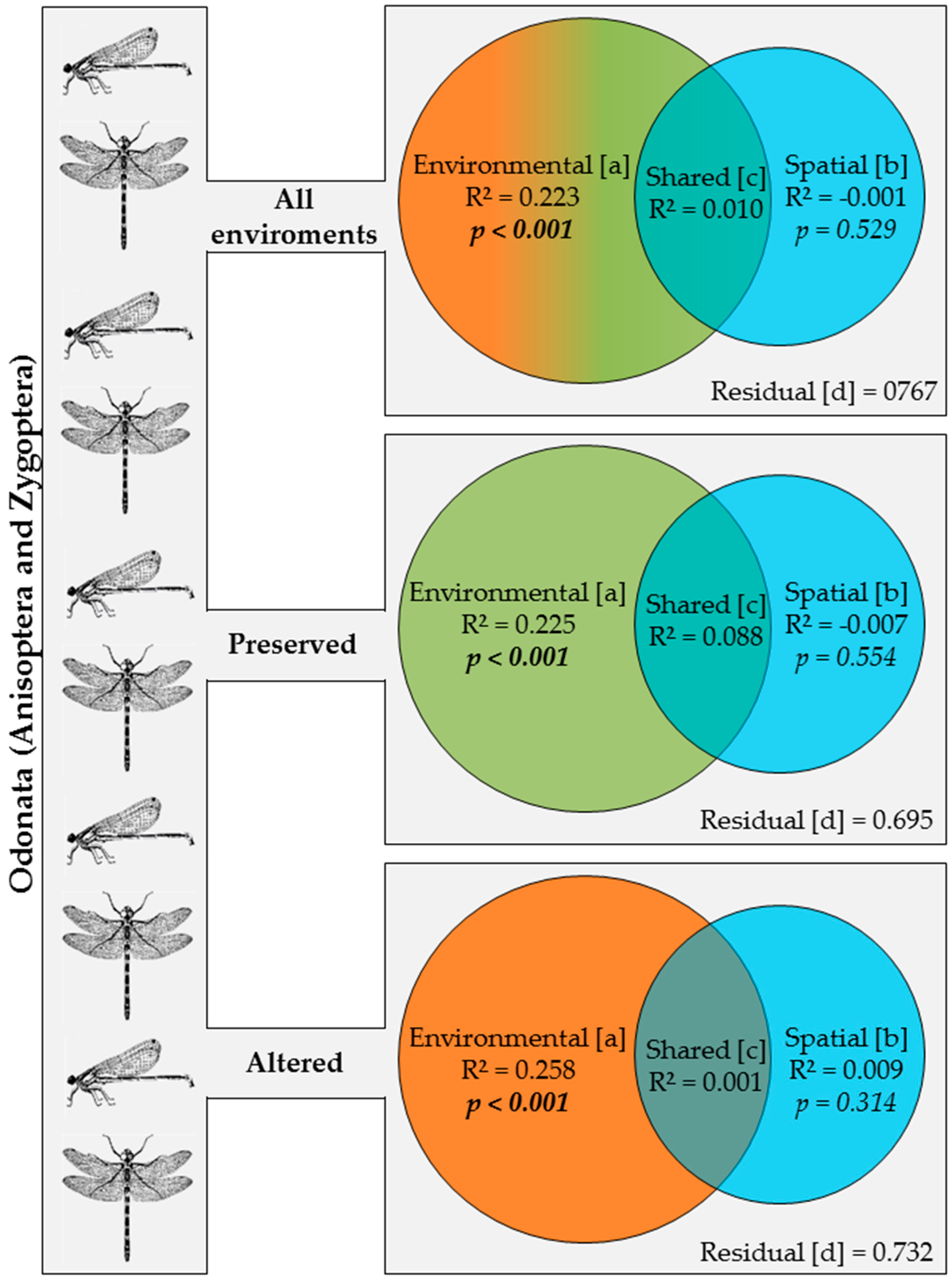
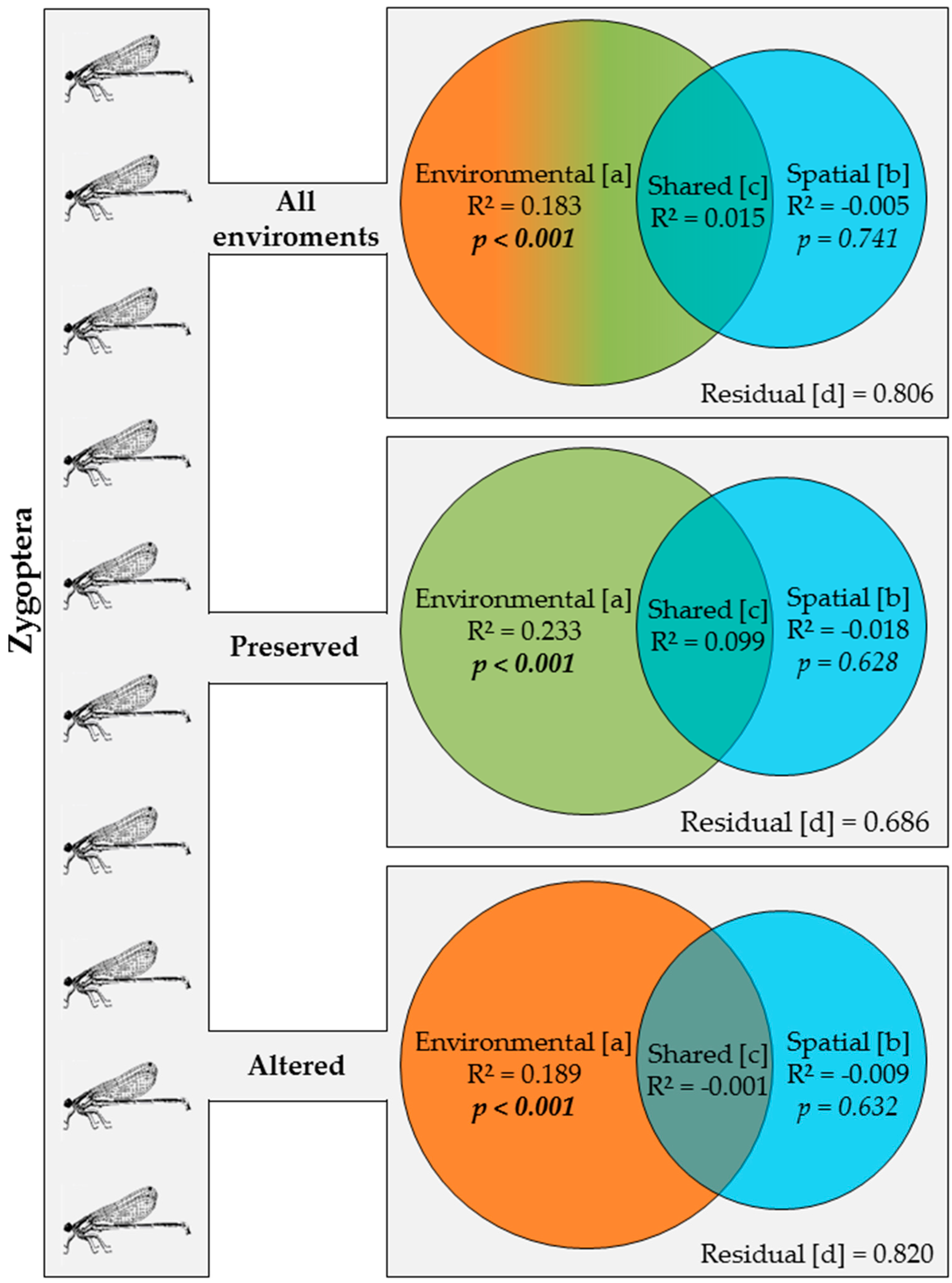
3.3. Effects of Environment and Space on Odonata Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loreau, M. Linking biodiversity and ecosystems: Towards a unifying ecological theory. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Márquez, J.C.; Kolasa, J. Local and Regional Processes in Community Assembly. PLoS ONE 2013, 8, e54580. [Google Scholar] [CrossRef]
- Pianka, E. Evolutionary Ecology; Harper Collins College Publishers: New York, NY, USA, 1994; p. 484. [Google Scholar]
- Juen, L.; De Marco, P. Odonate biodiversity in terra-firme forest streamlets in Central Amazonia: On the relative effects of neutral and niche drivers at small geographical extents. Insect. Conserv. Diver. 2011, 4, 265–274. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 1957, 22, 415–427. [Google Scholar] [CrossRef]
- Hubbell, S.P. A Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Hubbell, S.P. Neutral theory in community ecology and the hypothesis of functional equivalence. Funct. Ecol. 2005, 19, 166–172. [Google Scholar] [CrossRef]
- Cassemiro, F.A.S.; Padial, A.A. Teoria neutra da biodiversidade e biogeografia: Aspectos teóricos, impactos na literatura e perspectivas. Oecologia Bras. 2008, 12, 706–719. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.J.; Chase, M.; Hoopes, M.F. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Reed, K.E.; Bidner, L.R. Primate communities: Past, present and possible future. Yearb. Phys. Anthropol. 2004, 47, 2–39. [Google Scholar] [CrossRef] [PubMed]
- Foote, A.L.; Hornung, C.L.R. Odonates as biological indicators of grazing effects on Canadian prairie wetlands. Ecol. Entomol. 2005, 30, 273–283. [Google Scholar] [CrossRef]
- Calvão, L.B.; Vital, M.V.C.; Juen, L.; Lima Filho, G.F.; Oliveira-Junior, J.M.B.; Pinto, N.S.; De Marco, P. Thermoregulation and microhabitat choice in Erythrodiplax latimaculata Ris males (Anisoptera: Libellulidae. Odonatologica 2013, 42, 97–108. [Google Scholar]
- Monteiro-Júnior, C.S.; Couceiro, S.R.M.; Hamada, N.; Juen, L. Effect of vegetation removal for road building on richness and composition of Odonata communities in Amazonia, Brazil. Int. J. Odonatol. 2013, 16, 135–144. [Google Scholar] [CrossRef]
- Dolný, A.; Bárta, D.; Lhota, S.; Rusdianto; Drozd, P. Dragonflies (Odonata) in the Bornean rain forest as indicators of changes in biodiversity resulting from forest modification and destruction. Trop. Zool. 2011, 24, 63–86. [Google Scholar]
