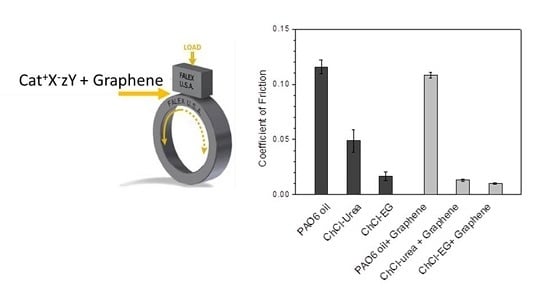
Reduction of the Coefficient of Friction of Steel-Steel Tribological Contacts by Novel Graphene-Deep Eutectic Solvents (DESs) Lubricants
Abstract
:1. Introduction
2. Materials and Methods
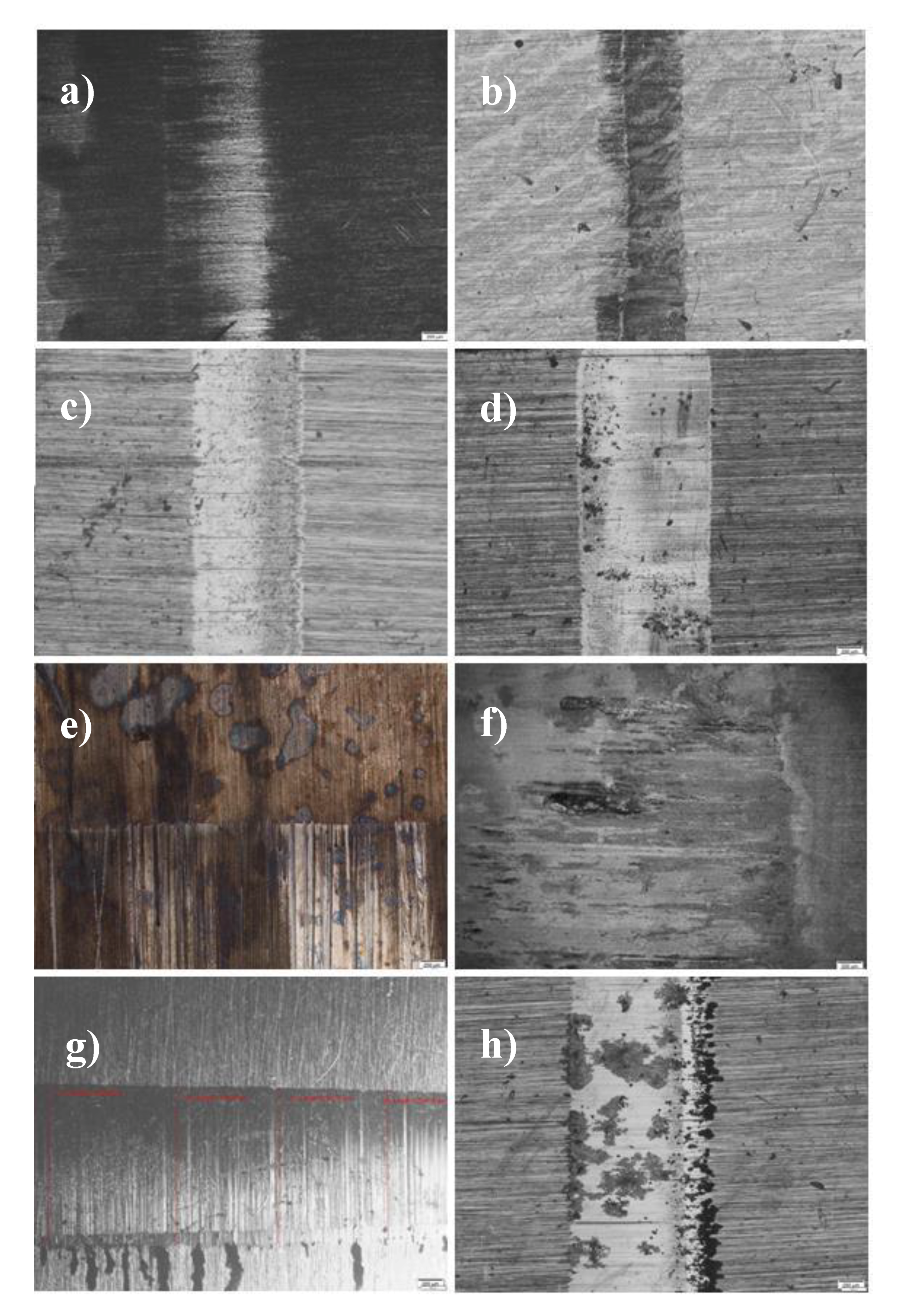
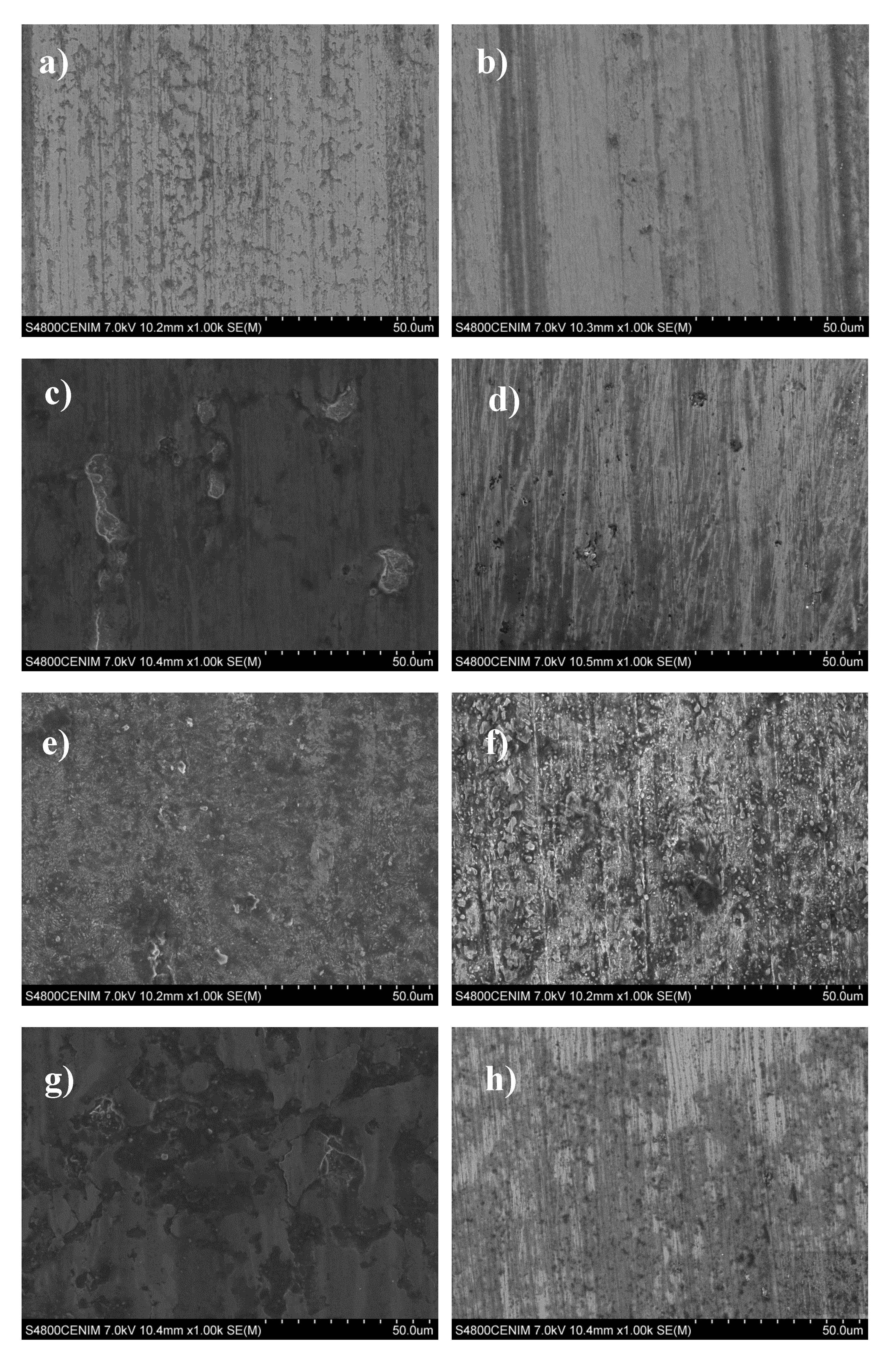
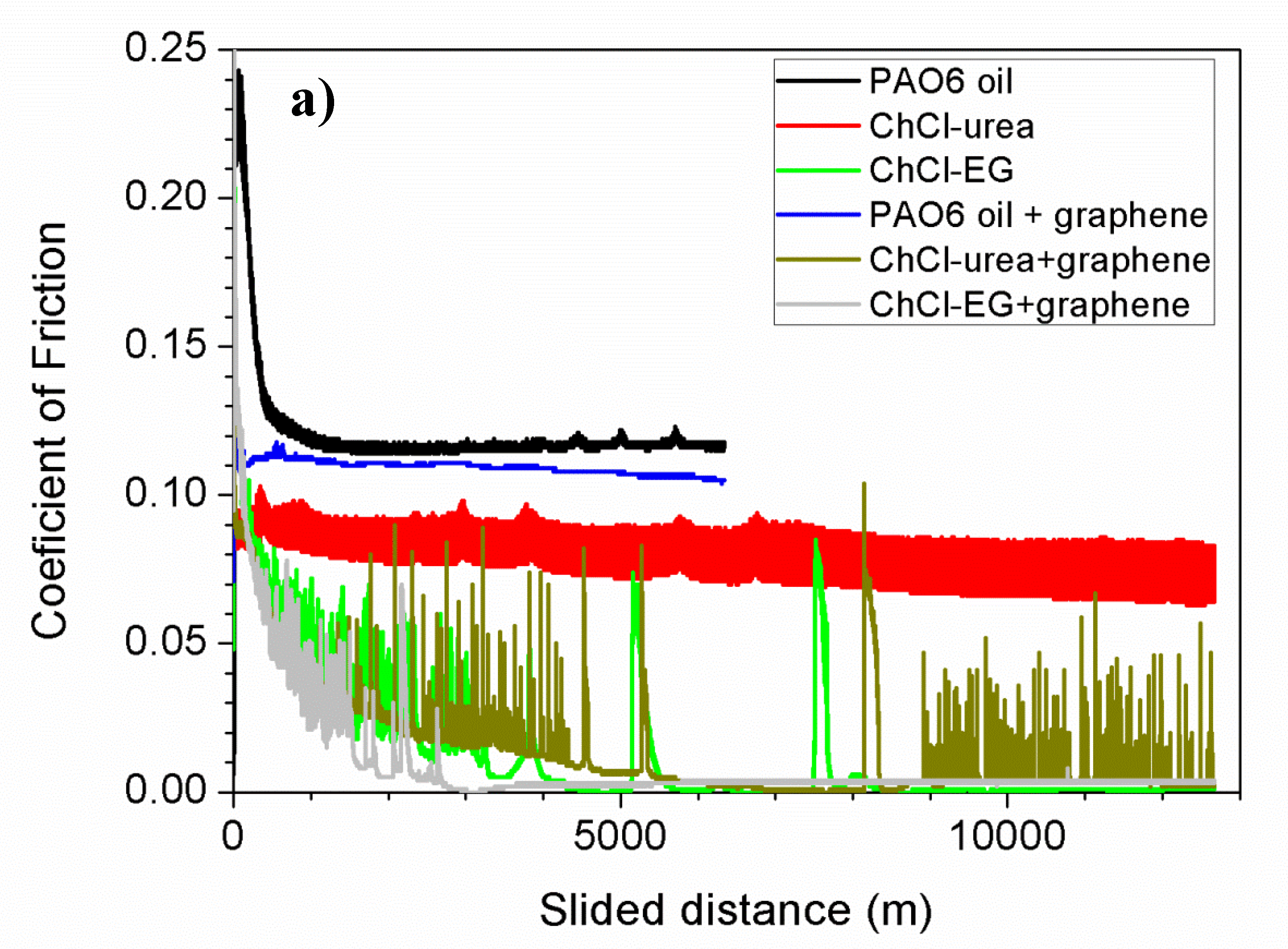
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ye, C.; Liu, W.; Chen, Y.; Yu, L. Room-temperature ionic liquids: A novel versatile lubricant. Chem. Commun. 2001, 2244–2245. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Bermúdez, M.D.; Jiménez, A.E.; Sanes, J.; Carrión, F.J. Ionic liquids as advanced lubricant fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Minami, I. Ionic liquids in tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Palacio, M.; Bhushan, B. A Review of Ionic Liquids for Green Molecular Lubrication in Nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- García, A.; González, R.; Hernández Battez, A.; Viesca, J.L.; Monge, R.; Fernández-González, A.; Hadfield, M. Ionic liquids as a neat lubricant applied to steel–steel contacts. Tribol. Int. 2014, 72, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Avilés, M.D.; Saurín, N.; Sanes, J.; Carrión, F.J.; Bermúdez, M.D. Ionanocarbon lubricants. The combination of ionic liquids and carbon nanophases in tribology. Lubricants 2017, 5, 14. [Google Scholar] [CrossRef]
