“Green” Synthesis of Nanocarbons for Reduced Friction and Wear
Abstract
1. Introduction
2. Materials and Methods
2.1. Lubricant Bases for Formulations
- ETRO IV (Group III) base oil, whose commercial family name when including further additives is SYNPLUS by RILUB SPA and which for simplicity’s sake will be called SYNPLUS in the following study.
- SN150 and BS150 mixture (Group I), whose lubricant family commercial name, when including further additives, is EUBUSH by RILUB SPA and which for simplicity’s sake will be called EUBUSH in the following study.
2.2. Carbon Nanotubes (CNTs) Preparation through a “Green” Approach
2.3. rGO Preparation through a “Green” Approach
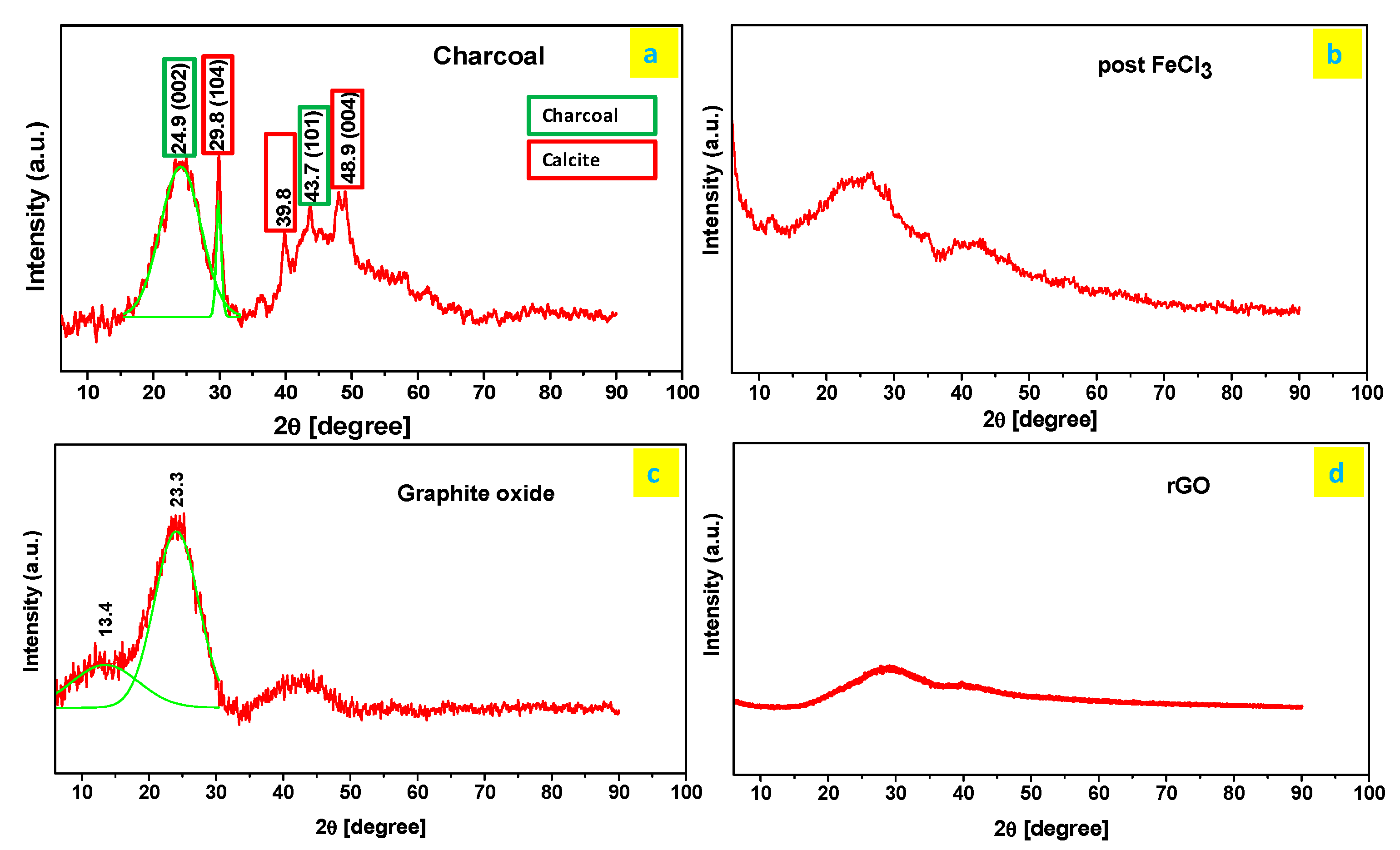
2.3.1. Graphitization of Charcoal
2.3.2. Graphite Oxide Preparation
2.3.3. Sonication and Reduction
2.4. Characterization Techniques
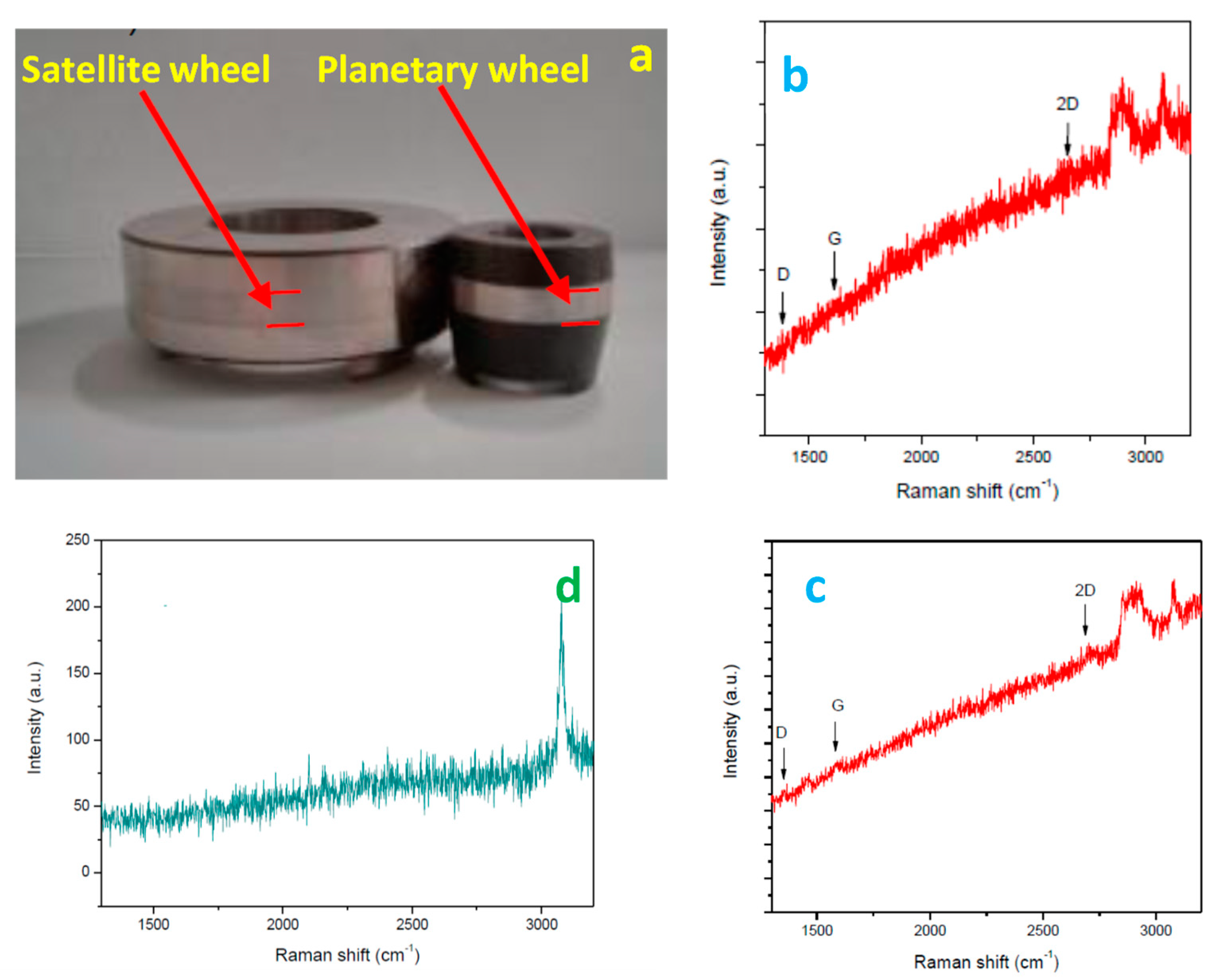
2.5. Tribological Tests
Test Description
3. Results and Discussion
3.1. Green Carbon Nanotubes (CNTs) Characterization
3.2. Green rGO Characterization
3.3. Lubricant Preparation and Stability Dispersion Tests
3.4. Tribological Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goyan, R.L.; Melley, R.E.; Wissner, P.A.; Ong, W.C. Biodegradable lubricants. Lubr. Eng. 1998, 54, 10. [Google Scholar]
- Zeng, X.; Li, J.; Wu, X.; Ren, T.; Liu, W. The Tribological Behaviors of Hydroxyl-containing Dithiocarbamate-triazine Derivatives as Additives in Rapeseed Oil. Tribol. Int. 2007, 40, 560–566. [Google Scholar] [CrossRef]
