Recent Advances in Preparation and Testing Methods of Engine-Based Nanolubricants: A State-of-the-Art Review
Abstract
:1. Introduction
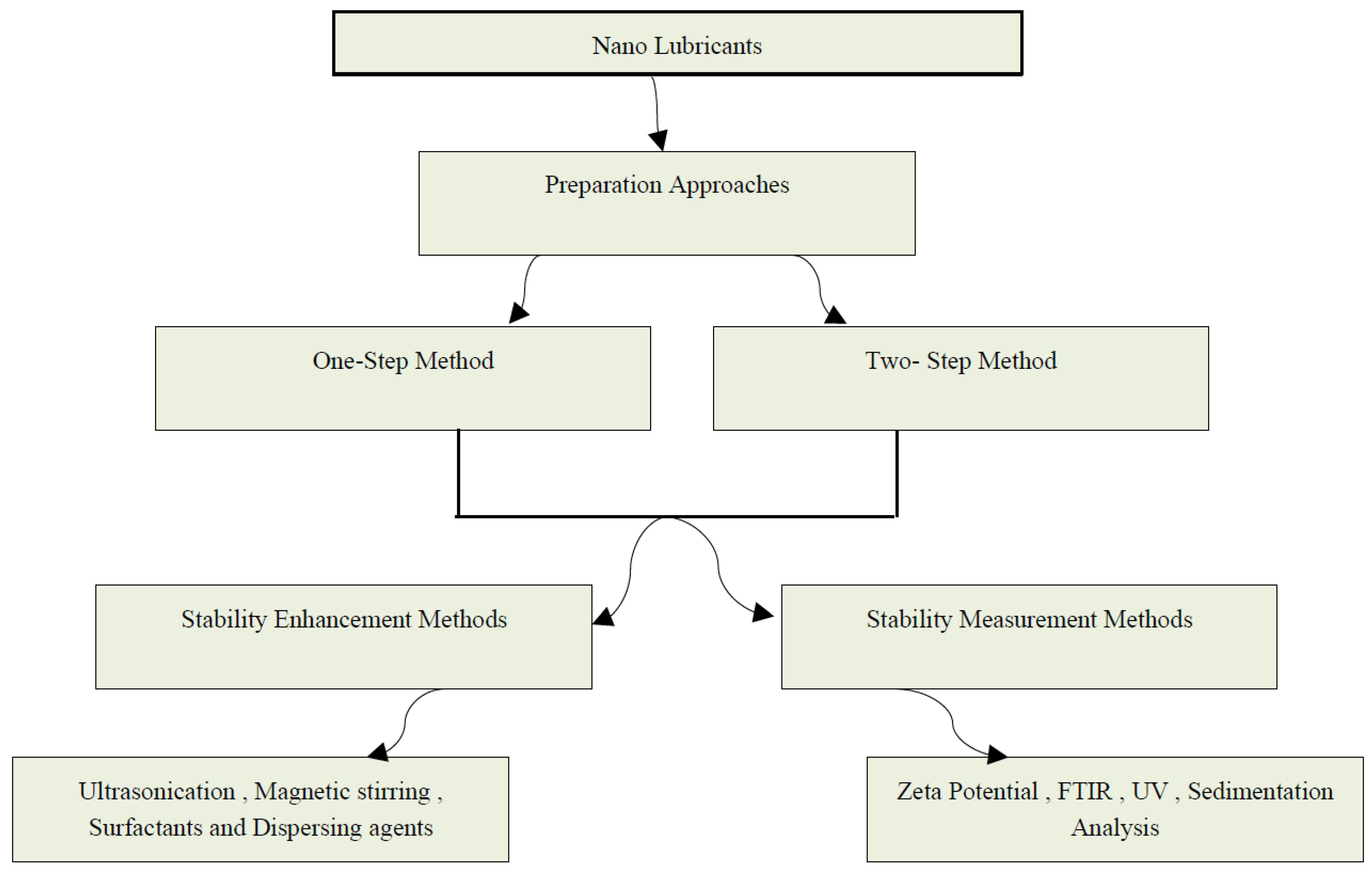
2. Preparation Methods and Dispersion Stability of Nanolubricants
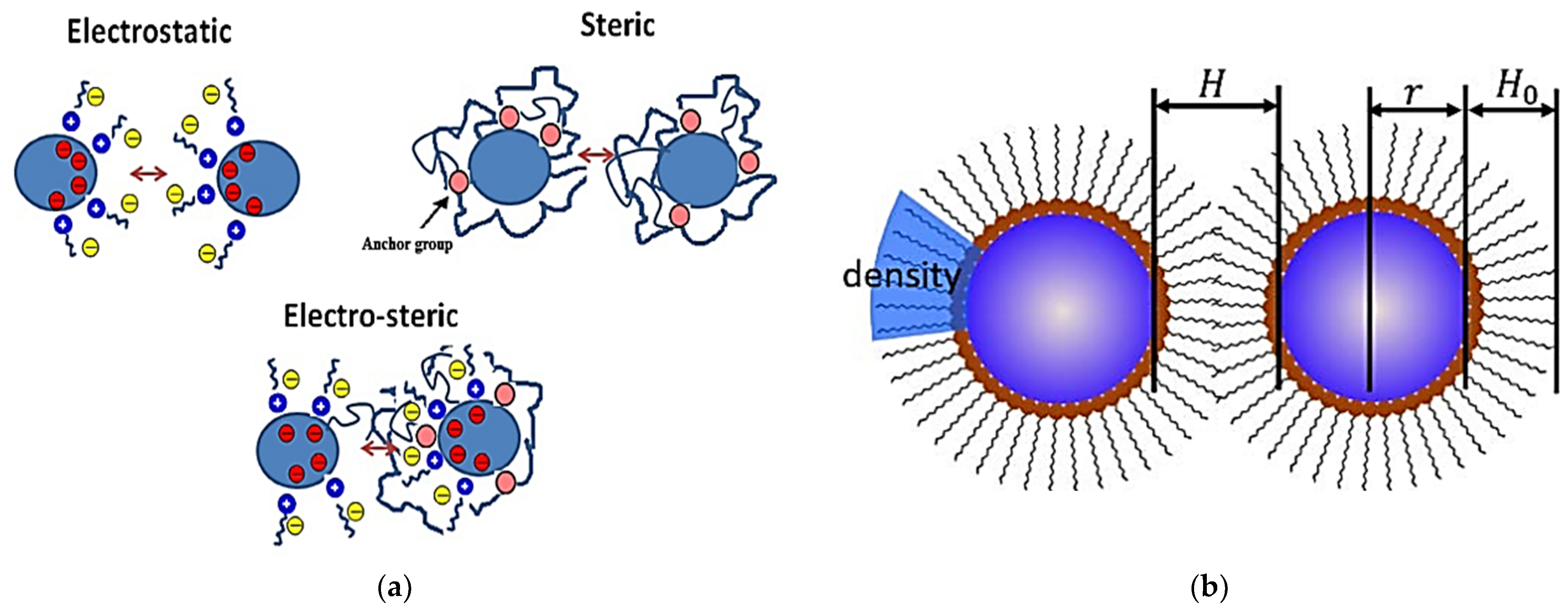
2.1. Basic Concepts
2.2. Preparation Methods of Nanolubricants
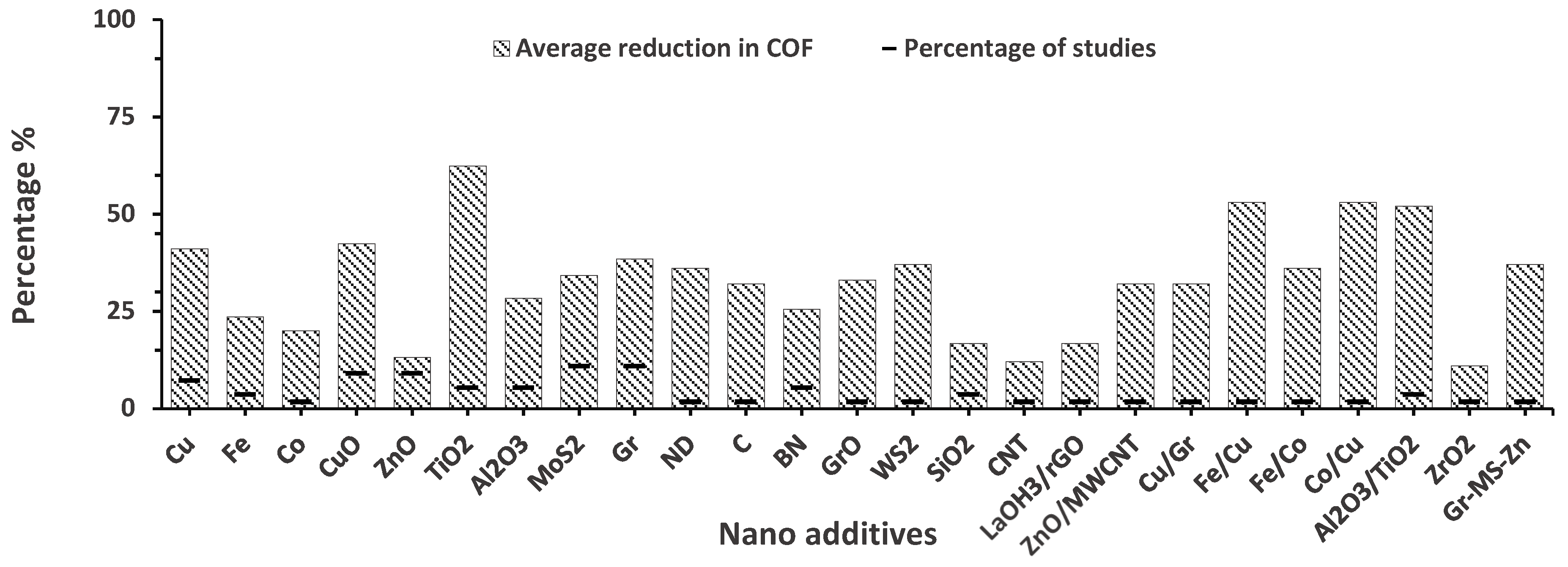
3. Tribological Performance of Nanolubricants
4. Rheological and Thermophysical Properties of Engine Lubricant-Based Nanofluids
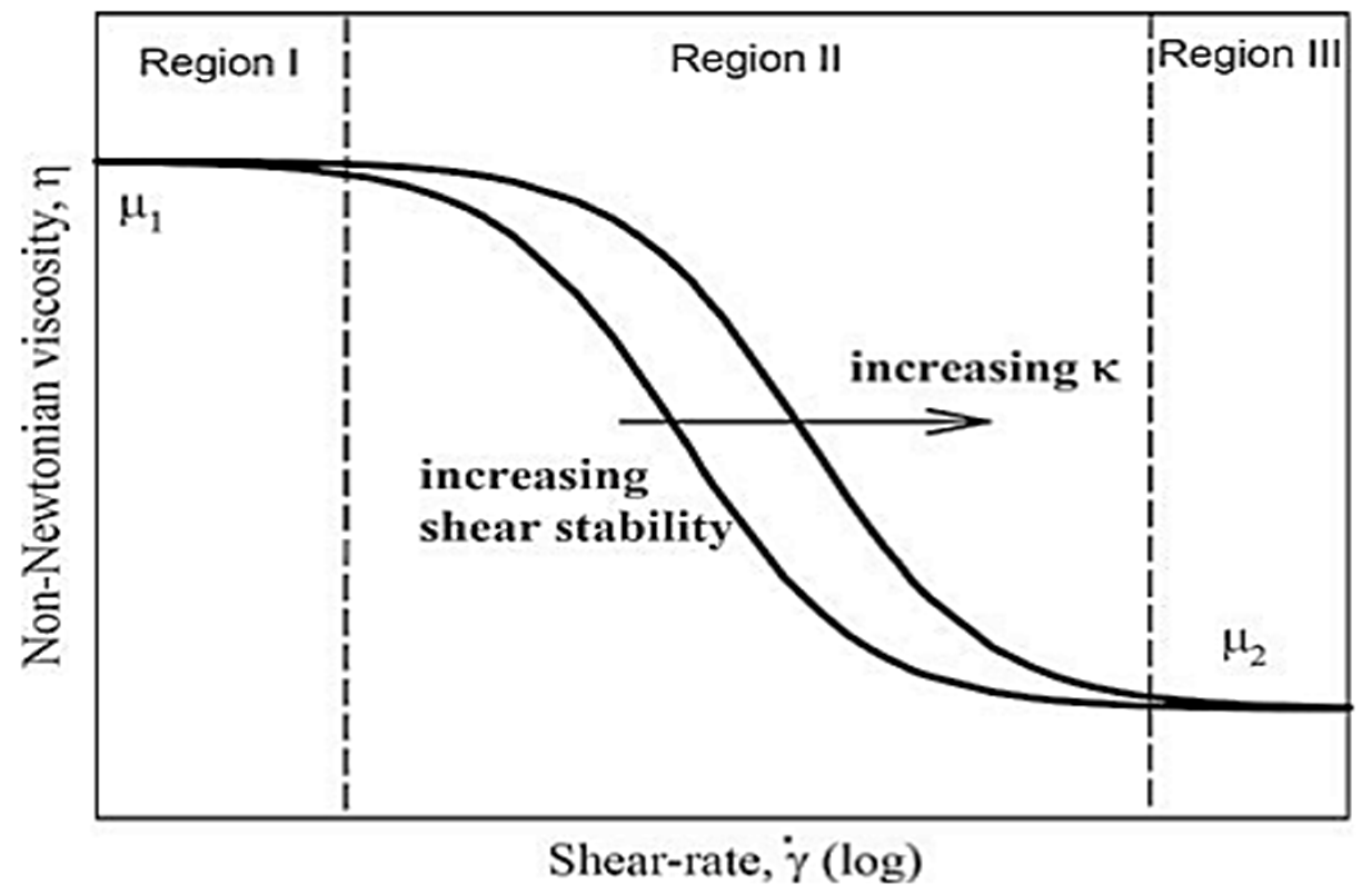
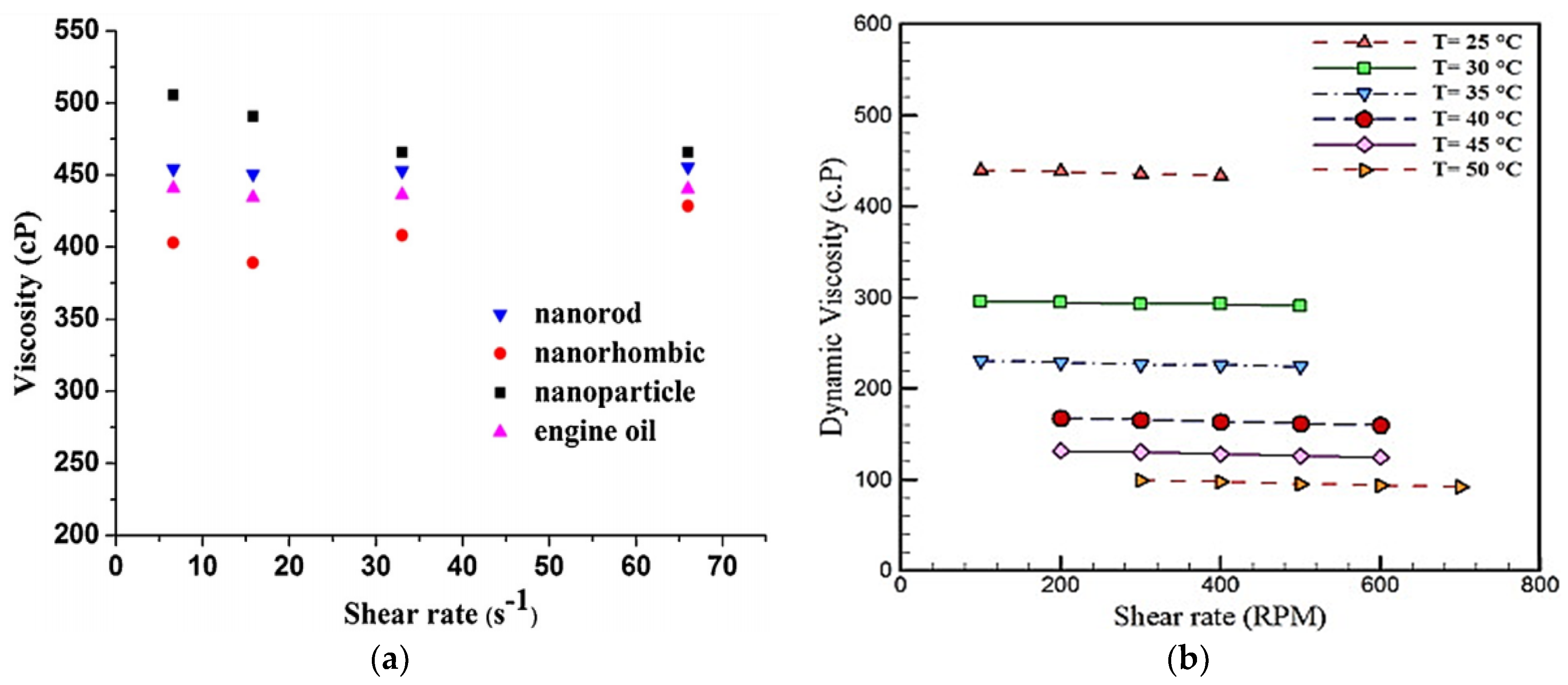
4.1. Viscosity
Prediction Models for the Viscosity
4.2. Heat Transfer Performance
4.2.1. Flash Point and Pour Point
4.2.2. Thermal Conductivity
4.2.3. Prediction Models for the Thermal Conductivity
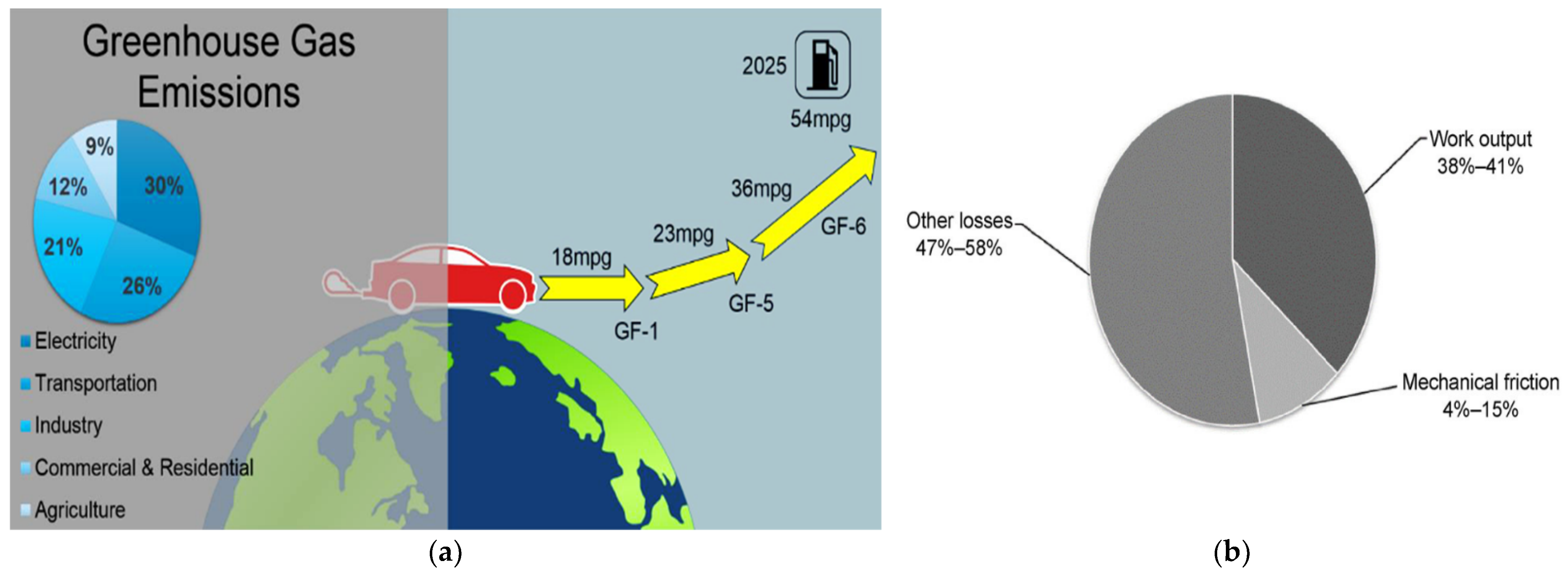
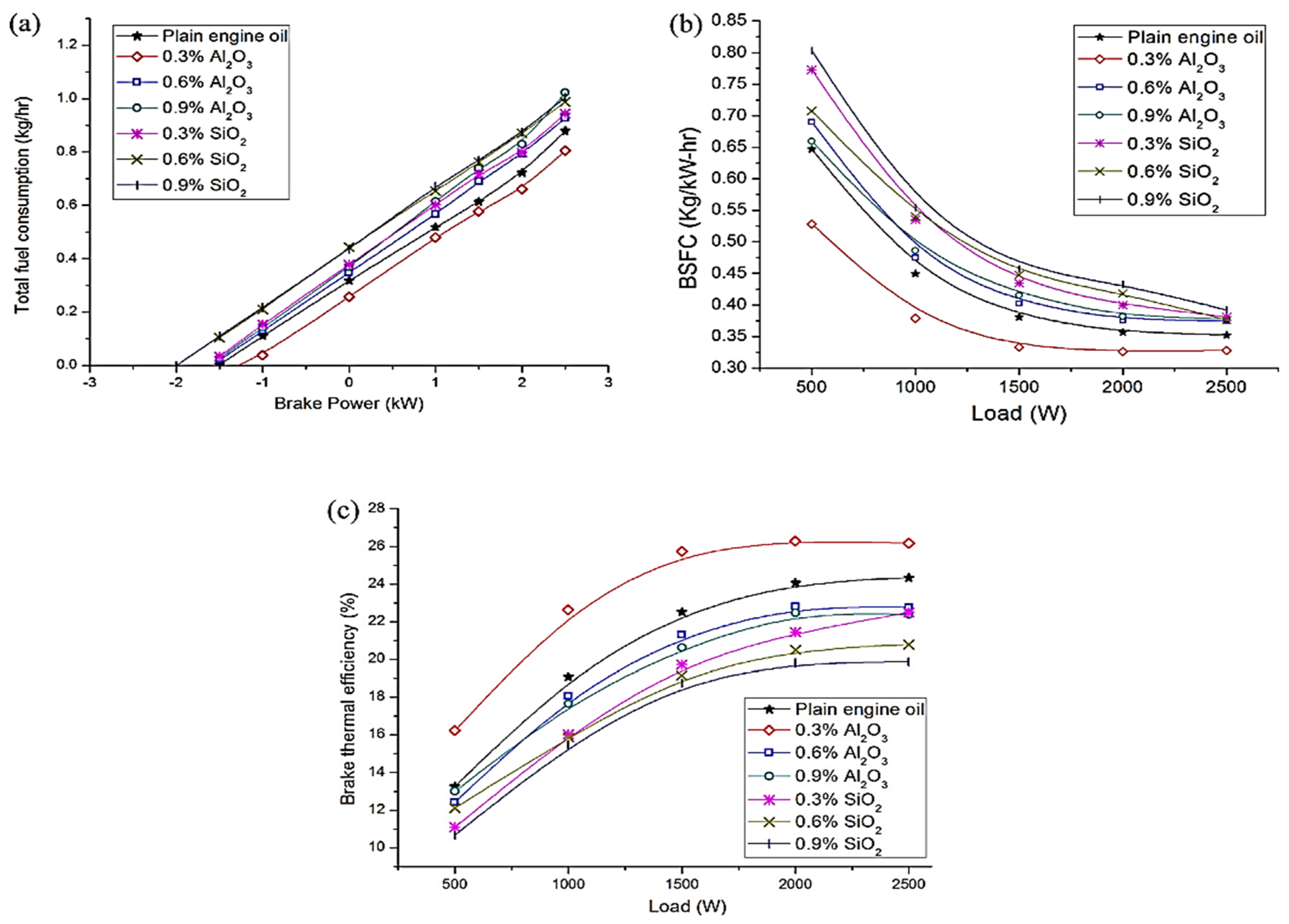
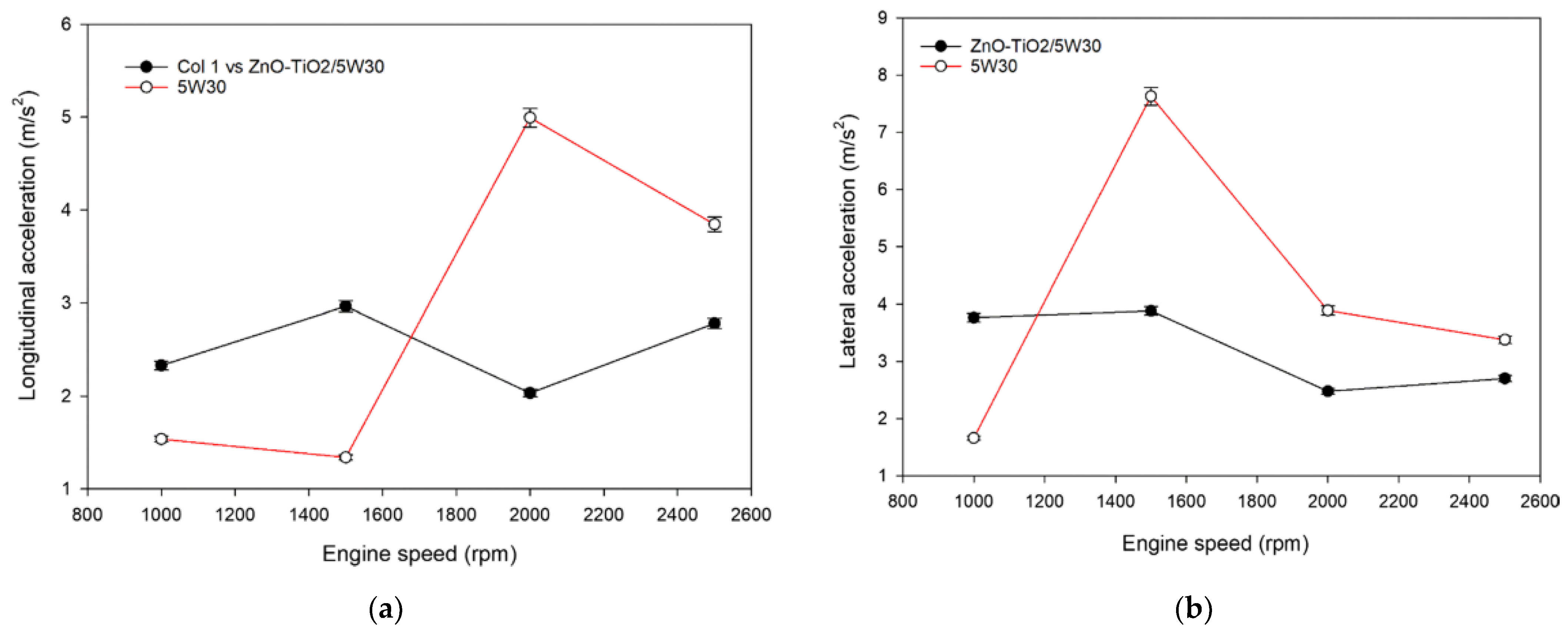
5. Fuel Economy and Emissions
6. Discussion
7. Future Directions and Challenges
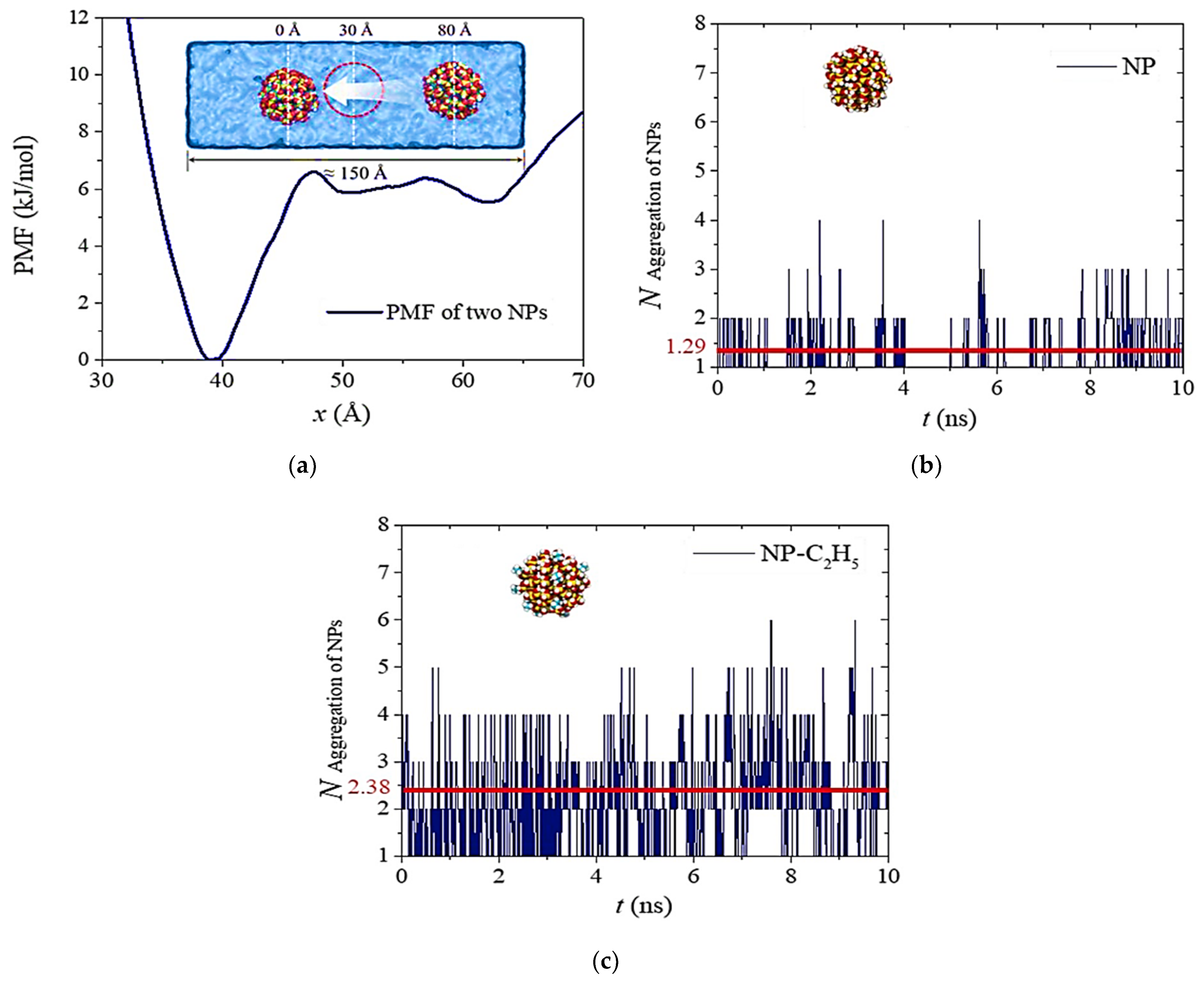
7.1. Molecular Dynamics Simulations
7.2. Ionic Based Nano Lubricants
7.3. Cost and Economics of Nanolubricants
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, M.K.A.; Xianjun, H.; Abdelkareem, M.A.A.; Elsheikh, A.H. Role of nanolubricants formulated in improving vehicle engines performance. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 563. [Google Scholar] [CrossRef]
- Shah, R.; Woydt, M.; Huq, N.; Rosenkranz, A. Tribology meets sustainability. Ind. Lubr. Tribol. 2020. [Google Scholar] [CrossRef]
- European Commission. EU Good Practice in Energy Efficiency, for a Sustainable, Safer and more Competion Goes to Overcome Friction; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Holmberg, K.; Erdemir, A. Tribology International The impact of tribology on energy use and CO 2 emission globally and in combustion engine and electric cars. Tribiol. Int. 2019, 135, 389–396. [Google Scholar] [CrossRef]
- Biberger, J.; Füßer, H. Tribology International Development of a test method for a realistic, single parameter-dependent analysis of piston ring versus cylinder liner contacts with a rotational tribometer. Tribiol. Int. 2017, 113, 111–124. [Google Scholar] [CrossRef]
- La, D.D.; Truong, T.N.; Pham, T.Q.; Vo, H.T.; Tran, N.T.; Nguyen, T.A.; Nadda, A.K.; Nguyen, T.T.; Chang, S.W.; Chung, W.J.; et al. Scalable fabrication of modified graphene nanoplatelets as an effective additive for engine lubricant oil. Nanomaterials 2020, 10, 877. [Google Scholar] [CrossRef]
- Abril, S.O.; Rojas, J.P.; Flórez, E.N. Numerical Methodology for Determining the Energy Losses in Auxiliary Systems and Friction Processes Applied to Low Displacement Diesel Engines. Lubricants 2020, 8, 103. [Google Scholar] [CrossRef]
- Knauder, C.; Allmaier, H.; Sander, D.E.; Sams, T. Investigations of the Friction Losses of Different Engine Concepts: Part 3: Friction Reduction Potentials and Risk Assessment at the Sub-Assembly Level. Lubricants 2020, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Knauder, C.; Allmaier, H.; Sander, D.E.; Sams, T. Investigations of the Friction Losses of Different Engine Concepts. Part 2: Sub-Assembly Resolved Friction Loss Comparison of Three Engines. Lubricants 2019, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Knauder, C.; Allmaier, H.; Sander, D.E.; Sams, T. Investigations of the Friction Losses of Different Engine Concepts. Part 1: A Combined Approach for Applying Subassembly-Resolved Friction Loss Analysis on a Modern Passenger-Car Diesel Engine. Lubricants 2019, 7, 39. [Google Scholar] [CrossRef] [Green Version]
- Aldana, P.U.; Vacher, B.; le Mogne, T.; Belin, M.; Thiebaut, B.; Dassenoy, F. Action Mechanism of WS2 Nanoparticles with ZDDP Additive in Boundary Lubrication Regime. Tribol. Lett. 2014, 56, 249–258. [Google Scholar] [CrossRef]
- Sharma, V.; Timmons, R.B.; Erdemir, A.; Aswath, P.B. Interaction of plasma functionalized TiO2 nanoparticles and ZDDP on friction and wear under boundary lubrication. Appl. Surf. Sci. 2019, 489, 372–383. [Google Scholar] [CrossRef]
- Hsu, C.-J.; Stratmann, A.; Rosenkranz, A.; Gachot, C. Enhanced Growth of ZDDP-Based Tribofilms on Laser-Interference Patterned Cylinder Roller Bearings. Lubricants 2017, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Gachot, C.; Hsu, C.; Suárez, S.; Grützmacher, P.; Rosenkranz, A.; Stratmann, A.; Jacobs, G. Microstructural and Chemical Characterization of the Tribolayer Formation in Highly Loaded Cylindrical Roller Thrust Bearings. Lubricants 2016, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Vyavhare, K.; Timmons, R.B.; Erdemir, A.; Edwards, B.L.; Aswath, P.B. Tribochemistry of fluorinated ZnO nanoparticles and ZDDP lubricated interface and implications for enhanced anti-wear performance at boundary lubricated contacts. Wear 2021, 474–475, 203717. [Google Scholar] [CrossRef]
- Ratoi, M.; Niste, V.B.; Walker, J.; Zekonyte, J. Mechanism of Action of WS2 Lubricant Nanoadditives in High-Pressure Contacts. Tribol. Lett. 2013, 52, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Bagi, S.; Aswath, P. Mechanism of Friction and Wear in MoS2 and ZDDP/F-PTFE Greases under Spectrum Loading Conditions. Lubricants 2015, 3, 687–711. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V. Development of Plasma Functionalized Nano-Additives for Oils and Study of Their Tribological Properties. Ph.D. Thesis, The University of Texas at Arlington, Arlington, TX, USA, 2017. [Google Scholar]
- Wong, V.W.; Tung, S.C. Overview of automotive engine friction and reduction trends–Effects of surface, material, and lubricant-additive technologies. Friction 2016, 4, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Srivyas, P.D.; Charoo, M.S. A Review on Tribological Characterization of Lubricants with Nano Additives for Automotive Applications. Tribol. Ind. 2018, 40, 594–623. [Google Scholar] [CrossRef]
- Tonk, R. The challenges and benefits of using carbon nano-tubes as friction modifier lubricant additives. Mater. Today Proc. 2020, 37, 3275–3278. [Google Scholar] [CrossRef]
- Kaleli, H.; Demirtaş, S.; Uysal, V.; Karnis, I.; Stylianakis, M.M.; Anastasiadis, S.H.; Kim, D.E. 920.Tribological performance investigation of a commercial engine oil incorporating reduced graphene oxide as additive. Nanomaterials 2021, 11, 386. [Google Scholar] [CrossRef]
- Tonk, R. The characterization analysis of multi walled carbon nanotubes coated with carboxylic group compounds to improve its dispersion properties in mineral base engine oils. Mater. Today Proc. 2020, 37, 3279–3282. [Google Scholar] [CrossRef]
- Shafi, W.K.; Charoo, M.S. An overall review on the tribological, thermal and rheological properties of nanolubricants, Tribology–Materials. Surf. Interfaces 2021, 15, 20–54. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H. Improving the heat transfer capability and thermal stability of vehicle engine oils using Al2O3/TiO2 nanomaterials. Powder Technol. 2020, 363, 48–58. [Google Scholar] [CrossRef]
- Charoo, M.S.; Hanief, M. Improving the tribological characteristics of a lubricating oil by nano sized additives. Mater. Today Proc. 2020, 28, 1205–1209. [Google Scholar] [CrossRef]
- Kałużny, J.; Waligórski, M.; Szymański, G.M.; Merkisz, J.; Różański, J.; Nowicki, M.; al Karawi, M.; Kempa, K. Reducing friction and engine vibrations with trace amounts of carbon nanotubes in the lubricating oil. Tribol. Int. 2020, 151. [Google Scholar] [CrossRef]
- Ivanov, M.; Shenderova, O. Nanodiamond-based nanolubricants for motor oils. Curr. Opin. Solid State Mater. Sci. 2017, 21, 17–24. [Google Scholar] [CrossRef]
- Kotia, A.; Chowdary, K.; Srivastava, I.; Ghosh, S.K.; Ali, M.K.A. Carbon nanomaterials as friction modifiers in automotive engines: Recent progress and perspectives. J. Mol. Liq. 2020, 310, 113200. [Google Scholar] [CrossRef]
- Vardhaman, B.S.A.; Amarnath, M.; Ramkumar, J.; Mondal, K. Enhanced tribological performances of zinc oxide/MWCNTs hybrid nanomaterials as the effective lubricant additive in engine oil. Mater. Chem. Phys. 2020, 253, 123447. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z. Experimental investigation of ZnO nanoparticles effects on thermophysical and tribological properties of diesel oil. Int. J. Hydrog. Energy 2020, 45, 23603–23614. [Google Scholar] [CrossRef]
- Mello, V.S.; Trajano, M.F.; Emilia, A.; Silva, D. Comparison Between the Action of Nano-Oxides and Conventional EP Additives in Boundary Lubrication. Lubricants 2020, 8, 54. [Google Scholar] [CrossRef]
- Esfe, M.H.; Mosaferi, M. Effect of MgO nanoparticles suspension on rheological behavior and a new correlation. J. Mol. Liq. 2020, 309, 112632. [Google Scholar] [CrossRef]