- Tiple, A.D.; Paunikae, S.; Talmale, S.S. Dragonflies and Damselflies (Odonata: Insecta) of Tropical Forest research Institute, Jabalpur, Madhya Padesh, Central India. J. Threat. Taxa 2012, 4, 2529–2533. [Google Scholar] [CrossRef]
- Simaika, J.P.; Samways, M.J. Using dragonflies to monitor and prioritize lotic systems: A South African perspective. Orgs. Diver. Evol. 2012, 12, 251–259. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.; Bae, M.; Hwang, S.; Noh, S.; Moon, J.; Park, Y. Distribution Patterns of Odonate Assemblages in Relation to Environmental Variables in Streams of South Korea. Insects 2018, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- McCauley, S.J. The role of local and regional processes in structuring larval dragonfly distributions across habitat gradients. Oikos 2007, 116, 121–133. Available online: https://www.jstor.org/stable/40234985 (accessed on 17 April 2014). [CrossRef]
- McCauley, S.J.; Davis, C.J.; Relyea, R.A.; Yurewicz, K.L.; Skelly, D.K.; Werner, E.E. Metacommunity patterns in larval odonates. Oecologia 2008, 158, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Brasil, L.S.; Oliveira-Junior, J.M.B.; Calvão, L.B.; Carvalho, F.G.; Monteiro-Júnior, C.S.; Dias-Silva, K.; Juen, L. Spatial, biogeographic and environmental predictors of diversity in Amazonian Zygoptera. Insect Conserv. Divers. 2018, 11, 174–184. [Google Scholar] [CrossRef]
- Alves-Martins, F.; Brasil, L.S.; Juen, L.; De Marco, P.; Stropp, J.; Hortal, J. Metacommunity patterns of Amazonian Odonata: The role of environmental gradients and major rivers. PeerJ 2019, 7, e6472. [Google Scholar] [CrossRef]
- Mendes, T.P.; Cabette, H.S.R.; Juen, L. Setting boundaries: Environmental and spatial effects on Odonata larvae distribution (Insecta). An. Acad. Bras. Cienc. 2015, 87, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felipe, T.R.A.; Súarez, Y.R. Caracterização e influência dos fatores ambientais nas assembleias de peixes de riachos em duas microbacias urbanas, Alto Rio Paraná. Biota Neotrop. 2012, 10, 143–151. [Google Scholar] [CrossRef]
- Callisto, M.; Moretti, M.; Goulart, M. Macroinvertebrados bentônicos como ferramenta para avaliar a saúde de riachos. Rev. Bras. Recur. Hídricos 2001, 6, 71–82. [Google Scholar] [CrossRef]
- Dodds, W.K. Freshwater Ecology: Concepts and Environmental Applications; Academic Press: Cambridge, UK, 2002; p. 569. [Google Scholar]