- Spikes, H. Additive-additive and additive-surface interactions in lubrication. Lubr. Sci. 1989, 2, 3–23. [Google Scholar] [CrossRef]
- Hamblin, P.; Kristen, U.; Chasan, D. Ashless antioxidants, copper deactivators and corrosion inhibitors: Their use in lubricating oils. Lubr. Sci. 1990, 2, 287–318. [Google Scholar] [CrossRef]
- Ingole, S.; Charanpahari, A.; Kakade, A.; Umare, S.S.; Bhatt, D.V.; Menghani, J. Tribological behavior of nano TiO2 as an additive in base oil. Wear 2013, 301, 776–785. [Google Scholar] [CrossRef]
- Ghalme, S.; Bhalerao, Y.J. Application of Nanoparticles as Additive for Lubricant Nano-Materials in Tribology. Recent Pat. Mater. Sci. 2017, 10, 88–96. [Google Scholar] [CrossRef]
- Tao, X.; Jiazheng, Z.; Kang, X. The ball-bearing effect of diamond nanoparticles as an oil additive. J. Phys. D 1993, 29, 2932–2937. [Google Scholar] [CrossRef]
- Shen, M.; Luo, J.; Wen, S. The tribological properties of oils added with diamond nanoparticles. Tribol. Trans. 2001, 44, 494–498. [Google Scholar] [CrossRef]
- Grierson, D.S.; Carpick, R.W. Nanotribology of carbon-based materials. Nano Today 2007, 2, 12–21. [Google Scholar] [CrossRef]
- Gong, Z.; Shi, J.; Zhang, B.; Zhang, J. Graphene nano scrolls responding to superlow friction of amorphous carbon. Carbon 2017, 116, 310–317. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Zhou, Y.; Lee, K.R.; Wang, A. Insights into friction dependence of carbon nanoparticles as oil-based lubricant additive at amorphous carbon interface. Carbon 2019, 150, 465–474. [Google Scholar] [CrossRef]