- Laad, M.; Jatti, V.K.S. Titanium oxide nanoparticles as additives in engine oil. J. King Saud Univ. Eng. Sci. 2016, 30, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Peng, R.; Du, H.; Shen, Y.; Li, Y.; Li, J.; Dong, G. The application of nano-MOS2 quantum dots as liquid lubricant additive for tribological behavior improvement. Nanomaterials 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karthikeyan, K.M.B.; Vijayanand, J.; Arun, K.; Rao, V.S. Thermophysical and wear properties of eco-friendly nano lubricants. Mater. Today Proc. 2020, 39, 285–291. [Google Scholar] [CrossRef]
- Yang, L.; Ji, W.; Mao, M.; Huang, J.N. An updated review on the properties, fabrication and application of hybrid-nanofluids along with their environmental effects. J. Clean. Prod. 2020, 257, 120408. [Google Scholar] [CrossRef]
- Mello, V.S.; Faria, E.A.; Alves, S.M.; Scandian, C. Enhancing Cuo nanolubricant performance using dispersing agents. Tribol. Int. 2020, 150, 106338. [Google Scholar] [CrossRef]
- Chen, Y.; Renner, P.; Liang, H. Dispersion of Nanoparticles in Lubricating Oil: A Critical Review. Lubricants 2019, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Rabaso, P.; Ville, F.; Dassenoy, F.; Diaby, M.; Afanasiev, P.; Cavoret, J.; Vacher, B.; le Mogne, T. Boundary lubrication: Influence of the size and structure of inorganic fullerene-like MoS2 nanoparticles on friction and wear reduction. Wear 2014, 320, 161–178. [Google Scholar] [CrossRef]
- Shaw, D. Introduction to Colloid and Surface Chemistry, 4th ed.; Elsevier: Oxford, UK; Boston, MA, USA, 2013. [Google Scholar] [CrossRef]
- Robins, M.; Fillery-Travis, A. Colloidal Dispersions; Russel, W.B., Saville, D.A., Schowalter, W.R., Eds.; Cambridge University Press: Cambridge, UK, 1989; Volume 17. [Google Scholar]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Azman, N.F.; Samion, S. Dispersion Stability and Lubrication Mechanism of Nanolubricants: A Review. Int. J. Precis. Eng. Manuf. Green Technol. 2019, 6, 393–414. [Google Scholar] [CrossRef]
- Babar, H.; Ali, H.M. Towards hybrid nanofluids: Preparation, thermophysical properties, applications, and challenges. J. Mol. Liq. 2019, 281, 598–633. [Google Scholar] [CrossRef]
- Shafi, W.K.; Charoo, M.S. NanoLubrication Systems: An Overview. Mater. Today Proc. 2018, 5, 20621–20630. [Google Scholar] [CrossRef]
- Asadi, A.; Aberoumand, S.; Moradikazerouni, A.; Pourfattah, F.; Żyła, G.; Estellé, P.; Mahian, O.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A. Recent advances in preparation methods and thermophysical properties of oil-based nanofluids: A state-of-the-art review. Powder Technol. 2019, 352, 209–226. [Google Scholar] [CrossRef]
- Jama, M.; Singh, T.; Gamaleldin, S.M.; Koc, M.; Samara, A.; Isaifan, R.J.; Atieh, M.A. Critical Review on Nanofluids: Preparation, Characterization, and Applications. J. Nanomater. 2016, 2016, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.K.A.; Xianjun, H. Colloidal stability mechanism of copper nanomaterials modified by ionic liquid dispersed in polyalphaolefin oil as green nanolubricants. J. Colloid Interface Sci. 2020, 578, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.A.; Xianjun, H. Role of bis2-ethylhexyl phosphate and Al2O3/TiO2 hybrid nanomaterials in improving the dispersion stability of nanolubricants. Tribol. Int. 2021, 155, 106767. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Shahram, J. Experimental investigation, model development and sensitivity analysis of rheological behavior of ZnO/10W40 nano-lubricants for automotive applications. Phys. Low-Dimens. Syst. Nanostruct. 2017, 90, 194–203. [Google Scholar] [CrossRef]
- Abdel-Rehim, A.A.; Akl, S.; Elsoudy, S. Investigation of the Tribological Behavior of Mineral Lubricant Using Copper Oxide Nano Additives. Lubricants 2021, 9, 16. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Turkson, R.F.; Peng, Z.; Chen, X. Enhancing the thermophysical properties and tribological behaviour of engine oils using nano-lubricant additivesAl2O3. RSC Adv. 2016, 6, 77913–77924. [Google Scholar] [CrossRef]
- Heredia-Cancino, J.A.; Ramezani, M.; Álvarez-Ramos, M.E. Effect of degradation on tribological performance of engine lubricants at elevated temperatures. Tribol. Int. 2018, 124, 230–237. [Google Scholar] [CrossRef]
- Kral, J.; Konecny, B.; Kral, J.; Madac, K.; Fedorko, G.; Molnar, V. Degradation and chemical change of longlife oils following intensive use in automobile engines. Measurement J. Int. Meas. Confed. 2014, 50, 34–42. [Google Scholar] [CrossRef]
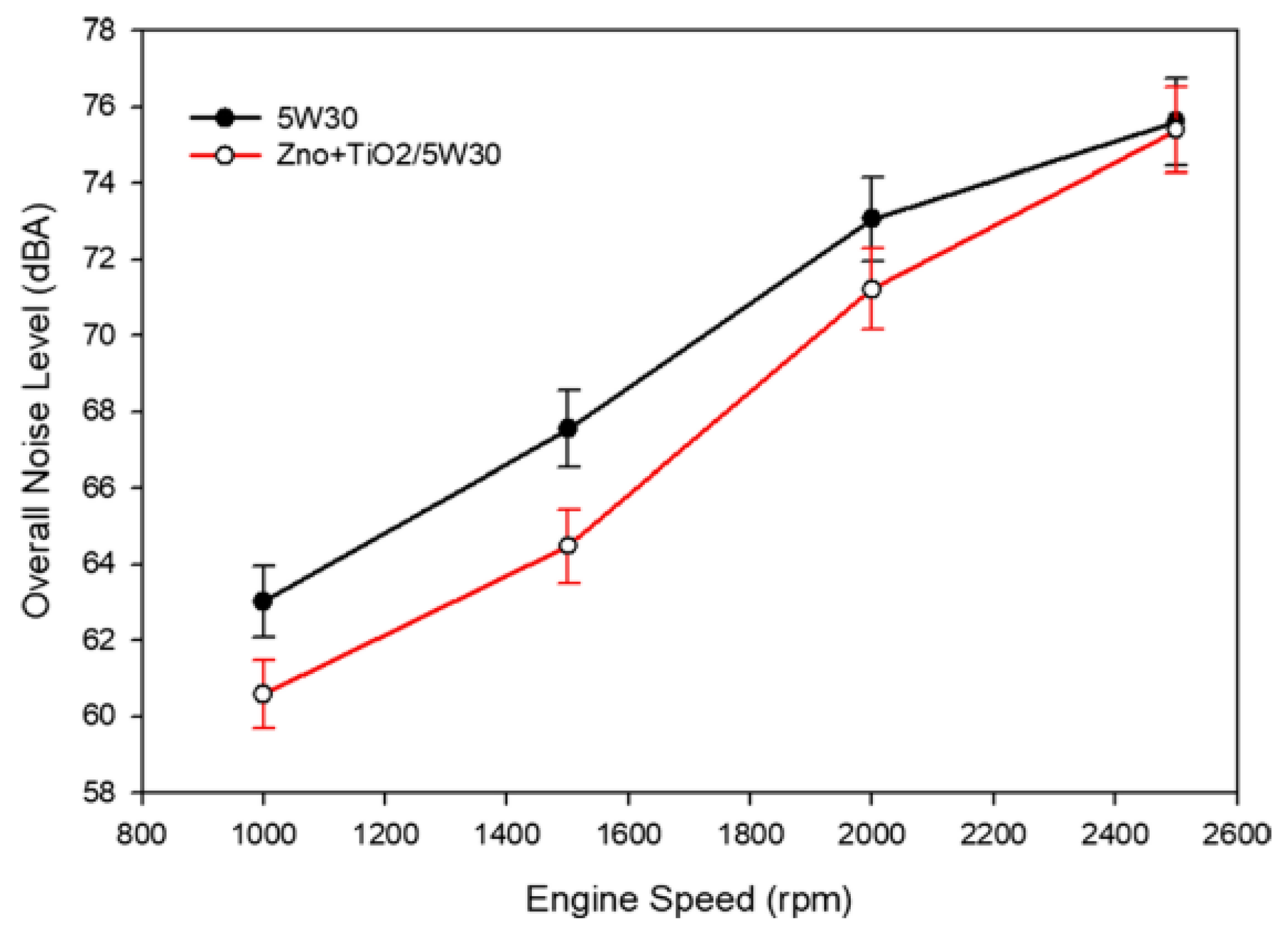
- Bharath, B.K.; Selvan, V.A.M. An Experimental Investigation on Rheological and Heat Transfer Performance of Hybrid Nanolubricant and Its Effect on the Vibration and Noise Characteristics of an Automotive Spark-Ignition Engine. Int. J. Thermophys. 2021, 42, 1–30. [Google Scholar] [CrossRef]
- Liu, K.N.; Zhang, Y.; Dai, F.; Sun, W. Improved heat transfer of the engine oil by changing it to hybrid nanofluid: Adding hybrid nano-powders. Powder Technol. 2021, 383, 56–64. [Google Scholar] [CrossRef]
- Gupta, H.; Rai, S.K.; Krishna, N.S.; Anand, G. Effect of silica nano-additive on flash point, pour point, rheological and tribological properties of lubricating engine oil: An experimental study. J. Dispers. Sci. Technol. 2021, 42, 622–632. [Google Scholar] [CrossRef]
- Singh, J.P.; Singh, S.; Nandi, T.; Ghosh, S.K. Development of graphitic lubricant nanoparticles based nanolubricant for automotive applications: Thermophysical and tribological properties followed by IC engine performance. Powder Technol. 2021, 387, 31–47. [Google Scholar] [CrossRef]
- Chouhan, A.; Sarkar, T.K.; Kumari, S.; Vemuluri, S.; Khatri, O.P. Synergistic lubrication performance by incommensurately stacked ZnO-decorated reduced graphene oxide/MoS2 heterostructure. J. Colloid Interface Sci. 2020, 580, 730–739. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Estellé, P. Experimental comparison between ZnO and MoS2 nanoparticles as additives on performance of diesel oil-based nano lubricant. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Wu, B.; Song, H.; Li, C.; Song, R.; Zhang, T.; Hu, X. Enhanced tribological properties of diesel engine oil with Nano-Lanthanum hydroxide/reduced graphene oxide composites. Tribol. Int. 2020, 141. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Hou, X.; Abdelkareem, M.A.A. Anti-wear properties evaluation of frictional sliding interfaces in automobile engines lubricated by copper/graphene nanolubricants. Friction 2019, 8, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, G.; Xie, G. Applied Surface Science Ultralow concentration of graphene oxide nanosheets as oil-based lubricant additives. Appl. Surf. Sci. 2019, 498, 143683. [Google Scholar] [CrossRef]
- Paul, G.; Shit, S.; Hirani, H.; Kuila, T.; Murmu, N.C. Tribological behavior of dodecylamine functionalized graphene nanosheets dispersed engine oil nanolubricants. Tribol. Int. 2019, 131, 605–619. [Google Scholar] [CrossRef]
- Ghasemi, R.; Fazlali, A.; Mohammadi, A.H. Effects of TiO 2 nanoparticles and oleic acid surfactant on the rheological behavior of engine lubricant oil. J. Mol. Liq. 2018, 268, 925–930. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Abdelkareem, M.A.A.; Gulzar, M.; Elsheikh, A.H. Novel approach of the graphene nanolubricant for energy saving via anti-friction/wear in automobile engines. Tribol. Int. 2018, 124, 209–229. [Google Scholar] [CrossRef]
- Kamal, M.; Ali, A.; Fuming, P.; Younus, H.A. Fuel economy in gasoline engines using Al2O3/TiO2 nanomaterials as nanolubricant additives. Appl. Energy 2018, 211, 461–478. [Google Scholar] [CrossRef]
- Demas, N.G.; Erck, R.A.; Lorenzo-martin, C.; Ajayi, O.O.; Fenske, G.R. Experimental Evaluation of Oxide Nanoparticles as Friction and Wear Improvement Additives in Motor Oil. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Sgroi, M.F.; Asti, M.; Gili, F.; Deorsola, F.A.; Bensaid, S.; Fino, D.; Kraft, G.; Garcia, I.; Dassenoy, F. Engine bench and road testing of an engine oil containing MoS2 particles as nano-additive for friction reduction. Tribol. Int. 2017, 105, 317–325. [Google Scholar] [CrossRef]
- Sepyani, K.; Afrand, M.; Esfe, M.H. An experimental evaluation of the effect of ZnO nanoparticles on the rheological behavior of engine oil. J. Mol. Liq. 2017, 236, 198–204. [Google Scholar] [CrossRef]
- Ran, X.; Yu, X.; Zou, Q. Effect of Particle Concentration on Tribological Properties of ZnO Nanofluids. Tribol. Trans. 2017, 60, 154–158. [Google Scholar] [CrossRef]
- Moghaddam, M.A.; Motahari, K. Experimental investigation, sensitivity analysis and modeling of rheological behavior of MWCNT-CuO 30–70/SAE40 hybrid nano-lubricant. Appl. Therm. Eng. 2017, 123, 1419–1433. [Google Scholar] [CrossRef]
- Wu, H.; Qin, L.; Dong, G.; Hua, M.; Yang, S.; Zhang, J. An investigation on the lubrication mechanism of MoS2 nano sheet in point contact: The manner of particle entering the contact area. Tribol. Int. 2017, 107, 48–55. [Google Scholar] [CrossRef]