- Cevasco, G.; Chiappe, C. Are ionic liquids a proper solution to current environmental challenges? Green Chem. 2014, 16, 2375–2385. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 2010–2011. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haerens, K.; Matthijs, E.; Chmielarz, A.; Van der Bruggen, B. The use of ionic liquids based on choline chloride for metal deposition: A green alternative? J. Environ. Manag. 2009, 90, 3245–3252. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Ferguson, J.L.; Gunaratne, H.Q.N.; Ferreira, R.; Leitao, M.C.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Novel biocompatible cholinium-based ionic liquids-toxicity and biodegradability. Green Chem. 2010, 12, 643–649. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef] [PubMed]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chimie 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Lawes, S.D.A.; Hainsworth, S.V.; Blake, P.; Ryder, K.S.; Abbott, A.P. Lubrication of Steel/Steel Contacts by Choline Chloride Ionic Liquids. Tribol. Lett. 2010, 37, 103–110. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ahmed, E.I.; Harris, R.C.; Ryder, K.S. Evaluating water miscible deep eutectic solvents (DESs) and ionic liquids as potential lubricants. Green Chem. 2014, 16, 4156–4161. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.J.; Mu, L.W.; Feng, X.; Lu, X.H. Friction and Wear Behavior of CF/PTFE Composites Lubricated by Choline Chloride Ionic Liquids. Tribol. Lett. 2013, 49, 413–420. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Penkov, O.; Kim, H.J.; Kim, H.J.; Kim, D.E. Tribology of graphene: A review. Int. J. Precis. Eng. Manuf. 2014, 15, 577–585. [Google Scholar] [CrossRef]