- Carvalho, F.G.; Pinto, N.S.; Oliveira-Júnior, J.M.B.; Juen, L. Effects of marginal vegetation removal on Odonata communities. Acta Limnol. Bras. 2013, 25, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Juen, L.; Oliveira-Junior, J.M.B.; Shimano, Y.; Mendes, T.P.; Cabette, H.S.R. Composição e riqueza de Odonata (Insecta) em riachos com diferentes níveis de conservação em um ecótone Cerrado-Floresta Amazônica. Acta Amaz. 2014, 44, 175–184. [Google Scholar] [CrossRef]
- Monteiro-Júnior, C.S.; Juen, L.; Hamada, N. Effects of urbanization on stream habitats and associated adult dragonfly and damselfly communities in central Brazilian Amazonia. Landsc. Urban. Plan. 2014, 127, 28–40. [Google Scholar] [CrossRef]
- Monteiro-Júnior, C.S.; Juen, L.; Hamada, N. Analysis of urban impacts on aquatic habitats in the central Amazon basin: Adult odonates as bioindicators of environmental quality. Ecol. Indic. 2015, 48, 303–311. [Google Scholar] [CrossRef]
- Oliveira-Junior, J.M.B.; Shimano, Y.; Gardner, T.A.; Hughes, R.M.; De Marco, P.; Juen, L. Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condition of small streams in the eastern Amazon. Austral. Ecol. 2015, 40, 733–744. [Google Scholar] [CrossRef]
- Oliveira-Junior, J.M.B.; De Marco, P.; Dias-Silva, K.; Leitão, R.P.; Leal, C.G.; Pompeu, P.S.; Gardner, T.A.; Hughes, R.M.; Juen, L. Effects of human disturbance and riparian conditions on Odonata (Insecta) assemb—Lages in eastern Amazon basin streams. Limnologica 2017, 66, 31–39. [Google Scholar] [CrossRef]
- Oliveira-Junior, J.M.B.; Juen, L. The Zygoptera/Anisoptera Ratio (Insecta: Odonata): A New Tool for Habitat Alterations Assessment in Amazonian Streams. Neotrop. Entomol. 2019, 48, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Rios, S.L.; Bailey, R.C. Relationship between riparian vegetation and stream benthic communities at three spatial scales. Hydrobiologia 2006, 553, 153–160. [Google Scholar] [CrossRef]
- Dumont, H.J.; Vierstraete, A.; Vanfleteren, J.R. A molecular phylogeny of the Odonata (Insecta). Syst. Entomol. 2010, 35, 6–18. [Google Scholar] [CrossRef]
- May, M.L. Odonata: Who They Are and What They Have Done for Us Lately: Classification and Ecosystem Services of Dragonflies. Insects 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Stoks, R.; Córdoba-Aguilar, A. Evolutionary ecology of Odonata: A complex life cycle perspective. Annu. Rev. Entomol. 2012, 57, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Corbet, P.S. Dragonflies: Behavior and Ecology of Odonata; Comstock Publ. Assoc.: Ithaca, NY, USA, 1999; p. 829. [Google Scholar]
- Oertli, B. The use of dragonflies in the assessment and monitoring of aquatic habitats. In Model. Organisms for Ecological and Evolutionary Research; Cordoba-Aguilar, A., Ed.; Oxford University Press: Oxford, UK, 2008; pp. 79–95. [Google Scholar]