- Esfe, M.H.; Afrand, M.; Yan, W.M.; Yarmand, H.; Toghraie, D.; Dahari, M. Effects of temperature and concentration on rheological behavior of MWCNTs/SiO220-80-SAE40 hybrid nano-lubricant. Int. Commun. Heat Mass Transf. 2016, 76, 133–138. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Mai, L.; Bicheng, C.; Turkson, R.F.; Qingping, C. Reducing frictional power losses and improving the scuffing resistance in automotive engines using hybrid nanomaterials as nano-lubricant additives. Wear 2016, 364–365, 270–281. [Google Scholar] [CrossRef]
- Asadi, M.; Asadi, A. Dynamic viscosity of MWCNT/ZnO-engine oil hybrid nanofluid: An experimental investigation and new correlation in different temperatures and solid concentrations. Int. Commun. Heat Mass Transf. 2016, 76, 41–45. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Yang, G.; Zhang, S.; Yu, L.; Zhang, P. Tribological properties of oleic acid-modified zinc oxide nanoparticles as the lubricant additive in poly-alpha olefin and diisooctyl sebacate base oils. RSC Adv. 2016, 6, 69836–69844. [Google Scholar] [CrossRef]
- Zheng, D.; Cai, Z.; Shen, M.; Li, Z.; Zhu, M. Investigation of the tribology behaviour of the graphene nanosheets as oil additives on textured alloy cast iron surface. Appl. Surf. Sci. 2016, 387, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Su, F.; Chen, Y. Supercritical Fluid Synthesis and Tribological Applications of Silver Nanoparticle-decorated Graphene in Engine Oil Nanofluid. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mungse, H.P.; Khatri, O.P. Dispersion and lubrication potential of nanosheets. RSC Adv. 2015, 25565–25571. [Google Scholar] [CrossRef]
- Koshy, C.P.; Rajendrakumar, P.K.; Thottackkad, M.V. Evaluation of the tribological and thermo-physical properties of coconut oil added with MoS2nanoparticles at elevated temperatures. Wear 2015, 330–331, 288–308. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, T.; Wang, J.; Ni, J.; Li, H.; Shao, X. Synthesis, characterization and tribological properties of Cu/reduced graphene oxide composites. Tribol. Int. 2015, 88, 17–24. [Google Scholar] [CrossRef]
- Zin, V.; Agresti, F.; Barison, S.; Colla, L.; Mercadelli, E.; Fabrizio, M.; Pagur, C. Tribological properties of engine oil with carbon nano-horns as nano-additives. Tribol. Lett. 2014, 55, 45–53. [Google Scholar] [CrossRef]
- Arumugam, S.; Sriram, G.; Ellappan, R. Bio-lubricant-biodiesel combination of rapeseed oil: An experimental investigation on engine oil tribology, performance, and emissions of variable compression engine. Energy 2014, 72, 618–627. [Google Scholar] [CrossRef]
- Wan, Q.; Jin, Y.; Sun, P.; Ding, Y. Rheological and tribological behaviour of lubricating oils containing platelet MoS2 nanoparticles. J. Nanopart. Res. 2014, 16, 2386. [Google Scholar] [CrossRef]
- Chen, T.; Xia, Y.; Jia, Z.; Liu, Z.; Zhang, H. Synthesis, Characterization, and Tribological Behavior of Oleic Acid Capped Graphene Oxide. J. Nanomater. 2014, 2014, 654145. [Google Scholar] [CrossRef]
- Mungse, H.P.; Khatri, O.P. Chemically functionalized reduced graphene oxide as a novel material for reduction of friction and wear. J. Phys. Chem. 2014, 118, 14394–14402. [Google Scholar] [CrossRef]
- Ettefaghi, E.o.l.; Ahmadi, H.; Rashidi, A.; Nouralishahi, A.; Mohtasebi, S.S. Preparation and thermal properties of oil-based nanofluid from multi-walled carbon nanotubes and engine oil as nano-lubricant. Int. Commun. Heat Mass Transf. 2013, 46, 142–147. [Google Scholar] [CrossRef]
- Ettefaghi, E.O.l.; Rashidi, A.; Ahmadi, H.; Mohtasebi, S.S.; Pourkhalil, M. Thermal and rheological properties of oil-based nanofluids from different carbon nanostructures. Int. Commun. Heat Mass Transf. 2013, 48, 178–182. [Google Scholar] [CrossRef]
- Demas, N.G.; Timofeeva, E.V.; Routbort, J.L.; Fenske, G.R. Tribological Effects of BN and MoS2 Nanoparticles Added to Polyalphaolefin Oil in Piston Skirt/Cylinder Liner Tests. Tribol. Lett. 2012, 47, 91–102. [Google Scholar] [CrossRef]
- Materials, A.C.S.A.; Madras, T.; Madras, T. Graphene-Based Engine Oil Nanofluids for Tribological Applications Graphene-Based Engine Oil Nanofluids for Tribological Applications. Tribol. Int. 2011, 4221–4227. [Google Scholar]
- Ahmed Ali, M.K.; Xianjun, H.; Essa, F.A.; Abdelkareem, M.A.A.; Elagouz, A.; Sharshir, S.W. Friction and Wear Reduction Mechanisms of the Reciprocating Contact Interfaces Using Nanolubricant Under Different Loads and Speeds. J. Tribol. 2018, 140. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, X.; Li, J. Graphene lubrication. Appl. Mater. Today 2020, 20, 100662. [Google Scholar] [CrossRef]
- Gulzar, M.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Zulkifli, N.W.M.; Mufti, R.A.; Zahid, R. Tribological performance of nanoparticles as lubricating oil additives. J. Nanopart. Res. 2016, 18, 223. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Mai, L.; Qingping, C.; Turkson, R.F.; Bicheng, C. Improving the tribological characteristics of piston ring assembly in automotive engines using Al2O3 and TiO2 nanomaterials as nano-lubricant additives. Tribol. Int. 2016, 103, 540–554. [Google Scholar] [CrossRef]
- Rajendhran, N.; Palanisamy, S.; Periyasamy, P.; Venkatachalam, R. Enhancing of the tribological characteristics of the lubricant oils using Ni-promoted MoS2 nanosheets as nano-additives. Tribol. Int. 2018, 118, 314–328. [Google Scholar] [CrossRef]
- Kheireddin, B.A. Tribological Properties of Nanoparticle Based Lubrication Systems. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2016. [Google Scholar]
- Ramón-Raygoza, E.D.; Rivera-Solorio, C.I.; Giménez-Torres, E.; Maldonado-Cortés, D.; Cardenas-Alemán, E.; Cué-Sampedro, R. Development of nanolubricant based on impregnated multilayer graphene for automotive applications: Analysis of tribological properties. Powder Technol. 2016, 302, 363–371. [Google Scholar] [CrossRef]
- Baskar, S.; Prabaharan, G.; Arumugam, S.; Nagabhooshanam, N. Modeling and Analysis of the Tribological Evaluation of Bearing Materials under the Influence of Nano Based Marine Lubricant Using D-Optimal Design. Mater. Today Proc. 2018, 5, 11548–11555. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Simionesie, D.; Schaschke, C. Graphite and hybrid nanomaterials as lubricant additives. Lubricants 2014, 2, 44–65. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Estellé, P. Viscosity, tribological and physicochemical features of ZnO and MoS2 diesel oil-based nanofluids: An experimental study. Fuel 2021, 293, 120481. [Google Scholar] [CrossRef]
- Tóth, D.; Szabó, I.; Kuti, R. Tribological Properties of Nano-Sized ZrO2 Ceramic Particles in Automotive Lubricants. FME Trans. 2020, 48, 36–43. [Google Scholar] [CrossRef]
- Thachnatharen, N.; Khalid, M.; Arulraj, A.; Sridewi, N. Materials Today: Proceedings. Tribological performance of hexagonal boron nitride hBN as nano-additives in military grade diesel engine oil. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Beheshti, A.; Huang, Y.; Ohno, K.; Blakey, I.; Stokes, J.R. Improving tribological properties of oil-based lubricants using hybrid colloidal additives. Tribol. Int. 2020, 144, 106130. [Google Scholar] [CrossRef]
- Avilés, M.D.; Pamies, R.; Sanes, J.; Bermúdez, M.D. Graphene-ionic liquid thin film nanolubricant. Nanomaterials 2020, 10, 535. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.Y.; Wang, S.R.; Wang, Y.; Wang, G.Q.; Yan, X.Y. The Influence of Nanocomposite Carbon additive on Tribological Behavior of Cylinder Liner/Piston Ring. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 491. [Google Scholar] [CrossRef]
- Asnida, M.; Hisham, S.; Awang, N.W.; Amirruddin, A.K.; Noor, M.M.; Kadirgama, K.; Ramasamy, D.; Najafi, G.; Tarlochan, F. Copper II oxide nanoparticles as additve in engine oil to increase the durability of piston-liner contact. Fuel 2018, 212, 656–667. [Google Scholar] [CrossRef]
- Borda, F.L.G.; de Oliveira, S.J.R.; Lazaro, L.M.S.M.; Leiróz, A.J.K. Experimental investigation of the tribological behavior of lubricants with additive containing copper nanoparticles. Tribol. Int. 2018, 117, 52–58. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Li, W.; Wu, P.-R.; Liu, Z. Study on structure-activity relationship between size and tribological properties of graphene oxide nanosheets in oil. J. Alloy. Compd. 2017, 722, 778–784. [Google Scholar] [CrossRef]
- Rasheed, A.K.; Khalid, M.; Javeed, A.; Rashmi, W.; Gupta, T.C.S.M.; Chan, A. Heat transfer and tribological performance of graphene nanolubricant in an internal combustion engine. Tribol. Int. 2016, 103, 504–515. [Google Scholar] [CrossRef]
- Ali, M.K.A.; Xianjun, H.; Elagouz, A.; Essa, F.A.; Abdelkareem, M.A.A. Minimizing of the boundary friction coefficient in automotive engines using Al2O3 and TiO2 nanoparticles. J. Nanopart. Res. 2016, 18, 377. [Google Scholar] [CrossRef]
- Scherge, M.; Böttcher, R.; Kürten, D.; Linsler, D. Multi-Phase Friction and Wear Reduction by Copper Nanopartices. Lubricants 2016, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Jeng, Y.-R.; Huang, Y.-H.; Tsai, P.-C.; Hwang, G.-L. Tribological Performance of Oil-Based Lubricants with Carbon-Fe Nanocapsules Additive. Tribol Trans. 2015, 58, 924–929. [Google Scholar] [CrossRef]