- Rui, L. Tribological behaviour of multi-walled carbon nanotube films. AIP Adv. 2014, 4, 031309. [Google Scholar] [CrossRef]
- Chebattina, K.R.R.; Srinivas, V.; Rao, N.M. Effect of Size of Multiwalled Carbon Nanotubes Dispersed in Gear Oils for Improvement of Tribological Properties. Adv. Tribol. 2018, 2018, 2328108. [Google Scholar] [CrossRef]
- Bhaumik, S.; Prabhu, S.; Singh, K.J. Analysis of Tribological Behavior of Carbon Nanotube Based Industrial Mineral Gear Oil 250 cSt Viscosity. Adv. Tribol. 2014, 2014, 341365. [Google Scholar] [CrossRef]
- Cornelio, J.A.C.; Cuervo, P.A.; Hoyos-Palacio, L.M.; Lara-Romero, J.; Toro, A. Tribological properties of Carbon Nanotubes as lubricant additive in oil and water for a wheel-rail system. J. Mater. Res. Technol. 2016, 5, 68–76. [Google Scholar] [CrossRef]
- Joly-Pottuz, L.; Dassenoy, F.; Vacher, B.; Martin, J.M.; Mieno, T. Ultralow friction and wear behaviour of Ni/Y-based single wall carbon nanotubes (SWNTs). Tribol. Int. 2004, 37, 1013–1018. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Shahnazar, S.; Bagheri, S.; Hamid, S.B.A. Enhancing lubricant properties by nanoparticle additives. Int. J. Hydrog. Energy 2016, 41, 3153–3170. [Google Scholar] [CrossRef]
- Guo, Y.B.; Zhang, S.W. The Tribological Properties of Multi-Layered Graphene as Additives of PAO2 Oil in Steel–Steel Contacts. Lubricants 2016, 4, 30. [Google Scholar] [CrossRef]
- He, Z.B.; Maurice, J.L.; Lee, C.S.; Cojocaru, C.S.; Pribat, D. Nickel catalyst faceting in plasma-enhanced direct current chemical vapor deposition of carbon nanofibers. Arabian J. Sci. Eng. Sect. B 2010, 35, 19–28. [Google Scholar]