- Butler, R.G.; de Maynadier, P.G. The significance of littoral and shoreline habitat integrity to the conservation of lacustrine damselflies (Odonata). J. Insect Conserv. 2008, 12, 23–36. [Google Scholar] [CrossRef]
- Miguel, T.B.; Oliveira-Junior, J.M.B.; Ligeiro, R.; Juen, L. Odonata (Insecta) as a tool for the biomonitoring of environmental quality. Ecol. Indic. 2017, 81, 555–566. [Google Scholar] [CrossRef]
- Gardner, T.A.; Ferreira, J.; Barlow, J.; Lees, A.C.; Parry, L.; Vieira, I.C.G.; Berenguer, E.; Abramovay, R.; Aleixo, A.; Andretti, C.; et al. A social and ecological assessment of tropical land-uses at multiple scales: The Sustainable Amazon Network. Philos. Trans. R. Soci. B 2013, 368, 20120166. [Google Scholar] [CrossRef] [PubMed]
- Heino, J.; Mykrä, H. Control of stream insect assemblages: Roles of spatial configuration and local environmental factors. Ecol. Entomol. 2008, 33, 614–622. [Google Scholar] [CrossRef]
- Shimano, Y.; Juen, L.; Salles, F.F.; Nogueira, D.S.; Cabette, H.S.R. Environmental and spatial processes determining Ephemeroptera (Insecta) structures in tropical streams. Ann. Limnol.-Int. J. Lim. 2013, 49, 31–41. [Google Scholar] [CrossRef]
- Costa, L.S.M.; Zanini Branco, C.C.; Bispo, P.C. O Papel dos Fatores Ambientais e Espaciais Sobre a Fauna de Ephemeroptera (Insecta) em Riachos de Mata Atlântica. EntomoBrasilis 2014, 7, 86–92. [Google Scholar] [CrossRef]
- De Marco, P.; Batista, J.D.; Cabette, H.S.R. Community Assembly of Adult Odonates in Tropical Streams: An Ecophysiological Hypothesis. PLoS ONE 2015, 10, e0123023. [Google Scholar] [CrossRef] [PubMed]
- Dutra, S.; De Marco, P. Bionomic differences in odonates and their influence on the efficiency of indicator species of environmental quality. Ecol. Indic. 2015, 49, 132–142. [Google Scholar] [CrossRef]
- Watrin, O.S.; Rocha, A.M.A. Levantamento da Vegetação Natural e do Uso da Terra no Município de Paragominas (PA) Utilizando Imagens TM/Landsat; EMBRAPA/CPATU. (EMBRAPA/CPATU); Boletim de Pesquisa: Belém, Brazil, 1992; p. 40. [Google Scholar]
- Nepstad, D.C.; Moutinho, P.R.S.; Dias-Filho, M.B.; Davidson, E.; Cardinot, G.; Markewitz, D.; Figueiredo, R.; Vianna, N.; Lefebvre, P.; Ray, D.; et al. The effects of rainfall exclusion on canopy processes and biogeochemistry of an Amazon forest. J. Geophys. Res. 2002, 107, 80–85. [Google Scholar] [CrossRef]
- Veloso, H.P.; Rangel Filho, A.L.R.; Lima, J.C.A. Classificação Da Vegetação Brasileira Adaptada a Um Sistema Universal; IBGE: Rio de Janeiro, Brazil, 1991; p. 123. [Google Scholar]