- Peña-Parás, L.; Taha-Tijerina, J.; Garza, L.; Maldonado-Cortés, D.; Michalczewski, R.; Lapray, C. Effect of CuO and Al2O3 nanoparticle additives on the tribological behavior of fully formulated oils. Wear 2015, 332–333, 1256–1261. [Google Scholar] [CrossRef]
- Padgurskas, J.; Rukuiza, R.; Prosyčevas, I.; Kreivaitis, R. Tribological properties of lubricant additives of Fe, Cu and Co nanoparticles. Tribol. Int. 2013, 60, 224–232. [Google Scholar] [CrossRef]
- Zhang, B.S.; Xu, B.S.; Xu, Y.; Gao, F.; Shi, P.J.; Wu, Y.X. CU nanoparticles effect on the tribological properties of hydrosilicate powders as lubricant additive for steelsteel contacts. Tribol. Int. 2011, 44, 878–886. [Google Scholar] [CrossRef]
- Abdullah, M.I.H.C.; Abdollah, M.F.B.; Amiruddin, H.; Tamaldin, N.; Nuri, N.R.M. Optimization of Tribological Performance of hBN/AL2O3Nanoparticles as Engine Oil Additives. Procedia Eng. 2013, 68, 313–319. [Google Scholar] [CrossRef] [Green Version]
- Ghaednia, H. An Analytical and Experimental Investigation of Nanoparticle Lubricants. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2014. [Google Scholar]
- Pe, L.; García-pineda, P.; Montemayor, O.E.; Nava, K.L.; Martini, A. Tribology International Effects of substrate surface roughness and nano/micro particle additive size on friction and wear in lubricated sliding. Tribol. Int. 2018, 119, 88–98. [Google Scholar] [CrossRef]
- Vaitkunaite, G.; Espejo, C.; Wang, C.; Thi, B.; Charrin, C.; Neville, A.; Morina, A. Tribology International MoS 2 tribofilm distribution from low viscosity lubricants and its effect on friction. Tribol. Int. 2020, 151, 106531. [Google Scholar] [CrossRef]
- Esfe, M.H.; Arani, A.A.A.; Esfandeh, S.; Afrand, M. Proposing new hybrid nano-engine oil for lubrication of internal combustion engines: Preventing cold start engine damages and saving energy. Energy 2019, 170, 228–238. [Google Scholar] [CrossRef]
- Stachowiak, G.; Batchelor, A.W. Engineering Tribology, 4th ed.; Elsevier: Butterworth, Malaysia, 2014. [Google Scholar]
- Wang, Q.J.; Chung, Y.-W. (Eds.) Encyclopedia of Tribology; Springer: Boston, MA, USA, 2013. [Google Scholar] [CrossRef]
- Chhabra, R.P. Non-Newtonian Fluids: An Introduction. In Rheology of Complex Fluids; Springer: New York, NY, USA, 2010; pp. 3–34. [Google Scholar] [CrossRef]
- Farbod, M.; asl, R.K.; abadi, A.R.N. Morphology dependence of thermal and rheological properties of oil-based nanofluids of CuO nanostructures. Colloids Surf. Physicochem. Eng. Asp. 2015, 474, 71–75. [Google Scholar] [CrossRef]
- Dardan, E.; Afrand, M.; Isfahani, A.H.M. Effect of suspending hybrid nano-additives on rheological behavior of engine oil and pumping power. Appl. Therm. Eng. 2016, 109, 524–534. [Google Scholar] [CrossRef]
- Asadi, A.; Asadi, M.; Rezaei, M.; Siahmargoi, M.; Asadi, F. The effect of temperature and solid concentration on dynamic viscosity of MWCNT/MgO 20–80–SAE50 hybrid nano-lubricant and proposing a new correlation: An experimental study. Int. Commun. Heat Mass Transf. 2016, 78, 48–53. [Google Scholar] [CrossRef]
- Hemmat Esfe, M.; Abbasian Arani, A.A.; Esfandeh, S. Improving engine oil lubrication in light-duty vehicles by using of dispersing MWCNT and ZnO nanoparticles in 5W50 as viscosity index improvers (VII). Appl. Therm. Eng. 2018, 143, 493–506. [Google Scholar] [CrossRef]
- Ma, J.; Shahsavar, A.; Al-Rashed, A.A.A.A.; Karimipour, A.; Yarmand, H.; Rostami, S. Viscosity, cloud point, freezing point and flash point of zinc oxide/SAE50 nanolubricant. J. Mol. Liq. 2020, 298, 112045. [Google Scholar] [CrossRef]
- Asadi, A.; Pourfattah, F. Heat transfer performance of two oil-based nanofluids containing ZnO and MgO nanoparticles; a comparative experimental investigation. Powder Technol. 2019, 343, 296–308. [Google Scholar] [CrossRef]
- Asadi, A.; Asadi, M.; Rezaniakolaei, A.; Rosendahl, L.A.; Wongwises, S. An experimental and theoretical investigation on heat transfer capability of Mg OH2/MWCNT-engine oil hybrid nano-lubricant adopted as a coolant and lubricant fluid. Appl. Therm. Eng. 2018, 129, 577–586. [Google Scholar] [CrossRef]
- Motahari, K.; Moghaddam, M.A.; Moradian, M. Experimental investigation and development of new correlation for influences of temperature and concentration on dynamic viscosity of MWCNT-SiO2 20-80/20W50 hybrid nano-lubricant. Chin. J. Chem. Eng. 2018, 26, 152–158. [Google Scholar] [CrossRef]
- Esfe, M.H.; Esfandeh, S. Investigation of rheological behavior of hybrid oil based nanolubricant-coolant applied in car engines and cooling equipments. Appl. Therm. Eng. 2018, 131, 1026–1033. [Google Scholar] [CrossRef]
- Esfe, M.H.; Rostamian, H.; Rejvani, M.; Emami, M.R.S. Rheological behavior characteristics of ZrO 2-MWCNT/10w40 hybrid nano-lubricant affected by temperature, concentration, and shear rate: An experimental study and a neural network simulating. Phys. Low-Dimens. Syst. Nanostruct. 2018, 102, 160–170. [Google Scholar] [CrossRef]
- Nadooshan, A.A.; Esfe, M.H.; Afrand, M. Evaluation of rheological behavior of 10W40 lubricant containing hybrid nano-material by measuring dynamic viscosity. Physica Low Dimens. Syst. Nanostruct. 2017, 92, 47–54. [Google Scholar] [CrossRef]
- Esfe, M.H.; Rostamian, H. Non-Newtonian power-law behavior of TiO 2 /SAE 50 nano-lubricant: An experimental report and new correlation. J. Mol. Liq. 2017, 232, 219–225. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A. Experimental study on synthesis, stability, thermal conductivity and viscosity of Cu–engine oil nanofluid. J. Taiwan Inst. Chem. Eng. 2017, 71, 315–322. [Google Scholar] [CrossRef]
- Alirezaie, A.; Saedodin, S.; Esfe, M.H.; Rostamian, S.H. Investigation of rheological behavior of MWCNT COOH-functionalized/MgO-Engine oil hybrid nanofluids and modelling the results with artificial neural networks. J. Mol. Liq. 2017, 241, 173–181. [Google Scholar] [CrossRef]
- Esfe, M.H.; Afrand, M.; Rostamian, S.H.; Toghraie, D. Examination of rheological behavior of MWCNTs/ZnO-SAE40 hybrid nano-lubricants under various temperatures and solid volume fractions. Exp. Therm. Fluid Sci. 2017, 80, 384–390. [Google Scholar] [CrossRef]
- Afrand, M.; Najafabadi, K.N.; Akbari, M. Effects of temperature and solid volume fraction on viscosity of SiO2-MWCNTs/SAE40 hybrid nanofluid as a coolant and lubricant in heat engines. Appl. Therm. Eng. 2016, 102, 45–54. [Google Scholar] [CrossRef]
- Vakili-Nezhaad, G.R.; Dorany, A. Investigation of the effect of multiwalled carbon nanotubes on the viscosity index of lube oil cuts. Chem. Eng. Commun. 2009, 196, 997–1007. [Google Scholar] [CrossRef]
- Desai, N.; Nagaraj, A.M.; Sabnis, N. Materials Today: Proceedings Analysis of thermo-physical properties of SAE20W40 engine oil by the addition of SiO 2 nanoparticles. Mater. Today Proc. 2021, 2–7. [Google Scholar] [CrossRef]
- Brinkman, H.C. The Viscosity of Concentrated Suspensions and Solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Esfe, M.H.; Arani, A.A.A.; Esfandeh, S. Experimental study on rheological behavior of monograde heavy-duty engine oil containing CNTs and oxide nanoparticles with focus on viscosity analysis. J. Mol. Liq. 2018, 272, 319–329. [Google Scholar] [CrossRef]
- Esfe, M.H.; Karimpour, R.; Arani, A.A.A.; Shahram, J. Experimental investigation on non-Newtonian behavior of Al2O3-MWCNT/5W50 hybrid nano-lubricant affected by alterations of temperature, concentration and shear rate for engine applications. Int. Commun. Heat Mass Transf. 2017, 82, 97–102. [Google Scholar] [CrossRef]
- Esfe, M.H.; Rostamian, H.; Sarlak, M.R.; Rejvani, M.; Alirezaie, A. Rheological behavior characteristics of TiO2-MWCNT/10w40 hybrid nano-oil affected by temperature, concentration and shear rate: An experimental study and a neural network simulating. Phys. Low-Dimens. Syst. Nanostruct. 2017, 94, 231–240. [Google Scholar] [CrossRef]
- Afrand, M.; Najafabadi, K.N.; Sina, N.; Safaei, M.R.; Kherbeet, A.S.; Wongwises, S.; Dahari, M. Prediction of dynamic viscosity of a hybrid nano-lubricant by an optimal artificial neural network. Int. Commun. Heat Mass Transf. 2016, 76, 209–214. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ozkaymak, M.; Sözen, A.; Menlik, T.; Fahed, A. Improving car radiator performance by using TiO2-water nanofluid. Eng. Sci. Technol. Int. J. 2018, 21, 996–1005. [Google Scholar] [CrossRef]
- Wrenick, S.; Sutor, P.; Pangilinan, H.; Schwarz, E.E. Heat Transfer Properties of Engine Oils. In Proceedings of the World Tribology Congress III, ASMEDC, Washington, DC, USA, 12–16 September 2005; Volume 1, pp. 595–596. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Mao, M.; Huang, J.n.; Ji, W. Enhancing the thermal conductivity of SAE 50 engine oil by adding zinc oxide nano-powder: An experimental study. Powder Technol. 2019, 356, 335–341. [Google Scholar] [CrossRef]