- Andrews, R.; Jacques, D.; Rao, A.M. Continuous production of aligned carbon nanotubes: A step closer to commercial realization. Chem. Phys. Lett. 1999, 303, 467–474. [Google Scholar] [CrossRef]
- Dasgupta, K.; Veugopalan, R.; Dey, G.K.; Sathiyamoorthy, D. Novel catalytic route to bulk production of high purity carbon nanotubes. J. Nanopart. Res. 2008, 10, 69–76. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.Q.; Zhao, M.Q.; Qian, W.Z.; Wei, F. Carbon nanotube mass production: Principles and processes. Chem. Sus. Chem. 2011, 4, 864–889. [Google Scholar] [CrossRef]
- D’Ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. Field Actions Sci. Rep. 2019, 19, 12–21. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Fernández-Merino, M.J.; Guardia, L.; Martínez-Alonso, A.; Tascón, J.M.D. Environmentally Friendly Approaches Toward the Mass Production of Processable Graphene from Graphite Oxide. J. Mater. Chem. 2011, 21, 298–306. [Google Scholar] [CrossRef]
- Casa, M.; Sarno, M.; Liguori, R.; Cirillo, C.; Rubino, A.; Bezzeccheri, E.; Liu, J.; Ciambelli, P. Conductive Adhesive Based on Mussel-Inspired Graphene Decoration with Silver Nanoparticles. J. Nanosci. Nanotechnol. 2018, 18, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-Friendly Method to Produce Graphene that Employs Vitamin C and Amino Acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar]