- Berenguer, E.; Ferreira, J.; Gardner, T.A.; Aragão, L.E.O.C.; Camargo, P.B.; Cerri, C.E.; Durigan, M.; Oliveira Junior, R.C.; Vieira, I.C.G.; Barlow, J. A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Chang. Biol. 2014, 20, 3713–3726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, N.G.M.; Lees, A.C.L.; Andretti, C.; Davis, B.; Solar, R.; Aleixo, A.; Barlow, J.; Ferreira, J.; Gardner, T.A. Avian biodiversity in multiple-use landscapes of the Brazilian Amazon. Biol. Conserv. 2013, 167, 339–348. [Google Scholar] [CrossRef]
- Putz, F.E.; Redford, H.K. The importance of defining ‘Forest’: Tropical forest degradation, deforestation, long-term phase shifts, and further transitions. Biotropica 2010, 42, 10–20. [Google Scholar] [CrossRef]
- May, M.L. Thermoregulation in adaptation to temperature in dragonflies (Odonata: Anisoptera). Ecol. Monogr. 1976, 46, 1–32. [Google Scholar] [CrossRef]
- May, M.L. Thermal adaptations of dragonflies, revisited. Adv. Odonatol. 1991, 5, 71–88. [Google Scholar]
- Fulan, J.A.; Henry, R. Temporal distribution of immature Odonata (Insecta) on Eichhornia azurea (Kunth) stands in the Camargo Lake, Paranapanema River, São Paulo. Rev. Bras. Entomol. 2007, 51, 224–227. [Google Scholar] [CrossRef]
- Baptista, D.F.; Dorvillé, L.F.M.; Buss, D.F.; Nessiamian, J.L. Spatial and temporal organization of aquatic insects assemblages in the longitudinal gradient of a tropical river. Rev. Bras. Biol. 2001, 61, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Heino, J. Taxonomic surrogacy; numerical resolution and responses of stream macroinvertebrate communities to ecological gradients: Are the inferences transferable among regions? Ecol. Indic. 2014, 36, 186–194. [Google Scholar] [CrossRef]
- Kaufmann, P.R.; Levine, P.; Robison, G.E.; Seeliger, C.; Peck, D.V. Quantifying Physical Habitat in Wadeable Streams; EPA/620/R-99/003; U.S. Environmental Protection Agency: Washington, DC, USA, 1999; p. 149.
- Peck, D.V.; Herlihy, A.T.; Hill, B.H.; Hughes, R.M.; Kaufmann, P.R.; Klemm, D.; Lazorchak, J.M.; McCormick, F.H.; Peterson, S.A.; Ringold, P.L.; et al. Environmental Monitoring and Assessment Program-Surface Waters Western Pilot Study: Field Operations Manual for Wadeable Streams; EPA/620/R-06/003; U.S. Environmental Protection Agency: Washington, DC, USA, 2006.
- De Marco, P.; Resende, D.C. Activity patterns and thermoregulation in a tropical dragonfly assemblage. Odonatologica 2002, 31, 129–138. Available online: http://natuurtijdschriften.nl/record/592390 (accessed on 10 March 2014).