- Deepak, S.N.; Ram, C.N. Physio-chemical study of traditional lubricant SAE 20 W40 and virgin coconut oil using TiO2 nano-additives. Mater. Today Proc. 2021, 42, 1024–1029. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A.; Moravej, M.; Aberoumand, H.; Javaherdeh, K. Experimental study on the rheological behavior of silver-heat transfer oil nanofluid and suggesting two empirical based correlations for thermal conductivity and viscosity of oil based nanofluids. Appl. Therm. Eng. 2016, 101, 362–372. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, R. Role of Brownian motion on the thermal conductivity enhancement of nanofluids. Appl. Phys. Lett. 2007, 91, 223102. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, A.; Nadeem, S. Metachronal wave analysis for non-Newtonian fluid under thermophoresis and Brownian motion effects. Res. Phys. 2017, 7, 2950–2957. [Google Scholar] [CrossRef]
- Harish, R.; Sivakumar, R. Effects of nanoparticle dispersion on turbulent mixed convection flows in cubical enclosure considering Brownian motion and thermophoresis. Powder Technol. 2021, 378, 303–316. [Google Scholar] [CrossRef]
- Asadi, A.; Asadi, M.; Rezaniakolaei, A.; Rosendahl, L.A.; Afrand, M.; Wongwises, S. Heat transfer efficiency of Al2O3-MWCNT/thermal oil hybrid nanofluid as a cooling fluid in thermal and energy management applications: An experimental and theoretical investigation. Int. J. Heat Mass Transf. 2018, 117, 474–486. [Google Scholar] [CrossRef]
- Asadi, A. A guideline towards easing the decision-making process in selecting an effective nanofluid as a heat transfer fluid. Energy Convers. Manag. 2018, 175, 1–10. [Google Scholar] [CrossRef]
- Ettefaghi, E.; Ahmadi, H.; Rashidi, A.; Mohtasebi, S.; Alaei, M. Experimental evaluation of engine oil properties containing copper oxide nanoparticles as a nanoadditive. Int. J. Ind. Chem. 2013, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Hemmati-Sarapardeh, A.; Varamesh, A.; Amar, M.N.; Husein, M.M.; Dong, M. On the evaluation of thermal conductivity of nanofluids using advanced intelligent models. Int. Commun. Heat Mass Transf. 2020, 118, 104825. [Google Scholar] [CrossRef]
- Sulgani, M.T.; Karimipour, A. Improve the thermal conductivity of 10w40-engine oil at various temperature by addition of Al 2 O 3 /Fe 2 O 3 nanoparticles. J. Mol. Liq. 2019, 283, 660–666. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Tamura, K.; Kasai, M.; Nakamura, Y.; Enomoto, T. Impact of Boundary Lubrication Performance of Engine Oils on Friction at Piston Ring-Cylinder Liner Interface. SAE Int. J. Fuels Lubr. 2014, 7, 875–881. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Sarma, P.K.; Srinivas, V.; Rao, V.D.; Kumar, A.K. Experimental study and analysis of lubricants dispersed with nano cu and tio2 in a four-stroke two wheeler. Nanoscale Res. Lett. 2011, 6, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotia, A.; Borkakoti, S.; Ghosh, S.K. Wear and performance analysis of a 4-stroke diesel engine employing nanolubricants. Particuology 2018, 37, 54–63. [Google Scholar] [CrossRef]
- Marcano, S.J.C.; Bensaid, S.; Deorsola, F.A.; Russo, N.; Fino, D. Nanolubricants for diesel engines: Related emissions and compatibility with the after-treatment catalysts. Tribol. Int. 2014, 72, 198–207. [Google Scholar] [CrossRef]
- Beig, G.; Sahu, S.K.; Singh, V.; Tikle, S.; Sobhana, S.B.; Gargeva, P.; Ramakrishna, K.; Rathod, A.; Murthy, B.S. Recent developments of nanoparticles additives to the consumables liquids in internal combustion engines: Part II: Nano-lubricants. Sci. Total Environ. 2019, 319, 114156. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Mamat, H. Nanofluids: Thermal Conductivity and Applications. Ref. Modul. Mater. Sci. Mater. Eng. 2019. [Google Scholar] [CrossRef]
- Sarno, M.; Scarpa, D.; Senatore, A.; Mustafa, W.A.A. rGO/GO nanosheets in tribology: From the state of the art to the future prospective. Lubricants 2020, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Ripoll, M.R.; Tomala, A.M.; Totolin, V.; Remškar, M. Performance of nanolubricants containing MoS 2 nanotubes during form tapping of zinc-coated automotive components. J. Manuf. Process. 2019, 39, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Basheer, A.A.; Kucherova, A.; Memetov, N.; Pasko, T.; Ovchinnikov, K.; Pershin, V.; Kuznetsov, D.; Galunin, E.; Grachev, V.; et al. Advances in carbon nanomaterials as lubricants modifiers. J. Mol. Liq. 2019, 279, 251–266. [Google Scholar] [CrossRef]
- Yamakov, V.; Wolf, D.; Phillpot, S.R.; Mukherjee, A.K.; Gleiter, H. Deformation-mechanism map for nanocrystalline metals by molecular-dynamics simulation. Nat. Mater. 2004, 3, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.; Mondal, P.; Padmanabhan, K.A. Plastic deformation of nanocrystalline materials. Nanostruct. Mater. 1997, 9, 603–606. [Google Scholar] [CrossRef]
- Fan, G.J.; Choo, H.; Liaw, P.K.; Lavernia, E.J. A model for the inverse Hall–Petch relation of nanocrystalline materials. Mater. Sci. Eng. 2005, 409, 243–248. [Google Scholar] [CrossRef]
- Schiotz, J. A Maximum in the Strength of Nanocrystalline Copper. Science 2003, 301, 1357–1359. [Google Scholar] [CrossRef] [Green Version]
- Bernholc, J.; Brenner, D.; Nardelli, M.B.; Meunier, V.; Roland, C. Mechanical and Electrical Properties of Nanotubes. Annu. Rev. Mater. Res. 2002, 32, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Yu, C.; Zhang, L.; Xie, G.; Guo, D.; Luo, J. Intelligent lubricating materials: A review. Compos. Part B Eng. 2020, 202, 108450. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.; Guo, Y.; Qi, H.; Che, Q.; Zhang, G. PEEK reinforced with low-loading 2D graphitic carbon nitride nanosheets: High wear resistance under harsh lubrication conditions. Compos. Part A Appl. Sci. Manuf. 2018, 109, 507–516. [Google Scholar] [CrossRef]
- Rodriguez, A.; Jaman, M.S.; Acikgoz, O.; Wang, B.; Yu, J.; Grützmacher, P.G.; Rosenkranz, A.; Baykara, M.Z. The potential of Ti3C2TX nano-sheets MXenes for nanoscale solid lubrication revealed by friction force microscopy. Appl. Surf. Sci. 2021, 535, 147664. [Google Scholar] [CrossRef]
- Wang, F.F.; Liu, Z.; Cheng, Z.L. High performance of MOF-structured lubricating material with nano- and micro-sized morphologies. Mater. Lett. 2019, 248, 222–226. [Google Scholar] [CrossRef]
- Wyatt, B.C.; Rosenkranz, A.; Anasori, B. 2D MXenes: Tunable Mechanical and Tribological Properties. Adv. Mater. 2021, 33, 2007973. [Google Scholar] [CrossRef]
- Malaki, M.; Varma, R.S. Mechanotribological Aspects of MXene-Reinforced Nanocomposites. Adv. Mater. 2020, 32, 202003154. [Google Scholar] [CrossRef]
- Yin, X.; Jin, J.; Chen, X.; Rosenkranz, A.; Luo, J. Ultra-Wear-Resistant MXene-Based Composite Coating via in Situ Formed Nanostructured Tribofilm. ACS Appl. Mater. Interfaces 2019, 11, 32569–32576. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Dong, S.; Ye, Z.; Wei, Y. Synthesis and tribological property of Ti3C2TX nanosheets. J. Mater. Sci. 2017, 52, 2200–2209. [Google Scholar] [CrossRef]
- Yang, J.; Chen, B.; Song, H.; Tang, H.; Li, C. Synthesis, characterization, and tribological properties of two-dimensional Ti3C2. Cryst. Res. Technol. 2014, 49, 926–932. [Google Scholar] [CrossRef]
- Hu, C.; Bai, M.; Lv, J.; Li, X. Molecular dynamics simulation of mechanism of nanoparticle in improving load-carrying capacity of lubricant film. Comput. Mater. Sci. 2015, 109, 97–103. [Google Scholar] [CrossRef]
- Chen, J.; Dai, F.; Zhang, L.; Xu, J.; Liu, W.; Zeng, S.; Xu, C.; Chen, L.; Dai, C. Molecular insights into the dispersion stability of graphene oxide in mixed solvents: Theoretical simulations and experimental verification. J. Colloid Interface Sci. 2020, 571, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Bai, M.; Lv, J.; Wang, P.; Zhang, L.; Li, X. Molecular dynamics simulation of nanofluid’s flow behaviors in the near-wall model and main flow model. Microfluid. Nanofluid. 2014, 17, 581–589. [Google Scholar] [CrossRef]
- Mirzaamiri, R.; Akbarzadeh, S.; Ziaei-Rad, S.; Shin, D.G.; Kim, D.E. Molecular dynamics simulation and experimental investigation of tribological behavior of nanodiamonds in aqueous suspensions. Tribol. Int. 2021, 156, 106838. [Google Scholar] [CrossRef]
- Hu, C.; Bai, M.; Lv, J.; Kou, Z.; Li, X. Tribology International Molecular dynamics simulation on the tribology properties of two hard nanoparticles diamond and silicon dioxide confined by two iron blocks. Tribiol. Int. 2015, 90, 297–305. [Google Scholar] [CrossRef]
- Fang, T.; Zhang, Y.; Yan, Y.; Dai, C.; Zhang, J. Molecular insight into the aggregation and dispersion behavior of modified nanoparticles. J. Pet. Sci. Eng. 2020, 191, 107193. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, Z.; Qian, Q.; Song, J.; Chen, Q.; Yang, J.; Wang, C.; Tu, Y. Association of Lennard-Jones particles in nanoconfined aqueous solution: Theory and molecular dynamics simulations. Phys. Stat. Mech. Appl. 2021, 563, 125414. [Google Scholar] [CrossRef]