- Thorenz, A.; Wietschel, L.; Stindt, D.; Tuma, A. Assessment of agroforestry residue potentials for the bioeconomy in the European Union. J. Clean. Prod. 2018, 176, 348–359. [Google Scholar] [CrossRef]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv. 2014, 4, 20441–20448. [Google Scholar] [CrossRef]
- Sarno, M.; Sannino, D.; Leone, C.; Ciambelli, P. Evaluating the effects of operating conditions on the quantity, quality and catalyzed growth mechanisms of CNTs. J. Mol. Catal. Chem. 2012, 357, 26–38. [Google Scholar] [CrossRef]
- Ciambelli, P.; Sannino, D.; Sarno, M.; Fonseca, A.; Nagy, J.B. Hydrocarbon decomposition in alumina membrane: An effective way to produce carbon nanotubes bundles. J. Nanosci. Nanotechnol. 2004, 4, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Altavilla, C.; Sarno, M.; Ciambelli, P.; Senatore, A.; Petrone, V. New ‘chimie douce’ approach to the synthesis of hybrid nanosheets of MoS2 on CNT and their anti-friction and anti-wear properties. Nanotechnology 2013, 24, 125601. [Google Scholar] [CrossRef] [PubMed]
- Buseck, P.R.; Beyssac, O. From Organic Matter to Graphite: Graphitization. Elements 2014, 10, 421–426. [Google Scholar] [CrossRef]
- Casiraghi, C.; Ferrari, A.C.; Robertson, J. Raman Spectroscopy of hydrogenated amorphous carbons. Phys. Rev. B 2005, 72, 085401. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Sarno, M.; Cirillo, C.; Ciambelli, P. Selective graphene covering of monodispersed magnetic nanoparticles. Chem. Eng. J. 2014, 246, 27–38. [Google Scholar] [CrossRef]