- Ferreira-Peruquetti, P.; De Marco, P. Efeito da alteração ambiental sobre comunidades de odonata em riachos de Mata Atlântica de Minas Gerais, Brasil. Rev. Bras. Zool. 2002, 19, 32–37. [Google Scholar] [CrossRef]
- Lencioni, F.A.A. The Damselflies of Brazil: An Illustrated Guide—Coenagrionidae; All Print Editora: São Paulo, Brazil, 2006; p. 419. [Google Scholar]
- Borror, D.J. A key to the New World genera of Libellulidae (Odonata). Ann. Entomol. Soc. Am. 1945, 38, 168–194. [Google Scholar] [CrossRef]
- Belle, J. A synopsis of the species of Phyllocycla Calvert with description of four new taxa and a key to the genera of the neotropical Gomphidae (Odonata, Gomphidae). Tijdschr. Voor Entomol. 1988, 131, 73–102. [Google Scholar]
- Belle, J. Higher classification of the South-American Gomphidae (Odonata). Zool. Meded. 1996, 70, 298–324. Available online: https://www.repository.naturalis.nl/document/149750 (accessed on 17 April 2014).
- Garrison, R.W. A synopsis of the genus Hetaerina with descriptions of four new species (Odonata: Calopterigidae). Trans. Amer. Entomol. Soci. 1990, 116, 175–259. [Google Scholar] [CrossRef]
- Lencioni, F.A.A. The Damselflies of Brazil: An Illustrated Guide—The Non Coenagrionidae Families; All Print Editora: São Paulo, Brazil, 2005; p. 332. [Google Scholar]
- Garrison, R.W.; Von Ellenrieder, N.; Louton, J.A. Dragonfly Genera of the New Word: An illustrated and Annotated Key to the Anisoptera; The Johns Hopkins University Press: Baltimore, MA, USA, 2006; p. 368. [Google Scholar]
- Garrison, R.W.; Von Ellenrieder, N.; Louton, J.A. Damselfly genera of the New World. In Baltimore, An Illustrated and Annotated Key to the Zygoptera; The Johns Hopkins University Press: Baltimore, MA, USA, 2010; p. 490. [Google Scholar]
- Nessimian, J.L.; Venticinque, E.; Zuanon, J.; De Marco, P.; Gordo, M.; Fidelis, L.; Batista, J.D.; Juen, L. Land use, habitat integrity, and aquatic insect assemblages in Central Amazonian streams. Hydrobiologia 2008, 614, 117–131. [Google Scholar] [CrossRef]
- Pereira, L.R.; Cabette, H.S.R.; Juen, L. Trichoptera as bioindicators of habitat integrity in the Pindaíba river basin, Mato Grosso (Central Brazil). Ann. Limnol.-Int. J. Lim. 2012, 48, 295–302. [Google Scholar] [CrossRef]
- Giehl, N.F.S.; Dias-Silva, K.; Juen, L.; Batista, J.D.; Cabette, H.S.R. Taxonomic and Numerical Resolutions of Nepomorpha (Insecta: Heteroptera) in Cerrado Streams. PLoS ONE 2014, 9, e103623. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P. All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol. Model. 2002, 153, 51–68. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; Bini, L.M. Modelling geographical patterns in species richness using eigenvector-based spatial filters. Glob. Ecol. Biogeogr. 2005, 14, 177–185. [Google Scholar] [CrossRef]
- Rangel, T.F.L.V.B.; Diniz-Filho, J.A.F.; Bini, L.M. Towards an integrated computational tool for spatial analysis in macroecology and biogeography. Glob. Ecol. Biogeogr. 2006, 15, 321–327. [Google Scholar] [CrossRef]
- Ferreira, W.R.; Ligeiro, R.; Macedo, D.; Hughes, R.M.; Kaufmann, P.R.; Oliveira, L.G.; Callisto, M. Importance of environmental factors for the richness and distribuition of benthic macroinvertebrates in tropical headwater streams. Freshw. Sci. 2014, 33, 860–871. [Google Scholar] [CrossRef]
- Jackson, D.A. Stopping rules in principal components analysis: A comparison of heuristical and statistical approaches. Ecology 1993, 74, 2204–2214. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Legendre, P. Estimating and controlling for spatial structure in the study of ecological communities. Glob. Ecol. Biogeogr. 2010, 19, 174–184. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Legendre, P.; Dray, S.; Borcard, D. Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef]
- Borcard, D.; Legendre, P.; Drapeau, P. Partialling out the spatial component of ecological variation. Ecology 1992, 73, 45–55. Available online: http://www.jstor.org/stable/1940179 (accessed on 22 April 2014). [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B. The vegan package. Community Ecol. Package 2007, 10, 631–637. Available online: http://www.R-project.org (accessed on 12 May 2014).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; p. 498. [Google Scholar] [CrossRef]
- R Development Core Team. Importance of Environmental Factors for the Richness and Distribuition of Benthic Macroinvertebrates in Tropical Headwater Streams; R Foundation for Statistical Computing: Vienna, Austria, 2011; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 12 May 2014).