- Abu-Hamdeh, N.H.; Almatrafi, E.; Hekmatifar, M.; Toghraie, D.; Golmohammadzadeh, A. Molecular dynamics simulation of the thermal properties of the Cu-water nanofluid on a roughed Platinum surface: Simulation of phase transition in nanofluids. J. Mol. Liq. 2020, 327, 114832. [Google Scholar] [CrossRef]
- Hu, C.; Bai, M.; Lv, J.; Liu, H.; Li, X. Applied Surface Science Molecular dynamics investigation of the effect of copper nanoparticle on the solid contact between friction surfaces. Appl. Surf. Sci. 2014, 321, 302–309. [Google Scholar] [CrossRef]
- Hu, C.; Lv, J.; Bai, M.; Zhang, X.; Tang, D. Molecular dynamics simulation of effects of nanoparticles on frictional heating and tribological properties at various temperatures. Friction 2020, 8, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lu, B.; Wu, Y.; Wang, J.; Zhang, X.; Wang, L.; Xi, D. Molecular dynamics simulation and experimental study on the lubrication of graphene additive films. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2020, 234, 1–16. [Google Scholar] [CrossRef]
- Hu, C.; Bai, M.; Lv, J.; Wang, P.; Li, X. Molecular dynamics simulation on the friction properties of nanofluids confined by idealized surfaces. Tribol. Int. 2014, 78, 152–159. [Google Scholar] [CrossRef]
- Hu, C.; Yi, C.; Bai, M.; Lv, J.; Tang, D. Molecular dynamics study of the frictional properties of multilayer MoS 2. RSC Adv. 2020, 10, 17418–17426. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Marian, M.; Profito, F.J.; Aragon, N.; Shah, R. The use of artificial intelligence in tribology—A perspective. Lubricants 2021, 9, 2. [Google Scholar] [CrossRef]
- Friedrich, K.; Reinicke, R.; Zhang, Z. Wear of polymer composites. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2002, 216, 415–426. [Google Scholar] [CrossRef]
- Argatov, I. Artificial Neural Networks ANNs as a Novel Modeling Technique in Tribology. Front. Mech. Eng. 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Mahrova, M.; Pagano, F.; Pejakovic, V.; Valea, A.; Kalin, M.; Igartua, A.; Tojo, E. Pyridinium based dicationic ionic liquids as base lubricants or lubricant additives. Tribol. Int. 2015, 82, 245–254. [Google Scholar] [CrossRef]
- Zhou, Y.; Leonard, D.N.; Guo, W.; Qu, J. Understanding Tribofilm Formation Mechanisms in Ionic Liquid Lubrication. Sci. Rep. 2017, 7, 8426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shameem, A.C.M.; Samad, B.A.; Koshy, G.; Suresh, T.G.A.; Mana, A.P. Assessment of the tribological performance of ionic liquid additive in engine oil for automotive application. J. Tribol. 2019, 21, 21–34. [Google Scholar]
- Barnhill, W.C. Tribological Testing and Analysis of Ionic Liquids as Candidate Anti-Wear Additives for Next-Generation Engine Lubricants. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2016. [Google Scholar]
- Bapat, A.P.; Erck, R.; Seymour, B.T.; Zhao, B.; Cosimbescu, L. Lipophilic polymethacrylate ionic liquids as lubricant additives. Eur. Polym. J. 2018, 108, 38–47. [Google Scholar] [CrossRef]
- González, R.; Viesca, J.L.; Battez, A.H.; Had, M.; Fernández-gonzález, A.; Bartolomé, M. Two phosphonium cation-based ionic liquids as lubricant additive to a polyalphaolefin base oil. J. Mol. Liq. 2019, 293, 111536. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic liquids as lubricant additives: A review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef]
- Oulego, P.; Blanco, D.; Ramos, D.; Viesca, J.L.; Díaz, M.; Battez, A.H. Environmental properties of phosphonium, imidazolium and ammonium cation-based ionic liquids as potential lubricant additives. J. Mol. Liq. 2018, 272, 937–947. [Google Scholar] [CrossRef]
- Guo, J.; Barber, G.C.; Schall, D.J.; Zou, Q.; Jacob, S.B. Tribological properties of ZnO and WS2 nanofluids using different surfactants. Wear 2017, 382–383, 8–14. [Google Scholar] [CrossRef]
- Wciślik, S. A simple economic and heat transfer analysis of the nanoparticles use. Chem. Pap. 2017, 71, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Alirezaie, A.; Hajmohammad, M.H.; Ahangar, M.R.H.; Esfe, M.H. Price-performance evaluation of thermal conductivity enhancement of nanofluids with different particle sizes. Appl. Therm. Eng. 2018, 128, 373–380. [Google Scholar] [CrossRef]
- Maji, N.C.; Krishna, H.P.; Chakraborty, J. Low-cost and high-throughput synthesis of copper nanopowder for nanofluid applications. Chem. Eng. J. 2018, 353, 34–45. [Google Scholar] [CrossRef]
- Esfe, M.H.; Arani, A.A.A.; Badi, R.S.; Rejvani, M. ANN modeling, cost performance and sensitivity analyzing of thermal conductivity of DWCNT–SiO2/EG hybrid nanofluid for higher heat transfer. J. Therm. Anal. Calorim. 2018, 131, 2381–2393. [Google Scholar] [CrossRef]
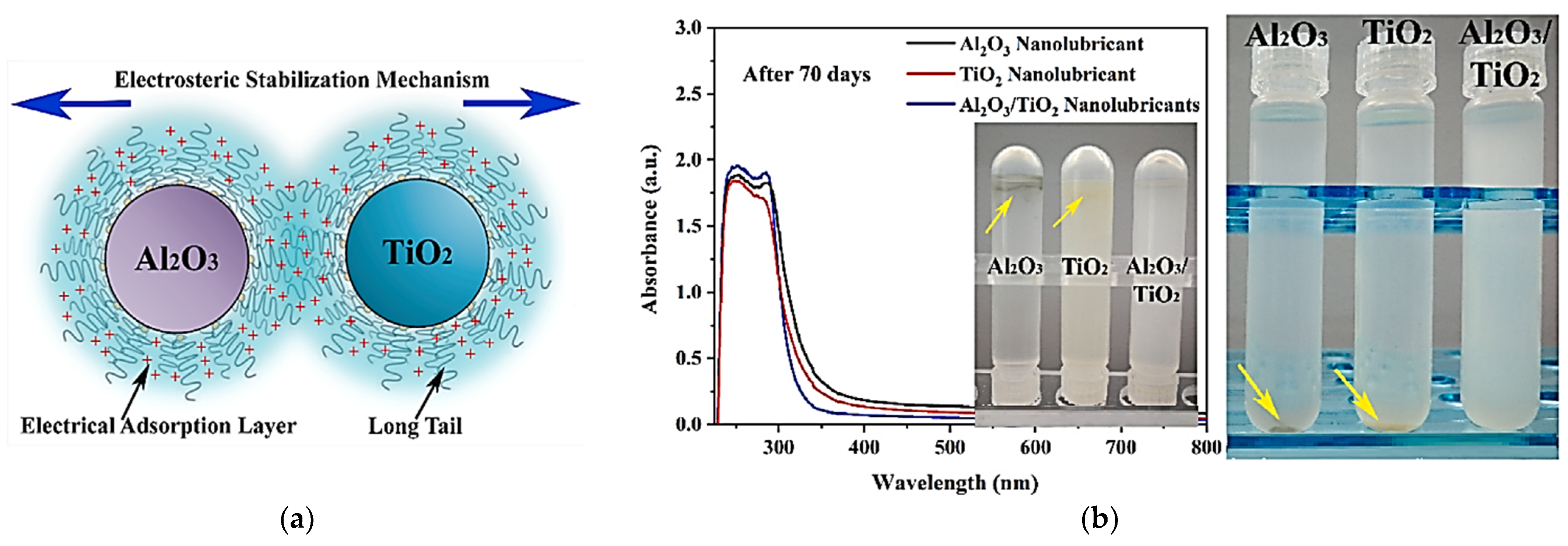
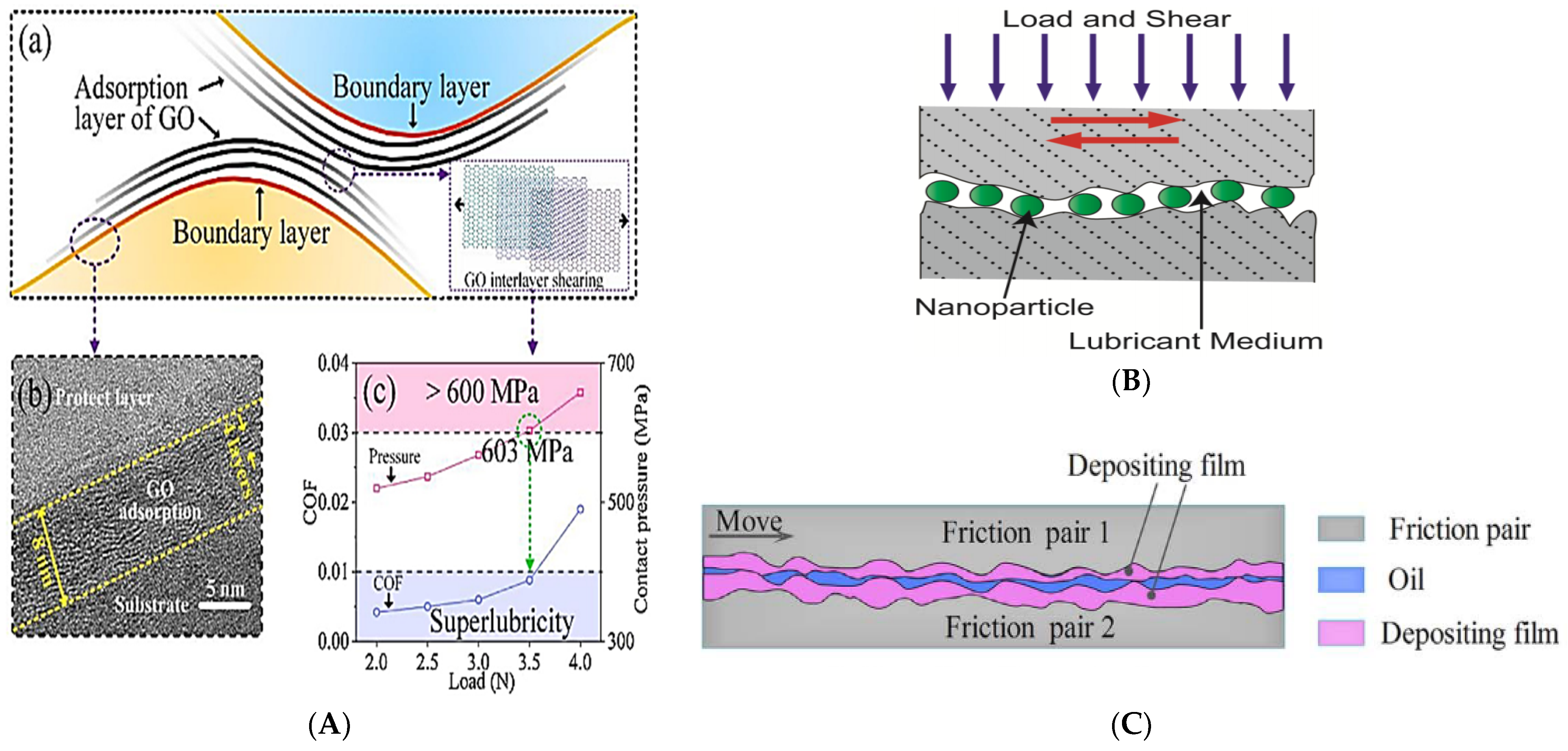
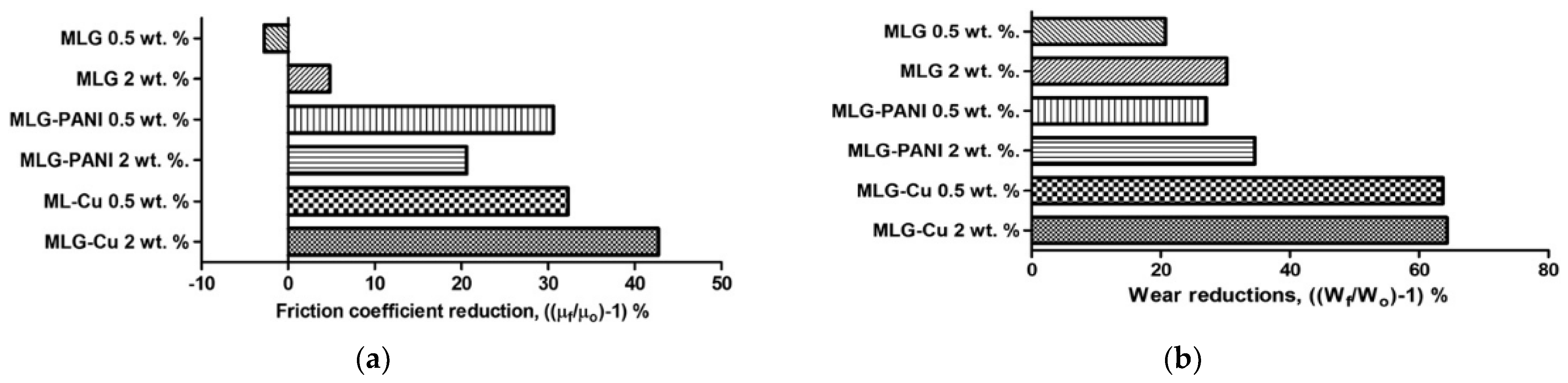
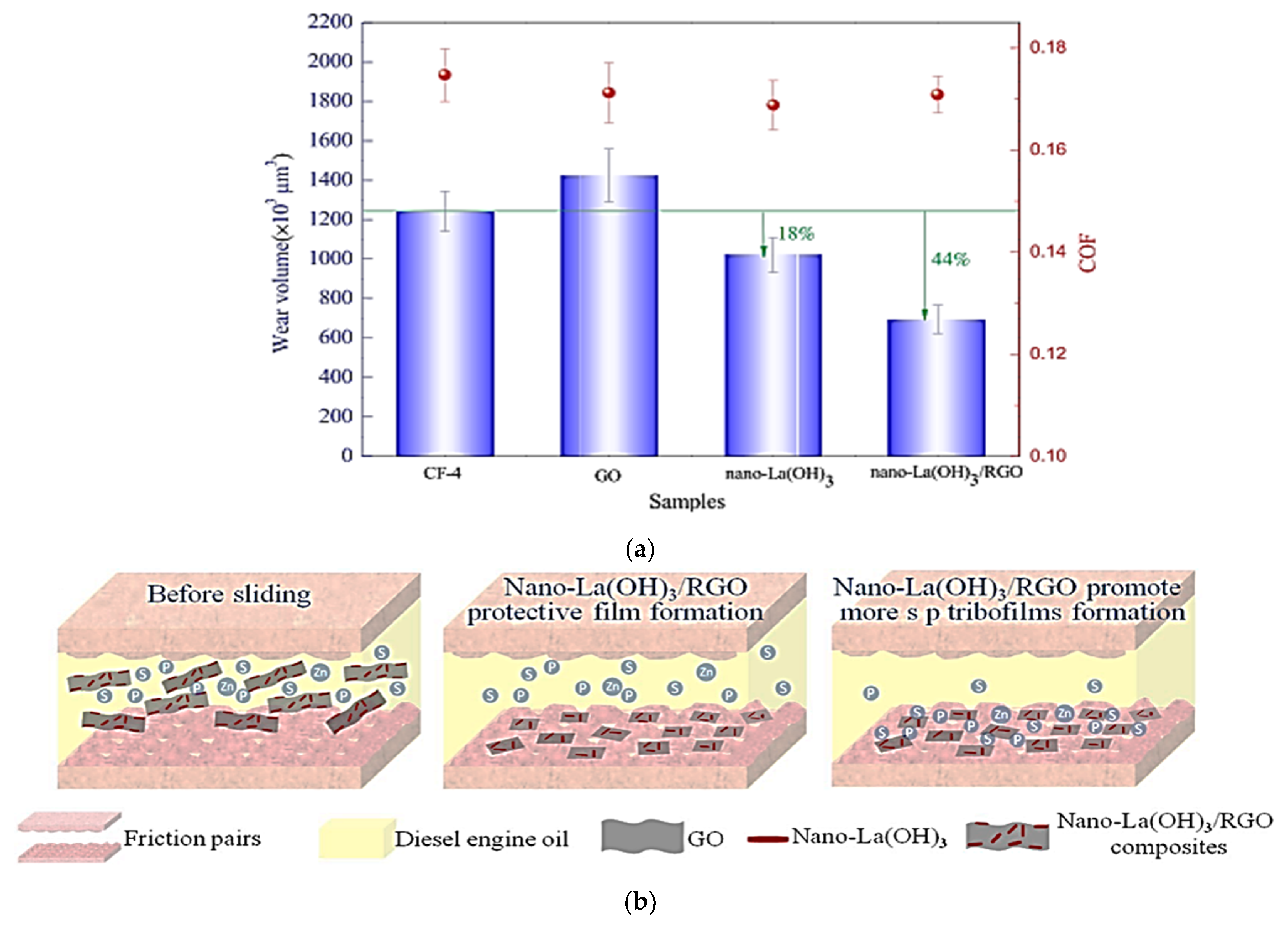
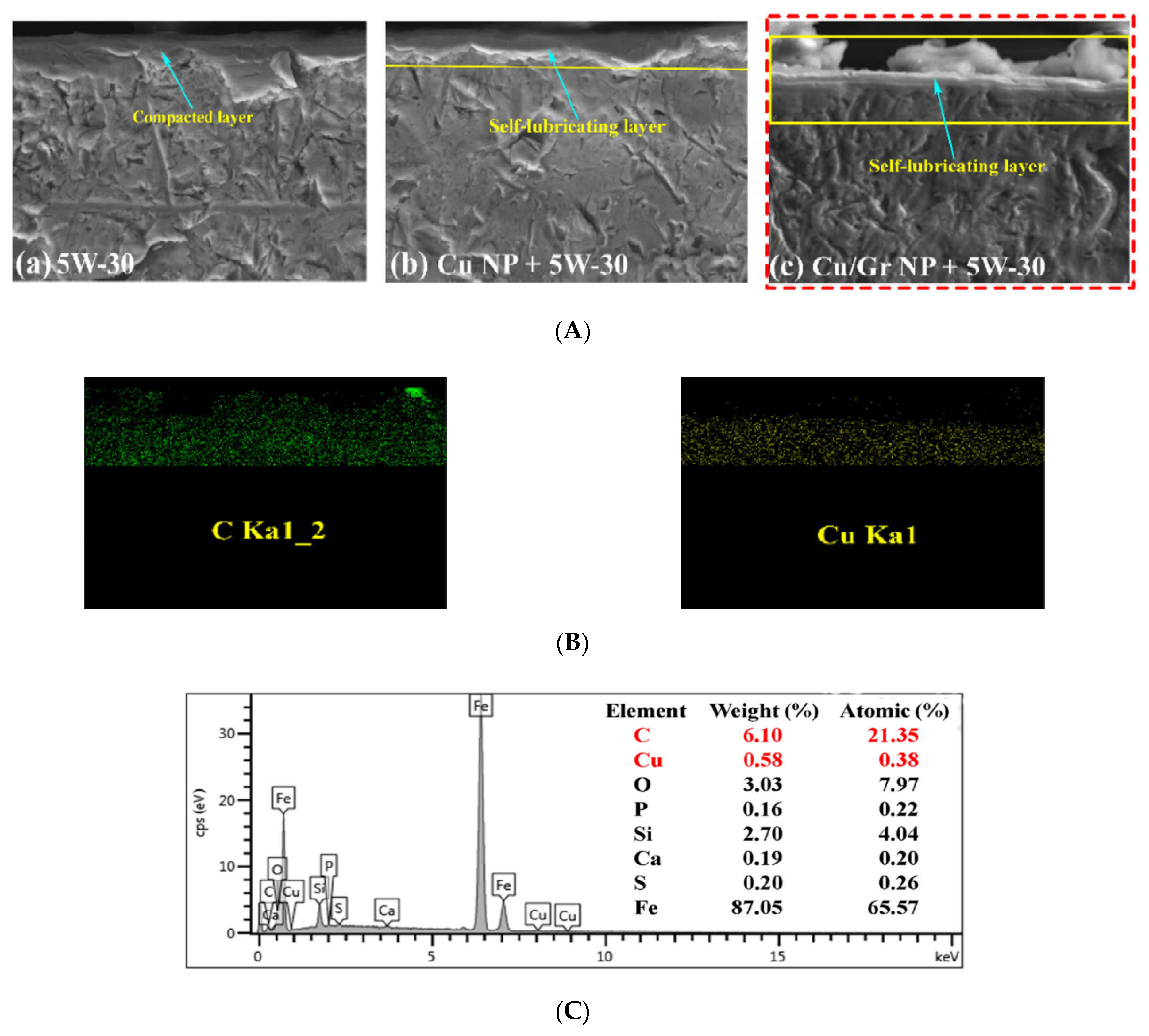
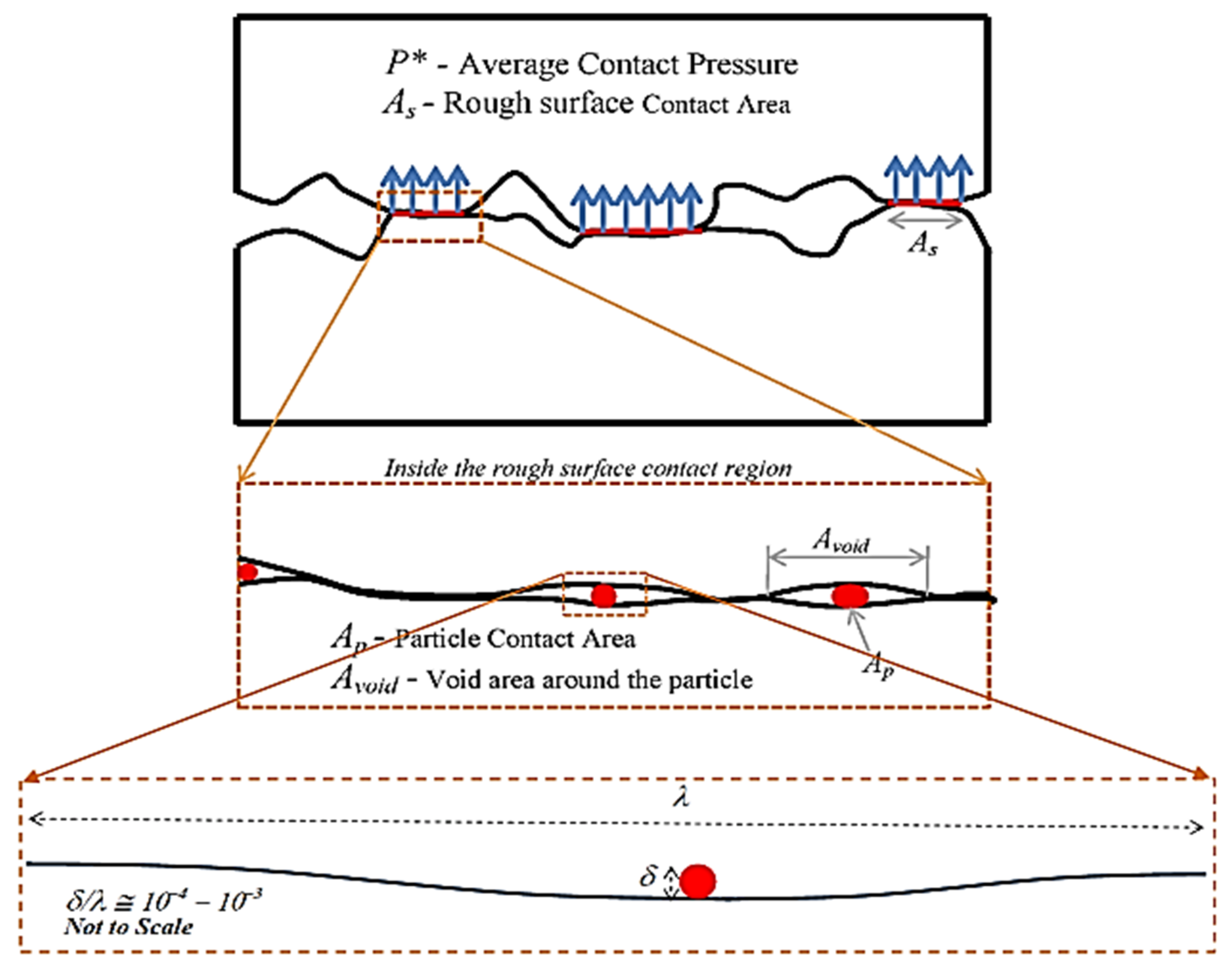

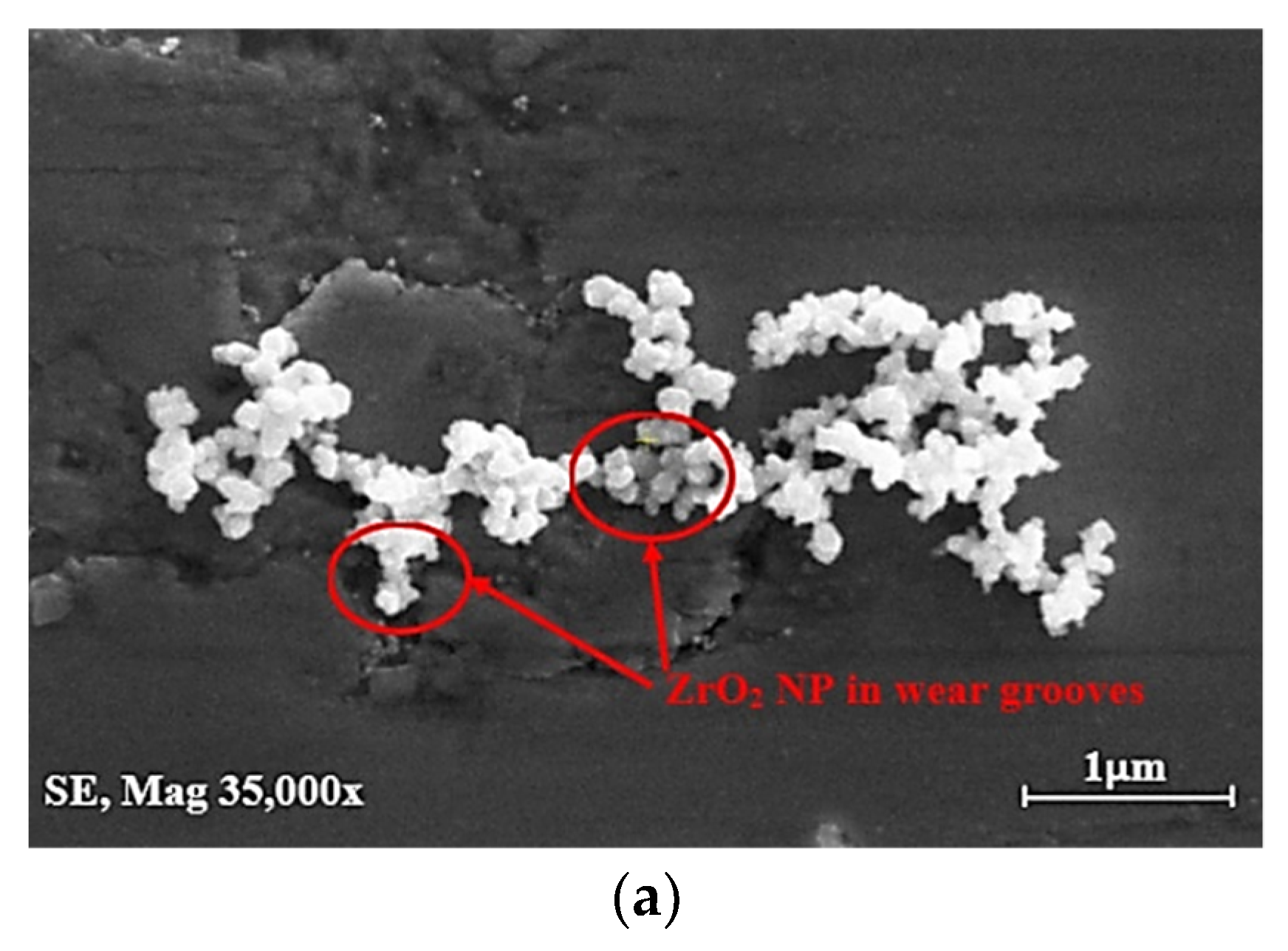
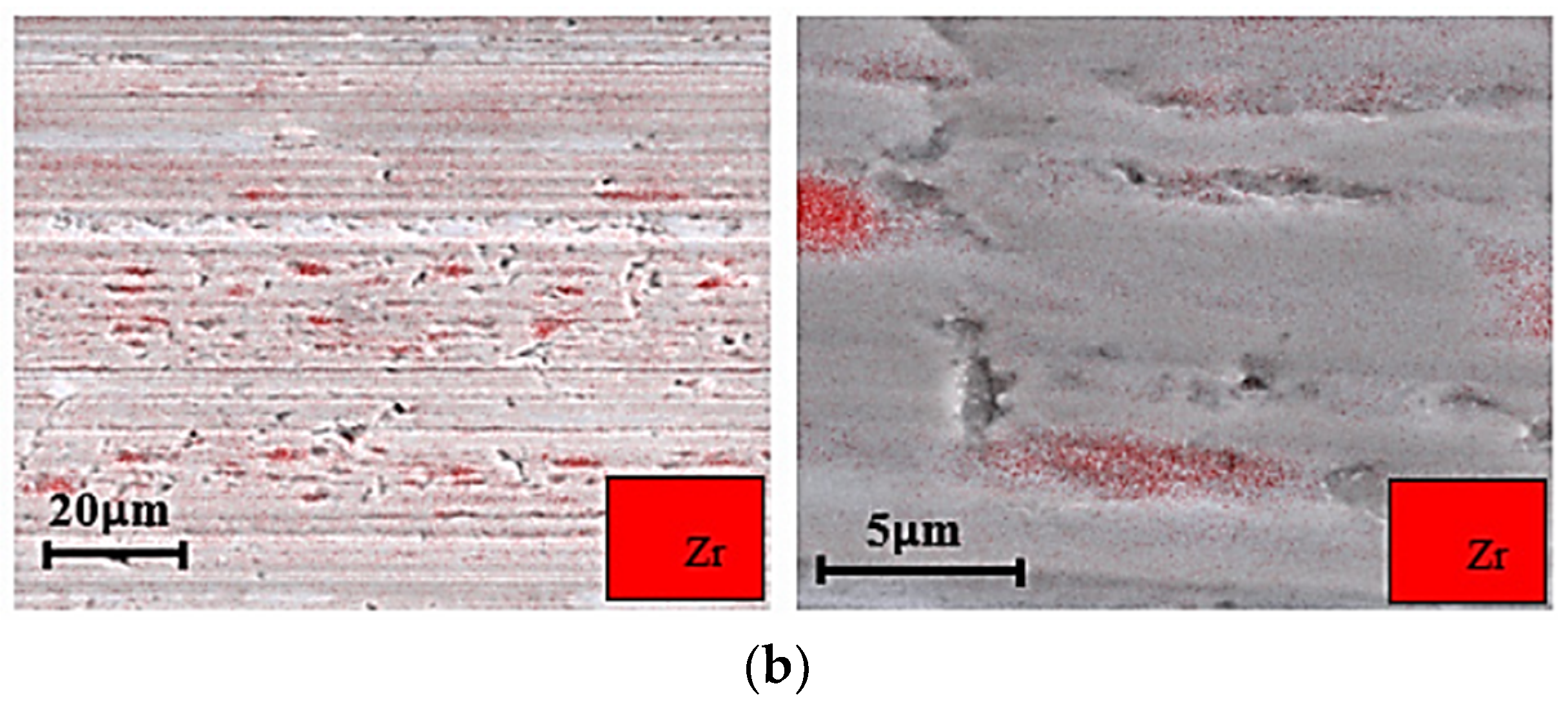








| Ase Lubricant | Nano-Additive | Preparation Method | Optimal Concentration in Terms of Stability | Dispersing Agents | Testing of Depression | Stable Condition/Duration | Processing Method/Processing Time | Reference | Year |
|---|---|---|---|---|---|---|---|---|---|
| Base as lubricant/PAO6 | Two-step method | 0.1 wt.% | HDEHP | Sedimentation, Zeta potential, FTIR, UV | Stable/70 days | Ultrasonic dispersion/6 h Magnetic stirrer | [50] | 2021 | |
| SAE 5 W-30 | Two-step method | All concentrations | Oleic acid | Sedimentation | Stable/1 week | Ultrasonic dispersion/2 h Magnetic stirrer | [56] | 2021 | |
| SAE 20 W-40 | Two-step method | 0.1 | - | Zeta potential | Stable/72 h | Ultrasonic dispersion/3 h | [57] | 2021 | |
| SAE 40 | Two-step method | All concentrations | - | Sedimentation | Stable/1 month | Ultrasonic dispersion/40 min Magnetic stirrer/30 min | [58] | 2021 | |
| SAE 30 | Graphite | 0.3 wt.% | 0.3 wt.% | Tween-80, Ethylene glycol | Sedimentation, zeta potential, | Stable/ 15 days | Ultrasonic dispersion/1.5 h | [59] | 2021 |
| Base as lubricant/paroline oil | One-step method | All concentrations | Not reported | Sedimentation | Stable/10 days | Ultrasonic dispersion | [35] | 2020 | |
| Base as lubricant/HD 50 | Gr | Two-step method | 0.01 wt.% | Sodium dodecyl persulfate (SDS), Oleic acid (OA) | Sedimentation, FTIR, XRD | Stable/30 days | Magnetic stirrer/3 h, Ultrasonic dispersion/12 h | [6] | 2020 |
| SAE 10W-40 | Gr-MS-Zn | One-step method | 0.5 wt.% | PEHA | Sedimentation | Stable/30 days | Not reported | [60] | 2020 |
| SAE 10W-40 | ZnO/MWCNT | Two-step method | 0.25 wt.% | Oleic acid (OA) | Sedimentation | Stable/15 days | Ultrasonic dispersion/2 h | [30] | 2020 |
| SAE 40 | Two-step method | 0.1 wt.% | Not reported | Sedimentation | Stable-ZnO/6 days | Ultrasonic bath/45 min | [61] | 2020 | |
| SAE 5W-30 | MWCNT | Two-step method | 0.03 wt.% | Liqui Moly | Not reported | Stable | Not reported | [27] | 2020 |
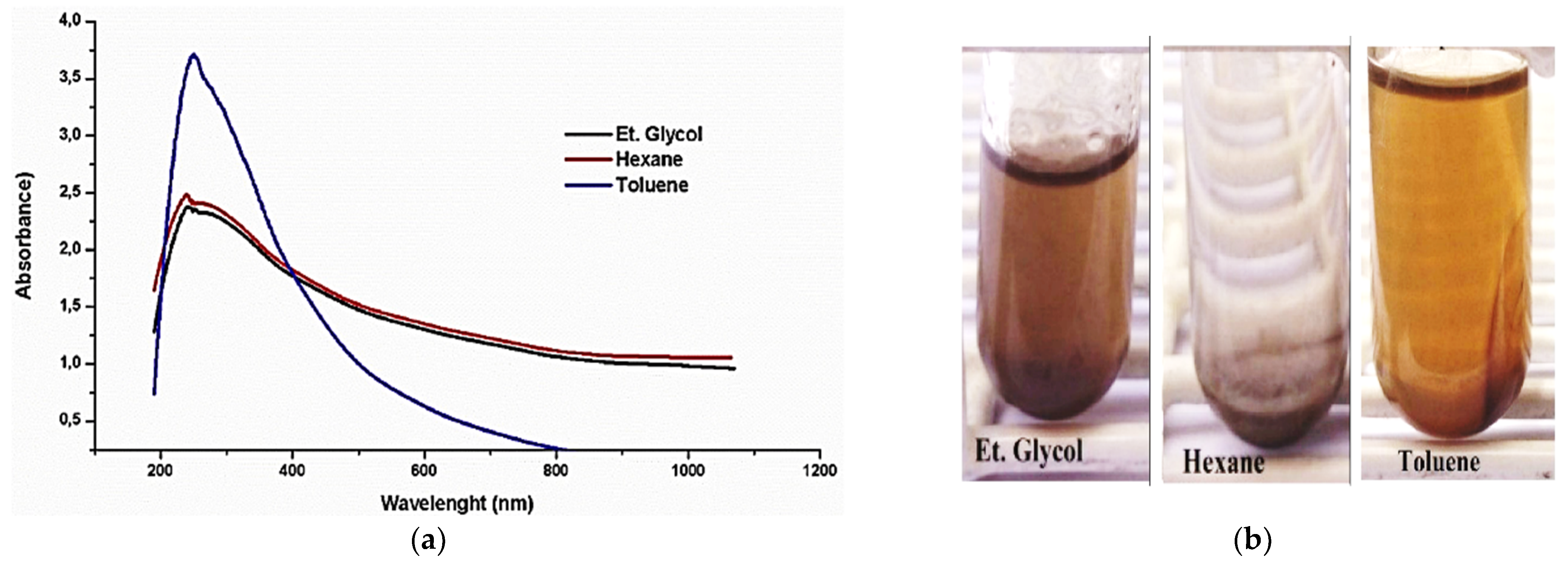
| Base as lubricant/PAO | CuO | One-step method | 0.1 wt.% | Toluene/OA, Hexane/OA, Et.Glycol/OA | Sedimentation, UV | Sable-Toluene/30 days | Magnetic stirrer/7 h | [38] | 2020 |
| SAE 20W-50 | La(OH)3/rGO | One-step method | Not reported | Not reported | Sedimentation, XRD | Stable/28 days | Ultrasonic dispersion/1 h | [62] | 2020 |
| SAE 5W-30 | Cu/Gr | Two-step method | 0.4 wt.% | Oleic acid (OA) | UV | Stable/11 days | Magnetic stirrer/4 h | [63] | 2019 |
| Base as lubricant/hexadecane | GO | Two-step method | Oleylamine | Sedimentation | Stable | Ultrasonic dispersion | [64] | 2019 | |
| SAE 5W-30 | GO | Two-step method | 0.1 wt.% | Dodecylamine, Ethanol, Xylene | UV, Sedimentation, XRD | Stable/32 Days | Ultrasonic dispersion/6 h | [65] | 2019 |
| SAE 10W-40 | Two-step method | Not reported | Oleic acid (OA) | Sedimentation, Zeta sizer | Not stable | Magnetic stirrer/15 min Ultrasonic dispersion/20 min | [66] | 2018 | |
| SAE 5W-30 | Gr | Two-step method | 0.4 wt.% | Oleic acid (OA) | Sedimentation, UV | Stable/11 days | Magnetic stirrer/4 h | [67] | 2018 |
| SAE 5W-30 | Two-step method | 0.1 wt.% | Oleic acid (OA) | Not reported | Not reported | Magnetic stirrer/4 h | [68] | 2018 | |
| SAE 10W-40 | ZnO | Two-step method | 0.25–2 vol.% | Not reported | Sedimentation | Stable/72 h | Magnetic stirrer/2 h, Ultrasonic dispersion | [51] | 2017 |
| Base as lubricant/PAO | Two-step method | 1 wt.% | Oleic acid (OA) | Not reported | Not stable | Magnetic stirrer | [69] | 2017 | |
| SAE 5W-30 | Two-step method | - | Not reported | Not reported | Not reported | Magnetic stirrer, Ultrasonic dispersion | [70] | 2017 | |
| SAE 50 | ZnO | Two-step method | 0.125–1.5 vol.% | Not reported | Sedimentation | Stable/2 days | Ultrasonic dispersion/5 h | [71] | 2017 |
| Base as lubricant/60SN | ZnO | Two-step method | 0.5 wt.% | Oleic acid (OA) | Sedimentation | Stable/12 h | Magnetic stirrer/20 min, Ultrasonic dispersion/30 min | [72] | 2017 |
| SAE 40 | MWCNT/CuO | Two-step method | 0.0625–1 vol.% | Not reported | Sedimentation | Stable/1 week | Magnetic stirrerUltrasonic dispersion | [73] | 2017 |