- Sarno, M.; Cirillo, C.; Scudieri, C.; Polichetti, M.; Ciambelli, P. Electrochemical Applications of Magnetic Core−Shell Graphene Coated FeCo Nanoparticles. Ind. Eng. Chem. Res. 2016, 55, 3157–3166. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.W.; Jung, W.G. Facile and safe graphene preparation on solution based platform. Ind. Eng. Chem. Res. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Chia, C.H.; Joseph, S.D.; Rawal, A.; Linser, R.; Hook, J.M.; Munroe, P. Microstructural characterization of white charcoal. J. Anal. Appl. Pyrolysis 2014, 109, 215–221. [Google Scholar] [CrossRef]
- Bera, A.; Ojha, K.; Mandal, A. Synergistic Effect of Mixed Surfactant Systems on Foam Behavior and Surface Tension. J. Surfactants Deterg. 2013, 16, 621–630. [Google Scholar] [CrossRef]
- Mohammad, D.E.; Negm, N.A.; Mishrif, M.R. Micellization and Interfacial Interaction Behaviors of Gemini Cationic Surfactants–CTAB Mixed Surfactant Systems. J. Surfactants Deterg. 2013, 16, 723–731. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L. Effect of SDBS–Tween 80 mixed surfactants on the distribution of polycyclic aromatic hydrocarbons in soil–water system. J. Soil. Sediment. 2010, 10, 1123–1130. [Google Scholar] [CrossRef]
- Rosen, M.J. Molecular interaction and synergism in binary mixtures of surfactants. ACS Symp. Ser. 1986, 311, 144–162. [Google Scholar]
- Holland, M.; Rubingh, D.N. Mixed surfactants systems. ACS Symp. Ser. 1992, 50, 2–30. [Google Scholar]