- Chase, J.M.; Bentgtsson, J. Increasing spatio-temporal scales: Metacommunity ecology. In Community Ecology: Processes, Models and Applications; Verhoef, H.A., Morin, P.J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 57–68. [Google Scholar]
- Cottenie, K. Integrating environmental and spatial processes in ecological community dynamics. Ecol. Lett. 2005, 8, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, T.; Bini, L.M.; Roque, F.O.; Couceiro, S.R.M.; Trivino-Strixino, S.; Cottenie, K. Common and rare species respond to similar niche processes in macroinvertebrate metacommunities. Ecography 2012, 35, 183–192. [Google Scholar] [CrossRef]
- Landeiro, V.L.; Bini, L.M.; Melo, A.S.; Pes, A.M.O.; Magnusson, W.E. The roles of dispersal limitation and environmental conditions in controlling caddisfly (Trichoptera) assemblages. Freshw. Biol. 2012, 57, 1554–1564. [Google Scholar] [CrossRef]
- Corkum, L.D. Patterns of benthic invertebrate assemblages in rivers of north-western North America. Freshw. Biol. 1989, 21, 191–205. [Google Scholar] [CrossRef]
- Lammert, M.; Allan, J.D. Assessing biotic integrity of streams: Effects of scale in measuring the influence of land use/ cover and habitat structure on fish and macroinvertebrates. Environ. Manag. 1999, 23, 257–270. [Google Scholar] [CrossRef]
- Göthe, E.; Angeler, D.G.; Sandin, L. Metacommunity structure in a small boreal stream network. J. Anim. Ecol. 2013, 82, 449–458. [Google Scholar] [CrossRef]
- Nabout, J.C.; Siqueira, T.; Bini, L.M.; Nogueira, I.S. No evidence for environmental and spatial processes in structuring phytoplankton communities. Acta Oecol. 2009, 35, 720–726. [Google Scholar] [CrossRef]
- Schulz, G.; Siqueira, T.; Stefan, G.; Roque, F.O. Passive and active dispersers respond similarly to environmental and spatial processes: An example from metacommunity dynamics of tree hole invertebrates. Fundam. Appl. Limnol. 2012, 181, 315–326. [Google Scholar] [CrossRef]
- Death, R.G. Predicting invertebrate diversity from disturbance regimes in Forest streams. Oikos 2002, 97, 18–30. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; p. 302. [Google Scholar]
- Beisner, B.E.; Peres-Neto, P.R.; Lindstro, E.S.; Barnett, A.; Longhi, M.L. The role of environmental and spatial processes in structuring lake communities from bacteria to fish. Ecology 2006, 87, 2985–2991. [Google Scholar] [CrossRef]
- Feld, C.K.; Hering, D. Community structure or function: Effects of environmental stress on benthic macroinvertebrates at different spatial scales. Freshw. Biol. 2007, 52, 1380–1399. [Google Scholar] [CrossRef]
- McPeek, M.A. Ecological factors limiting the distributions and abundances of Odonata. Dragonflies & Damselflies. In Model Organisms for Ecological and Evolutionary Research; Córdoba-Aguilar, A., Ed.; Oxford University Press: Oxford, UK, 2008; pp. 51–62. [Google Scholar]
- Landeiro, V.L.; Magnusson, W.E.; Melo, A.S.; Espírito-Santo, H.M.V.; Bini, L.M. Spatial eigenfunction analyses in stream networks: Do watercourse and overland distances produce different results? Freshw. Biol. 2011, 56, 1184–1192. [Google Scholar] [CrossRef]
- Tscharntke, T.; Steffan-Dewenter, I.; Kruess, A.; Thies, C. Contribution of small habitat fragments to conservation of insect communities of grassland-cropland landscapes. Ecol. Appl. 2002, 12, 354–363. [Google Scholar] [CrossRef]
- Resende, D.C. Residence advantage in heterospecific territorial disputes of Erythrodiplax Brauer species (Odonata, Libellulidae). Rev. Bras. Entomol. 2010, 54, 110–114. [Google Scholar] [CrossRef]
- Clark, T.E.; Samways, M.J. Dragonflies (Odonata) as indicators of biotope quality in the Kruger National Park, South Africa. J. Appl. Ecol. 1996, 33, 1001–1012. [Google Scholar] [CrossRef]