| Base as lubricant/paraffin | Two-step method | 1 wt.% | ZDDP, Polyisobutylene amine succinimide (PIBS) | SEM | Stable | Ultrasonic dispersion/ 40 min | [74] | 2017 | |
| SAE 50 | Two-step method | 0.0625–2 vol.% | Not reported | Sedimentation | Stable | Magnetic stirrer/2 h, Ultrasonic dispersion | [75] | 2016 | |
| SAE 5W-30 | Two-step method | 0.25 wt.% | Oleic acid (OA) | UV, Zeta sizer | Stable/336 h | Magnetic stirrer/4 h | [76] | 2016 | |
| SAE 10W-40 | MWCNT/ZnO | Two-step method | 0.125–1 vol.% | Not reported | Sedimentation | Stable/1 week | Magnetic stirrer/2 h Ultrasonic dispersion/1 h | [77] | 2016 |
| Base as lubricant/PAO | ZnO | One-step method | 0.2–1.5 wt.% | Oleic acid (OA) | FTIR | Stable | Magnetic stirrer | [78] | 2016 |
| Base as lubricant/PAO | Gr | Two-step method | 0.01 wt.% | Span-80 | Sedimentation | Stable/4 weeks | Magnetic stirrer/10 min, ultrasonic dispersion/15 min | [79] | 2016 |
| SAE 10W-40 | GO, Ag, GNP | Two-step method | 0.06–0.1 wt.% | Stearic amine, ethanol, SDS, glucose | Sedimentation, FTIR | Stable/2 weeks | Ultrasonic dispersion/5 h | [80] | 2016 |
| SAE 10W-40 | GO | One- step method | Octadecylamine (ODA) | XRD, FTIR | Stable/30 days | Ultrasonic dispersion | [81] | 2015 | |
| Base as lubricant /500 N | One-step method | 0.58 wt.% | Sodium dodecyl persulfate (SDS), heptane | Sedimentation, UV | Stable/1 year | ultrasonic shaker/1 h | [82] | 2015 | |
| Base as lubricant/PAO | Cu/rGO | One-step method | 0.5–2 wt.% | Oleic acid (OA) | FTIR | Stable/ 7 days | Ultrasonic dispersion | [83] | 2015 |
| SAE 40 | C | Two-step method | 0.01 vol.% | Not reported | Zeta sizer | Stable/14 days | Ultrasonic dispersion/ 1 h | [84] | 2014 |
| Rapeseed oil/SAE20W40 | CuO | Two-step method | 0.5 wt.% | Not reported | UV | Stable/29 days | Rotary Shaker /5 h, ultrasonic dispersion/2 h | [85] | 2014 |
| SAE 15W-40 | Two-step method | All concentrations | Span-80 | Sedimentation | Stable/14 days | High shear homogenizer/ 30 min | [86] | 2014 | |
| Base as lubricant/PAO | Gr | Two-step method | 0.5–1.5 wt.% | Oleic acid (OA) | FTIR | Stable/ 7 days | Magnetic stirrer/30 min | [87] | 2014 |
| SAE 10W-40 | rGO | One-step method | Octadecylamine (ODA) | Sedimentation, XRD, zeta potential, FTIR | Stable/30 days | Ultrasonic dispersion | [88] | 2014 | |
| SAE 20W-50 | MWCNT | One-Step method | 0.1 wt.% | SOCL2, DMF, THF, dda | Sedimentation, XRD | Stable/720 h | Ultrasonic dispersion, Magnetic stirrer | [89] | 2013 |
| SAE 20W-50 | MWCNT | Two-step method | 0.1 wt.% | Dodecylamine | Sedimentation | Stable/720 h | planetary ball mill/3 h | [90] | 2013 |
| Base as lubricant/PAO | Two-step method | 3 wt.% | Benzethonium chloride | Sedimentation | Stable | Ultrasonic dispersion | [91] | 2012 | |
| 500 W | GO | Two-step method | DMF | FTIR | Stable | Ultrasonic dispersion/1 h | [92] | 2011 |
| Base Lubricant | Nano Additive | Reduction in COF % | Tribological Configuration | Testing Load/Speed | Testing Temperature °C | Concentration wt.% | Effective Concentration wt.% | Reference | Year |
|---|---|---|---|---|---|---|---|---|---|
| SAE-40 | ZnO | 5 | Pin-on-Disc | 20–75 N | Ambient Temperature | 0.1, 0.4, 0.7 | 0.4 | [102] | 2021 |
| 16 | 150 rpm | 0.7 | |||||||
| SAE-30 | Graphite | 91.6 | Pin-on-Disc | 20–50 N 300 rpm | Ambient Temperature | 0.3 | 0.3 | [59] | 2021 |
| SAE-40 | 18.46 | Pin-on-Disc | 120–180 N 120 rpm | Ambient Temperature | 0.1–1 vol.% | 0.1 vol.% | [58] | 2021 | |
| Base as lubricant—Group III | 11 | Ball-on-Disc | 50–100 N 50 Hz | 100 | 0.1–0.6 | 0.4 | [103] | 2021 | |
| SAE 20 W-50 | hBN | 20.5 | Four-ball Tribometer | 392.5 N 1200 rpm | 75 | 0.025 vol.% | 0.025 vol.% | [104] | 2021 |
| Base as lubricant—Group III | ZnO | 7.8 | Cylinder-on-flat | 500–530 Mpa 300 rpm | 100 | 0.33 | 0.33 | [15] | 2021 |
| SAE 20W-50 | Boron nitride (BN) | 56 | Ball-on-Disc | 40–100 N 150 rpm | Ambient Temperature | 0.5 | 0.5 | [26] | 2020 |
| Tungsten disulphide (WS2) | 37 | ||||||||
| Graphene (Gr) | 2.5 | ||||||||
| SAE 10W-40 | Gr-MS-Zn | 37 | Four-ball Tribometer | 392.5 N 1200 rpm | 75 | 0.025–0.1 | 0.05 | [60] | 2020 |
| SAE 20W-50 | Nano-lanthanum hydroxide/reduced graphene oxide (LaOH3/rGO) | 16.7 | Ball-on-Disc | 1.96–5.88 N 0.1 m/s | 40–80 | 0.05, 0.1 | 0.1 | [62] | 2020 |
| SAE 10W-30 | Hairy silica particles (HSP) | 15 | Ball-on-Three-plate testing module | 2 N 0.05 m/s | 25–100 | 0.1, 0.3, 0.5, 1 | 0.3 | [105] | 2020 |
| SAE 10W-40 | ZnO/MWCNT | 32 | Ball-on-Disc | 35–55 N 5–15 Hz | Ambient Temperature | 0.25, 0.50, 0.75, and 1 | 0.25 | [30] | 2020 |
| SAE 40 | ZnO | 5.9 | Pin-on-disc | 75 N | Ambient Temperature | 0.1, 0.4, and 0.7 | 0.4 | [61] | 2020 |
| 12 | 150 rpm | 0.7 | |||||||
| Base as lubricant—Paroline oil | 64 | Ball-on-disc | 6 N 1.5 Hz | Ambient Temperature | 0.1, 0.2, 0.3 and 0.5 | 0.3 | [35] | 2020 | |
| Base as lubricant—Ionic liquid | Gr | 40 | Pin-on-disc | 0.5 N 0.01 m/s | Ambient Temperature | 0.5 | 0.5 | [106] | 2020 |
| Diesel oil | ZnO | 5.86 | Pin-on-disc | 75 N | Ambient Temperature | 0.1, 0.4, 0.7 | 0.4 | [31] | 2020 |
| Base as lubricant—PAO 40 | CuO | 24 | Ball-on-disc | 10 N 0.01 m/s | 50 | 0.5 | 0.5 | [32] | 2020 |
| SAE 5W-30 | Gr | 40 | Ball-on-Plate | 10–50 N 3 mm/s | Ambient Temperature | 0.01, 0.05, 0.1 | 0.1 | [65] | 2019 |
| SAE 5W-30 | Cu/Gr | 26–32 | Piston Ring/Cylinder Liner | 90–368 N 0.154–0.6 m/s | 100 | 0.03, 0.2, 0.4, and 0.6 | 0.4 | [63] | 2019 |
| SN/GF-5 lubricant | C | 32 | piston ring/cylinder liner interface | 50–400 N 10 Hz | 100 | 1, 3 and 5 | 3 | [107] | 2019 |
| SAE 5W-30 | 53 | piston ring/cylinder liner interface | 250 N 0.5 m/s | Ambient Temperature | 0.1 | 0.1 | [68] | 2018 | |
| SAE 5W-30 | Graphene (Gr) | 29–35 | Piston Ring/Cylinder Liner | 90–368 N 0.154–0.6 m/s | 70–90 | 0.03, 0.2, 0.4, 0.6 | 0.4 | [67] | 2018 |
| SAE 10W-30 | Copper oxide (CuO) | 76 | Piston skirt-Liner Contact Tester | 2–9 N 200–300 rpm | Ambient Temperature | 0.005–0.01 | 0.008 | [108] | 2018 |
| SAE 10W-30 | 86 | Pin-on-Disc | 40–60 N 1 m/s | Ambient Temperature | 0.3, 0.4, 0.5 | 0.5 | [34] | 2018 | |
| Base as lubricant—SN 500 | 21 | Four-ball Tribometer | 392 N 1200 rpm | 75 | 0.1–0.5 | 0.5 | [97] | 2018 | |
| Base as lubricant—Mineral | Cu | 40–60 | Four-ball Tribometer, Pin-on-Disc | 392 N 1200 rpm | 40–100 | 0.3, 3 | 0.3 | [109] | 2018 |
| Base as lubricant—SN 500 | Graphene oxide (GrO) | 33 | Four-ball Tribometer | 147 N 1200 rpm | Ambient Temperature | 0.04 | 0.04 | [110] | 2017 |
| Base as lubricant—Liquid paraffin | 12.2–35.3 | Ball-on-Disk | 30 N 0.036 m/s | Ambient Temperature | 1 | 1 | [74] | 2017 | |
| SAE 5W-30 | ND | 36 | Block-on0ring | 30 kg 200 rpm | Ambient Temperature | 0.016 | 0.016 | [28] | 2017 |
| Base as lubricant—60SN base oil | ZnO | 41 | Four-ball Tribometer | 500 N 1000 rpm | Ambient Temperature | 0.2, 0.5, 0.5, 0.8,and 1 | 0.5 | [72] | 2017 |
| SAE 5W-30 | 48–50 | Piston Ring/Cylinder Liner | 30–250 N 50–800 rpm | 100 | 0.05, 0.1, 0.25, 0.5 | 0.25 | [96] | 2016 | |
| SAE 20W-50 | Gr | 21 | Four-ball Tribometer | 400 N 1200 rpm | 75 | 0.01 | 0.01 | [111] | 2016 |
| SAE 5W-30 | 51 | piston ring/cylinder liner interface | 40–230 N 0.5–1.45 m/s | 100 | 0.05, 0.1, 0.25 and 0.5 | 0.1 | [76] | 2016 | |
| SAE 5 W- 30 | 35 | piston ring/cylinder liner interface | 185–340 | 60 | 0.25 | 0.25 | [112] | 2016 | |
| 51 | 0.25–0.66 m/s | ||||||||
| Chevron Taro 30 DP 40 | Cu | 18.2 | Pin-on-disc | 0.1–180 mN | 25 | 0.3 vol.% | 3 vol.% | [113] | 2016 |
| 0.02 mm/s | 3 vol.% | ||||||||
| Base as lubricant—Mineral | Fe–Carbon capsules | 8 | Block-on-ring | 650 N 1.65 m/s | Ambient Temperature | 0.01–0.1 | 0.07 | [114] | 2015 |
| SAE 75 W- 85 | CuO | 14 | Four-ball Tribometer | 7000 N | Ambient Temperature | 0.5, 1 and 2 | 2 | [115] | 2015 |
| - | 500 rpm | - | |||||||
| SAE 40 | SWCNH | 12 | Ball-on-disc | 600 MPa 0.001–1.8 m/s | 25,40,60 and 80 | 0.005, 0.01 and 0.02 | 0.01 | [84] | 2014 |
| SAE 10 | CuO | 18 | Four-ball Tribometer, Pin-on-Disc | 150 N 1420 rpm | Ambient Temperature | 0.5, 0.25 | All concentrations | [116] | 2013 |
| Cu | 49 | 0.5 | |||||||
| Fe | 39 | 0.5 | |||||||
| Co | 20 | 0.5 | |||||||
| Fe/Cu | 53 | 0.25/0.25 | |||||||
| Fe/Co | 36 | 0.25/0.25 | |||||||
| Co/Cu | 53 | 0.25/0.25 | |||||||
| Base as lubricant—PAO 10 | 57 | Piston Skirt/Cylinder Liner | 250 N | 20–100 | 3 | 3 | [91] | 2012 | |
| BN | No improvement | 2 Hz | |||||||
| SAE 15 W-40 | Cu | 37 | Ball-on-disc | 50 N 10–30 Hz | Ambient Temperature | 0.0125, 0.025, 0.0375 and 0.05 | 0.0375 | [117] | 2011 |
| Base Lubricant | Nano Additive | Testing Temperature °C | Testing Shear Rate | Concentration | Viscosity Behaviour | Reference | Year |
|---|---|---|---|---|---|---|---|
| SAE 5W-30 | MgO | 5–55 | 0.1–1.5 vol.% | non-Newtonian | [33] | 2020 | |
| SAE 50 | ZnO | 25–65 | 0.125–1.5 vol.% | Newtonian | [130] | 2020 | |