- Kałużny, J.; Merkisz-Guranowska, A.; Giersig, M.; Kempa, K. Lubricating Performance Of Carbon Nanotubes In Internal Combustion Engines—Engine Test Results For CNT Enriched Oil. Int. J. Automot. Technol. 2017, 18, 1047–1059. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 2013, 54, 454–459. [Google Scholar] [CrossRef]
- Sarno, M.; Casa, M. Green and one-step synthesis for Ag/graphene hybrid supercapacitor with remarkable performance. J. Phys. Chem. Solids 2018, 120, 241–249. [Google Scholar] [CrossRef]
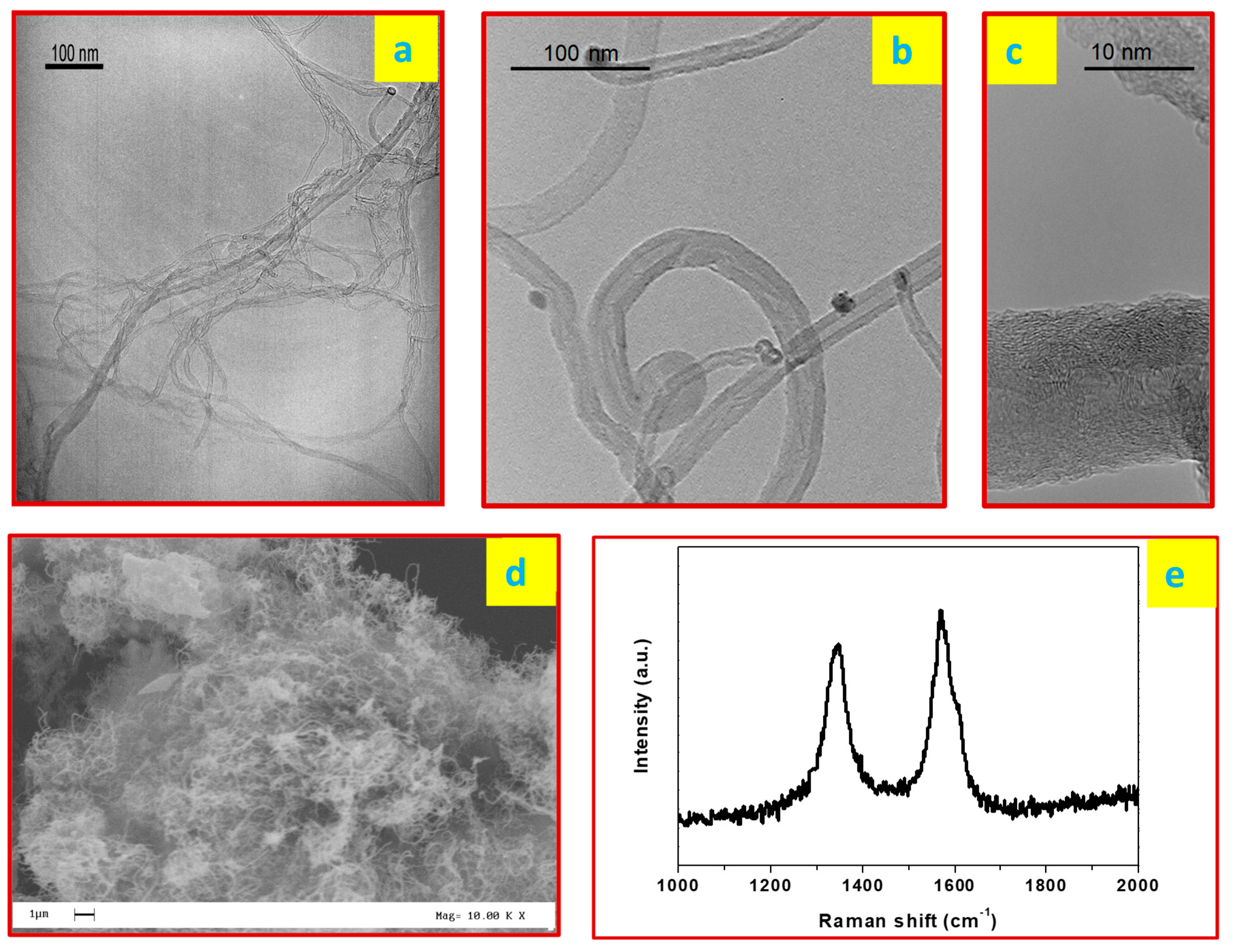

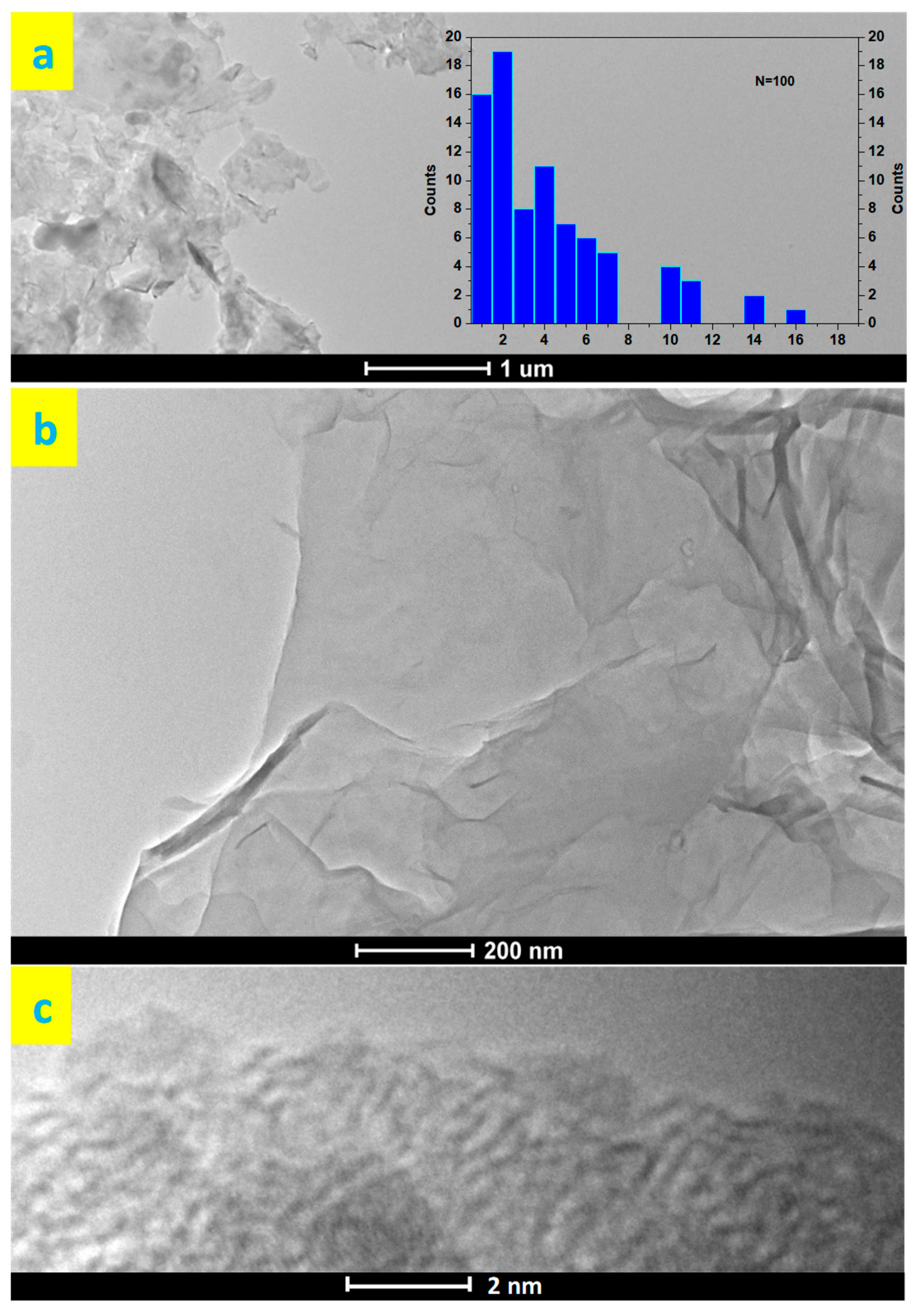
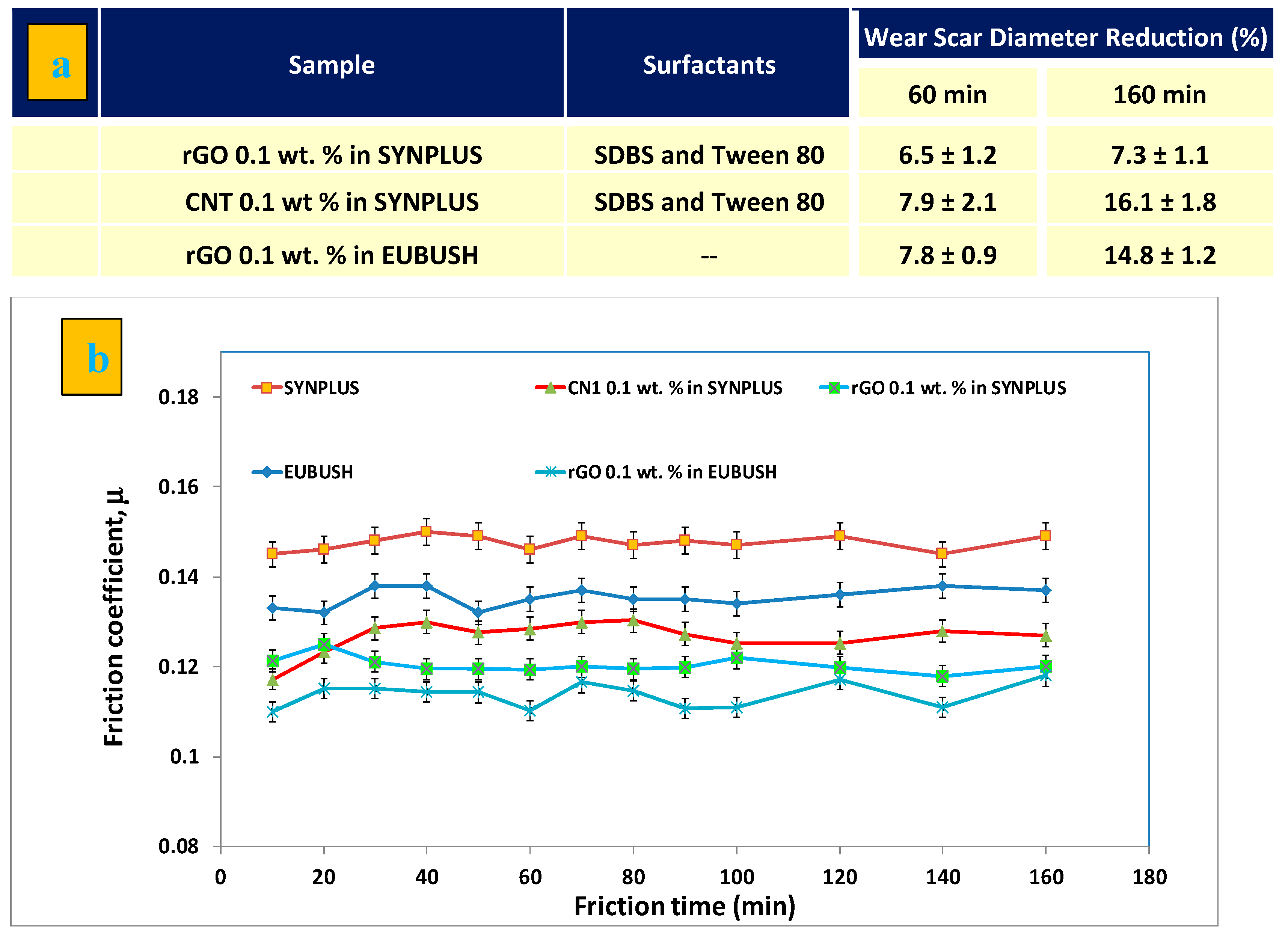
| Sample | Surfactant/Dispersant |
|---|---|
| CNT | 1,8 diaminonaphthalene |
| rGO | 1,8 diaminonaphthalene |
| CNT | SDBS |
| rGO | SDBS |
| CNT | SDBS and Tween 80 |
| rGO | SDBS and Tween 80 |
| CNT | HiTEC®646E Performance Additive—Polyisobutylene Succinimides |
| rGO | HiTEC®646E Performance Additive—Polyisobutylene Succinimides |
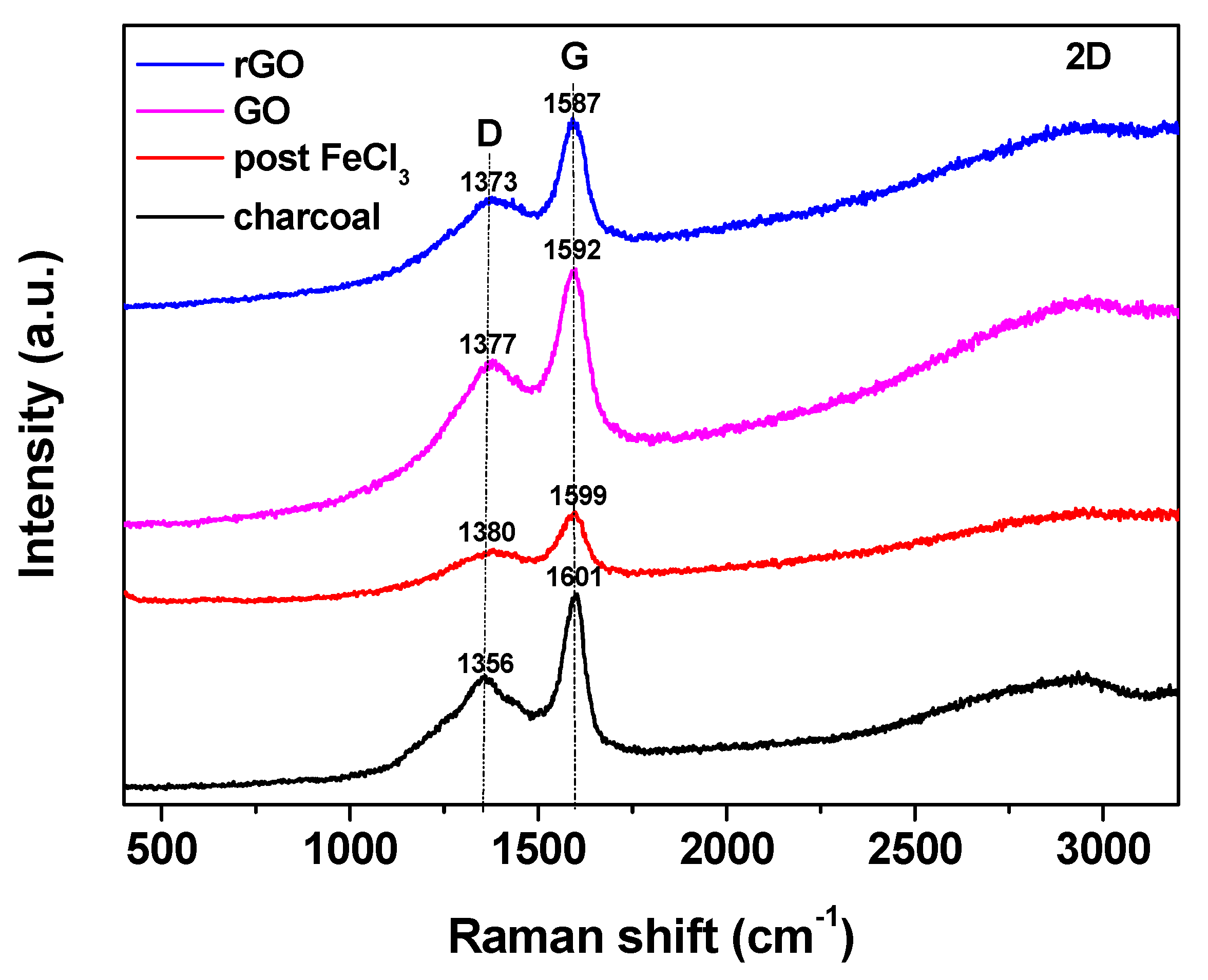
| Sample | D Band (cm−1) | G Band (cm−1) | ID/IG |
|---|---|---|---|
| rGO | 1373 | 1587 | 0.38 |
| GO | 1377 | 1592 | 0.55 |
| Post FeCl3 | 1380 | 1599 | 0.63 |
| charcoal | 1356 | 1601 | 0.72 |
| ID | Sample | Nanoadditive Concentration | Surfactant/Dispersant | A Ratio |
|---|---|---|---|---|
| 2 | rGO | 0.05% | / | 0.65 |
| 7 | rGO | 0.05% | 1,8 diaminonaphthalene | 0.67 |
| 8 | rGO | 0.1% | 1,8 diaminonaphthalene | 0.67 |
| 9 | rGO | 1% | 1,8 diaminonaphthalene | 0.65 |
| 16 | rGO | 0.05% | SDBS | 0.88 |
| 17 | rGO | 0.1% | SDBS | 0.74 |
| 18 | rGO | 1% | SDBS | 0.66 |
| 25 | rGO | 0.05% | SDBS and Tween 80 | 0.93 |