- Schindler, M.; Fesl, C.; Chovanec, A. Dragonfly associations (Insect: Odonata) in relation to habitat variables: A multivariate approach. Hydrobiologia 2003, 497, 169–180. [Google Scholar] [CrossRef]
- Karouzas, I.; Gritzalis, K.C. Local and regional factors determining aquatic and semi-aquatic bug (Heteroptera) assemblages in rivers and streams of Greece. Hydrobiologia 2006, 573, 199–212. [Google Scholar] [CrossRef]
- Pither, J.; Taylor, P.D. An experimental assessment of landscape connectivity. Oikos 1998, 83, 166–174. [Google Scholar] [CrossRef]
- Heinrich, B. The Hot-Blooded Insects: Strategies and Mechanism of Thermoregulation; Harvard University Press: Cambridge, MA, USA, 1993; p. 601. [Google Scholar] [CrossRef]
- Paulson, D. The importance of forest to Neotropical dragonflies. In Forest and Dragonflies; Cordero Rivera, A., Ed.; Pensoft Publishers: Sofia, Balkans, 2006; pp. 79–101. [Google Scholar]
- Heino, J. The importance of metacommunity ecology for environmental assessment research in the freshwater realm. Biol. Rev. 2013, 88, 166–178. [Google Scholar] [CrossRef]
- Nekola, J.C.; White, P.S. The distance decay of similarity in biogeography and ecology. J. Biogeogr. 1999, 26, 867–878. [Google Scholar] [CrossRef] [Green Version]
- Brow, B.L.; Swan, C.M. Dendritic network structure constrains metacommunity properties in riverine ecosystems. J. Anim. Ecol. 2010, 79, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Heino, J.; Grönroos, M.; Soininen, J.; Virtanen, R.; Muotka, T. Context dependency and metacommunity structuring in boreal headwater streams. Oikos 2012, 121, 537–544. [Google Scholar] [CrossRef]
- Astorga, A.; Oksanen, J.; Luoto, M.; Soininen, J.; Virtanen, R.; Muotka, T. Distance decay of similarity in freshwater communities: Do macro- and microorganisms follow the same rules? Glob. Ecol. Biogeogr. 2012, 21, 365–375. [Google Scholar] [CrossRef]

| Variables of the Habitat Integrity Index (HII) | Loading | |
|---|---|---|
| Axis 1 | Axis 2 | |
| Pattern of land use in the area outside the riparian vegetation zone (PLUORV) | 0.028 | 0.087 |
| Width of the riparian forest (WRF) | 0.141 * | 0.033 |
| Degree of preservation of the riparian forest (DPRF) | 0.133 * | 0.013 |
| Condition of the riparian forest within a radius of 10 m (CRF10) | 0.133 * | 0.012 |
| Retention devices (RD) | 0.079 | 0.059 |
| Sediments in the channel (SC) | 0.083 | 0.104 |
| Structure of the river bank (SRB) | 0.038 | 0.231 |
| Excavation under bank (EUB) | 0.081 | 0.040 |
| River bed (RB) | 0.008 | 0.262 |
| Areas of rapids, pools or meanders (ARPM) | 0.067 | 0.100 |
| Aquatic vegetation (AV) | 0.085 | 0.058 |
| Detritus (D) | 0.123 | 0.001 |
| Eigenvalue | 5.405 | 1.582 |
| Broken-stick | 5.404 | 6.987 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Junior, J.M.B.; Juen, L. Structuring of Dragonfly Communities (Insecta: Odonata) in Eastern Amazon: Effects of Environmental and Spatial Factors in Preserved and Altered Streams. Insects 2019, 10, 322. https://doi.org/10.3390/insects10100322
Oliveira-Junior JMB, Juen L. Structuring of Dragonfly Communities (Insecta: Odonata) in Eastern Amazon: Effects of Environmental and Spatial Factors in Preserved and Altered Streams. Insects. 2019; 10(10):322. https://doi.org/10.3390/insects10100322
Chicago/Turabian StyleOliveira-Junior, José Max Barbosa, and Leandro Juen. 2019. "Structuring of Dragonfly Communities (Insecta: Odonata) in Eastern Amazon: Effects of Environmental and Spatial Factors in Preserved and Altered Streams" Insects 10, no. 10: 322. https://doi.org/10.3390/insects10100322
APA StyleOliveira-Junior, J. M. B., & Juen, L. (2019). Structuring of Dragonfly Communities (Insecta: Odonata) in Eastern Amazon: Effects of Environmental and Spatial Factors in Preserved and Altered Streams. Insects, 10(10), 322. https://doi.org/10.3390/insects10100322