| SAE 10W-40 | ZnO, MgO | 5–75 | 5–1000 rpm | 0.125–1.5 vol.% | Newtonian for both nanolubricants | [131] | 2019 |
| Engine lubricant | 25–60 | 100–600 rpm | 0.125–1.5 vol.% | Newtonian | [132] | 2018 | |
| 20W-50 | 40–100 | 0.05–1 vol.% | Newtonian | [133] | 2018 | ||
| SAE 40 | MWCNT/MgO | 25–45 | 100–1000 rpm | 0.25–2 vol.% | non-Newtonian | [134] | 2018 |
| SAE 10W-40 | /MWCNT | 5–55 | 0.05–1 vol.% | non-Newtonian | [135] | 2018 | |
| SAE 10W-40 | 5–55 | 0.05–1 vol.% | non-Newtonian | [136] | 2017 | ||
| SAE 10W-40 | ZnO | 5–55 | 0.25–2 vol.% | Newtonian | [51] | 2017 | |
| SAE 50 | 25–50 | 0.125–1.5 vol.% | Newtonian/non-Newtonian | [137] | 2017 | ||
| Engine lubricant | Cu | 40–100 | 0.2–1 wt.% | Newtonian/non-Newtonian | [138] | 2017 | |
| SAE 50 | MWCNT/MgO | 20–50 | 0.0625–1 vol.% | non-Newtonian | [139] | 2017 | |
| SAE 40 | MWCNT/ZnO | 25–60 | 1333–13,333 rpm | 0.05–1 vol.% | Newtonian | [140] | 2017 |
| SAE 40 | 25–50 | 100–500 rpm | 0.625–2 vol.% | Newtonian/non-Newtonian | [75] | 2016 | |
| SAE 40 | /MWCNT | 25–50 | 0.0625–1 vol.% | Newtonian | [127] | 2016 | |
| SAE 40 | 25–60 | 0.0625–1 vol.% | Newtonian | [141] | 2016 | ||
| SAE 10W-40 | MWCNT/ZnO | 5–55 | 5–1000 rpm | 0.125–1 vol.% | Newtonian | [77] | 2016 |
| SAE 50 | MWCNT/MgO | 25–50 | 100–700 rpm | 0.25–2 | Newtonian | [128] | 2016 |
| SAE 20W-50 | CuO | 5–70 | 0.2–6 wt.% | Newtonian | [126] | 2015 |
| Base Lubricant | Nano Additive | Testing Temperature °C | Concentration | Effective Concentration | Most Viscosity Refinement | Reference | Year |
|---|---|---|---|---|---|---|---|
| SAE 40 | 40–100 | 0.1–0.7 wt.% | 0.7 wt.% | Increase by 9.57%/MOS2, 10.12%/ZnO, at 100 °C | [102] | 2021 | |
| SAE 20W-40 | 50–90 | 0.3–1.5 wt.% | 0.3 wt.% | Increase by 99%, at 90 °C | [143] | 2021 | |
| SAE 50 | ZnO | 25–65 | 0.125–1.5 vol.% | 1.5 vol.% | Increase by 25.3% | [130] | 2020 |
| SAE 40 | 40–100 | 0.1–0.7 wt.% | 0.7 wt.% | Increase by 9.58%/MOS2, 10.14%/ZnO, at 100 °C | [61] | 2020 | |
| SAE 5W-30 | MgO | 5–55 | 0.1–1.5 vol.% | 1.5 vol.% | Increase by 25% at 15 °C | [33] | 2020 |
| Engine lubricant | MgO and ZnO | 5–55 | 0.125–1 vol.% | 1.5 vol.% for both nanolubricants | Increase by 124.3%/ZnO, 75%/MgO, at 55 °C | [131] | 2019 |
| SAE 10W-40 | 5–55 | 0.05–1 vol.% | 1 vol.% | Increase by 31% at 55 °C | [135] | 2018 | |
| Engine lubricant | MWCNT/Mg | 25–60 | 0.25–2 vol% | 2 vol% | Increase by 60% at 60 °C | [132] | 2018 |
| SAE 20W-50 | °C | 40–100 | 0.05–1 vol.% | 1 vol.% | Increase by 171% at 100 °C | [133] | 2018 |
| Engine lubricant | Cu | 4–100 | 0.2–1 wt.% | 1 wt.% | Increase by 37% at 40 °C | [138] | 2017 |
| SAE 50 | MWCNT/MgO | 25–50 | 0.0625–1 vol.% | 1 vol.% | Decrease by 75% at 50 °C | [139] | 2017 |
| SAE 40 | MWCNT/CuO | 25–50 | 0.0625–1 vol.% | 1 vol.% | Increase by 29.47% at 30 °C | [73] | 2017 |
| SAE 40 | MWCNT/ZnO | 25–60 | 0.05–1 vol.% | 1 vol% | Increase by 33.3% at 40 °C | [140] | 2017 |
| SAE 40 | /MWCNT | 25–50 | 0.0625–1 vol.% | 1 vol.% | Increase by 46% at 35 °C | [127] | 2016 |
| SAE 50 | MWCNT/MgO | 25–50 | 0.25–2 vol.% | 2 vol% | Increase by 65% at 40 °C | [128] | 2016 |
| SAE 40 | 25–60 | 0.0625–1 vol.% | 1 vol.% | Increase by 37.4% at 60 °C | [141] | 2016 | |
| SAE 40 | MWCNT/SiO2 | 25–50 | 0.0625–2 vol.% | 0.5 vol.% | Increase by 1.7% at 40 °C | [75] | 2016 |
| SAE 10W-40 | MWCNT/ZnO | 5–55 | 0.125–1 vol.% | 1 vol.% | Increase by 55% at 55 °C | [77] | 2016 |
| Reference | Year | Base Lubricant | Nano Additive | Proposed Model | Accuracy of the Model |
|---|---|---|---|---|---|
| [129] | 2018 | SAE 5W-50 | MWCNT/ZnO | R2 = 0.9715 | |
| [133] | 2018 | SAE 20W-50 | Margin of deviation < 1% | ||
| [140] | 2017 | SAE 40 | MWCNT/ZnO | Maximum error = 2% | |
| [147] | 2017 | SAE 10W-40 | /MWCNT | Margin of deviation = 1.1% | |
| [51] | 2017 | SAE 10W-40 | ZnO | Margin of deviation < 1% | |
| [73] | 2017 | SAE 40 | MWCNT/CuO | - | |
| [128] | 2016 | SAE 50 | MWCNT/MgO | Maximum error = 8% | |
| [148] | 2016 | SAE 40 | Margin of deviation = 4% | ||
| [75] | 2016 | SAE 40 | Margin of deviation = 1.2% | ||
| [127] | 2016 | SAE 40 | /MWCNT | Margin of deviation = 2% |
| Base Lubricant | Nano Additive | Testing Temperature °C | Concentration | Effective Concentration | Improvement in Thermal Conductivity | Reference | Year |
|---|---|---|---|---|---|---|---|
| SAE 20 W-40 | 20–70 | 0.1–0.8 vol.% | 0.8 vol.% | Increase by 24.42% at 70 °C | [57] | 2021 | |
| SAE 50 | ZnO | 25–55 | 0.125–1.5 vol.% | 1.5 vol.% | Increase by 8.74% at 55 °C | [151] | 2019 |
| Engine oil | ZnO, MgO | 15–55 | 0.125–1.5 vol.% | 1.5 vol.% for both nanoparticles | Increase by 28%/ZnO, 32%/MgO, at 55 °C | [131] | 2019 |
| Engine oil | /MWCNT | 25–50 | 0.125–1.5 vol.% | 1.5 vol.% | Increase by 45% at 50 °C | [157] | 2018 |
| SAE 10W-40 | MWCNT/ZnO | 15–55 | 0.125 0 1 vol.% | 1 vol.% | Increase by 40% at 55 °C | [158] | 2018 |
| SAE 5 W-50 | 25–60 | 0.25–2 vol.% | 2 vol.% | Increase by 50% at 60 °C | [132] | 2018 | |
| SAE 50 | MWCNT/MgO | 25–50 | 0.25–2 vol.% | 2 vol.% | Increase by 62% at 50 °C | [128] | 2016 |
| Engine oil | Cu | 40–100 | 0.2–1 wt.% | 1 wt.% | Increase by 37% at 100 °C | [138] | 2016 |
| SAE 20W-50 | Gr | 10–180 | 0.01 wt.% | 0.01 wt.% | Increase by 23% at 80 °C | [111] | 2016 |
| SAE 20W-50 | CuO | 20 | 0.1–0.5 wt.% | 0.1 wt.% | Increase by 3% | [159] | 2013 |
| Reference | Year | Base Lubricant | Nano Additive | Proposed Model | Accuracy of the Model |
|---|---|---|---|---|---|
| [151] | 2019 | SAE 50 | ZnO | Margin of deviation < 1% | |
| [157] | 2018 | Engine oil | + 0.00026 T | Maximum error = 2% | |
| [132] | 2018 | SAE 5W-50 | + 0.003 T | Maximum error = 2% | |
| [128] | 2016 | SAE 50 | MWCNT/MgO | Maximum error = 3% |
| Reference | Year | Substantial Findings | Studied Property |
|---|---|---|---|
| [194] | 2020 | Addressing of effective method to quantify aggregation/dispersion range through molecular simulation | Dispersion Stability |
| [195] | 2021 | MD simulations stated that the transformation from dispersion state to the reversible state is related to the Lennard–Jones(LJ) particle number | |
| [190] | 2020 | Providing a facile method to produce high dispersion stability of GO suspensions. MD simulations indicated that the used surface agents could form a high reaggregation barrier on the surface of nanoparticles | |
| [196] | 2021 | By increasing the receiving heat flux of nanofluid, the thermal conductivity is improved. | Rheological and Thermophysical Characteristics |
| [191] | 2014 | Discussing the hypotheses behind the flow of nanofluids using near and main flow models | |
| [192] | 2021 | The common hypotheses of the ball-bearing lubrication mechanism have been confirmed through the MD simulations, which verified the rolling motion of nanoparticles. | Tribological Characteristics |
| [197] | 2014 | A clear investigation was concluded about the effect of sliding velocity and load capacity on the formation of the nano tribo-films at the contacted surfaces | |
| [198] | 2020 | Frictional heating and anti-wear properties of the friction pair is controlled through the existence of nanoparticles | |
| [199] | 2020 | Simulations of MD clarified that Gr nano additives could form a thick layer of tribo-film that can help in reducing the coefficient of friction and friction force | |
| [200] | 2014 | Nanofluids have a higher transition pressure than the base fluid, with an excellent load-carrying capacity | |
| [201] | 2020 | Theoretical guidance was implemented for the lubrication mechanism of MOS2 nanoparticles | |
| [193] | 2015 | Confirmation of the ball-bearing lubrication mechanism of nanoparticles under mild velocities and loads | |
| [189] | 2015 | The load-carrying capacity of nanofluid is improved regarding the base oil before the rupture of the lubricant film | |
| [120] | 2018 | Atomistic simulations confirmed the mending mechanism of nanolubricants through which nanoparticles fill in valleys of the sliding asperities |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akl, S.; Elsoudy, S.; Abdel-Rehim, A.A.; Salem, S.; Ellis, M. Recent Advances in Preparation and Testing Methods of Engine-Based Nanolubricants: A State-of-the-Art Review. Lubricants 2021, 9, 85. https://doi.org/10.3390/lubricants9090085
Akl S, Elsoudy S, Abdel-Rehim AA, Salem S, Ellis M. Recent Advances in Preparation and Testing Methods of Engine-Based Nanolubricants: A State-of-the-Art Review. Lubricants. 2021; 9(9):85. https://doi.org/10.3390/lubricants9090085
Chicago/Turabian StyleAkl, Sayed, Sherif Elsoudy, Ahmed A. Abdel-Rehim, Serag Salem, and Mark Ellis. 2021. "Recent Advances in Preparation and Testing Methods of Engine-Based Nanolubricants: A State-of-the-Art Review" Lubricants 9, no. 9: 85. https://doi.org/10.3390/lubricants9090085
APA StyleAkl, S., Elsoudy, S., Abdel-Rehim, A. A., Salem, S., & Ellis, M. (2021). Recent Advances in Preparation and Testing Methods of Engine-Based Nanolubricants: A State-of-the-Art Review. Lubricants, 9(9), 85. https://doi.org/10.3390/lubricants9090085