| 26 | rGO | 0.1% | SDBS and Tween 80 | 0.90 |
| 27 | rGO | 1% | SDBS and Tween 80 | 0.88 |
| 34 | rGO | 0.05% | PS | 0.91 |
| 35 | rGO | 0.1% | PS | 0.88 |
| 36 | rGO | 1% | PS | 0.81 |
| ID | Sample | Nano-Additive Concentration | Surfactant/Dispersant | COF Reduction (%) | Mean Wear Scar Diameter Reduction (%) |
|---|---|---|---|---|---|
| 28 | rGO | 0.05% | SDBS and Tween 80 | 8.2 ± 1.1 | 10.2 ± 0.2 |
| 29 | rGO | 0.1% | SDBS and Tween 80 | 18.3 ± 1.2 | 15.0 ± 0.4 |
| 30 | rGO | 1% | SDBS and Tween 80 | 6.0 ± 0.9 | 12.1 ± 0.3 |
| 31 | rGO | 0.05% | PS | 14.2 ± 1.2 | 13.4 ± 0.1 |
| 32 | rGO | 0.1% | PS | 16.3 ± 1.3 | 14.2 ± 0.3 |
| 33 | rGO | 1% | PS | 7.1 ± 1.1 | 9.1 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarno, M.; Senatore, A.; Scarpa, D.; Cirillo, C. “Green” Synthesis of Nanocarbons for Reduced Friction and Wear. Lubricants 2020, 8, 13. https://doi.org/10.3390/lubricants8020013
Sarno M, Senatore A, Scarpa D, Cirillo C. “Green” Synthesis of Nanocarbons for Reduced Friction and Wear. Lubricants. 2020; 8(2):13. https://doi.org/10.3390/lubricants8020013
Chicago/Turabian StyleSarno, Maria, Adolfo Senatore, Davide Scarpa, and Claudia Cirillo. 2020. "“Green” Synthesis of Nanocarbons for Reduced Friction and Wear" Lubricants 8, no. 2: 13. https://doi.org/10.3390/lubricants8020013
APA StyleSarno, M., Senatore, A., Scarpa, D., & Cirillo, C. (2020). “Green” Synthesis of Nanocarbons for Reduced Friction and Wear. Lubricants, 8(2), 13. https://doi.org/10.3390/lubricants8020013