- Arenas, M.A.; Ahuir-Torres, J.I.; García, I.; Carvajal, H.; de Damborenea, J. Tribological behaviour of laser textured Ti6Al4V alloy coated with MoS 2 and graphene. Tribol. Int. 2018, 128, 240–247. [Google Scholar] [CrossRef]
- Yu, B.; Liu, Z.; Zhou, F.; Liu, W.; Liang, Y. A novel lubricant additive based on carbon nanotubes for ionic liquids. Mater. Lett. 2008, 62, 2967–2969. [Google Scholar] [CrossRef]
- Zhao, W.; Zeng, Z.; Peng, S.; Wu, X.; Xue, Q.; Chen, J. Fabrication and investigation the microtribological behaviors of ionic liquid-graphene composite films. Tribol. Trans. 2013, 56, 480–487. [Google Scholar] [CrossRef]
- Fan, X.; Wang, L. High-performance lubricant additives based on modified graphene oxide by ionic liquids. J. Colloid Interface Sci. 2015, 452, 98–108. [Google Scholar] [CrossRef]
- Pamies, R.; Avilés, M.D.; Arias-Pardilla, J.; Espinosa, T.; Carrión, F.J.; Sanes, J.; Bermúdez, M.D. Antiwear performance of ionic liquid+graphene dispersions with anomalous viscosity-temperature behavior. Tribol. Int. 2018, 122, 200–209. [Google Scholar] [CrossRef]
- Avilés, M.D.; Carrión-Vilches, F.J.; Sanes, J.; Bermúdez, M.D. Diprotic Ammonium Succinate Ionic Liquid in Thin Film Aqueous Lubrication and in Graphene Nanolubricant. Tribol. Lett. 2019, 67, 26. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, S. Densities and viscosities of (choline chloride + urea) deep eutectic solvent and its aqueous mixtures in the temperature range 293.15 K to 363.15 K. J. Chem. Eng. Data 2014, 59, 2221–2229. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Đokić-Stojanović, D.R.; Đorđević, B.S.; Todorović, V.M.; Konststinović, S.S.; Veljković, V.B. The physico-chemical and thermodynamic properties of the choline chloride-based deep eutectic solvents. J. Serb. Chem. Soc. 2017, 82. [Google Scholar] [CrossRef]
- Mulia, K.; Putri, S.; Krisanti, E.; Nasruddin. Natural deep eutectic solvents (NADES) as green solvents for carbon dioxide capture. AIP Conf. Proc. 2017, 1823, 020022. [Google Scholar]
- Kinoshita, H.; Kondo, M.; Nishina, Y.; Fujii, M. Anti-wear effect of graphene oxide in lubrication by fluorine-containing ionic liquid for steel. Tribol. Online 2015, 10, 91–95. [Google Scholar] [CrossRef]
- Kinoshita, H.; Nishina, Y.; Alias, A.A.; Fujii, M. Tribological properties of monolayer graphene oxide sheets as water-based lubricant additives. Carbon 2014, 66, 720–723. [Google Scholar] [CrossRef]
- Guo, Y.B.; Zhang, S.W. The tribological properties of multi-layered graphene as additives of PAO2 oil in steel-steel contacts. Lubricants 2016, 4, 30. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Few layer graphene to reduce wear and friction on sliding steel surfaces. Carbon 2013, 54, 454–459. [Google Scholar] [CrossRef]

| Halide Salt | mp/°C | Hydrogen Bond Donor (HBD) | mp/°C | Salt:HBD (Molar Ratio) | Viscosity (cP) | Density (g/cm3) | Conductivity (mS/cm−1) | DES Tf °C |
|---|---|---|---|---|---|---|---|---|
| ChCl | 303 | Urea | 134 | 1:2 | 630–750 | 1.16 | 0.75 | 12 |
| ChCl | 303 | Ethylene glycol | −12.9 | 1:2 | 36 | 1.06–1.10 | 7.61 | - |
| ChCl | 303 | Malic acid | 130 | 1:1 | 446 | 1.23 | 0.1–10 | −56 |
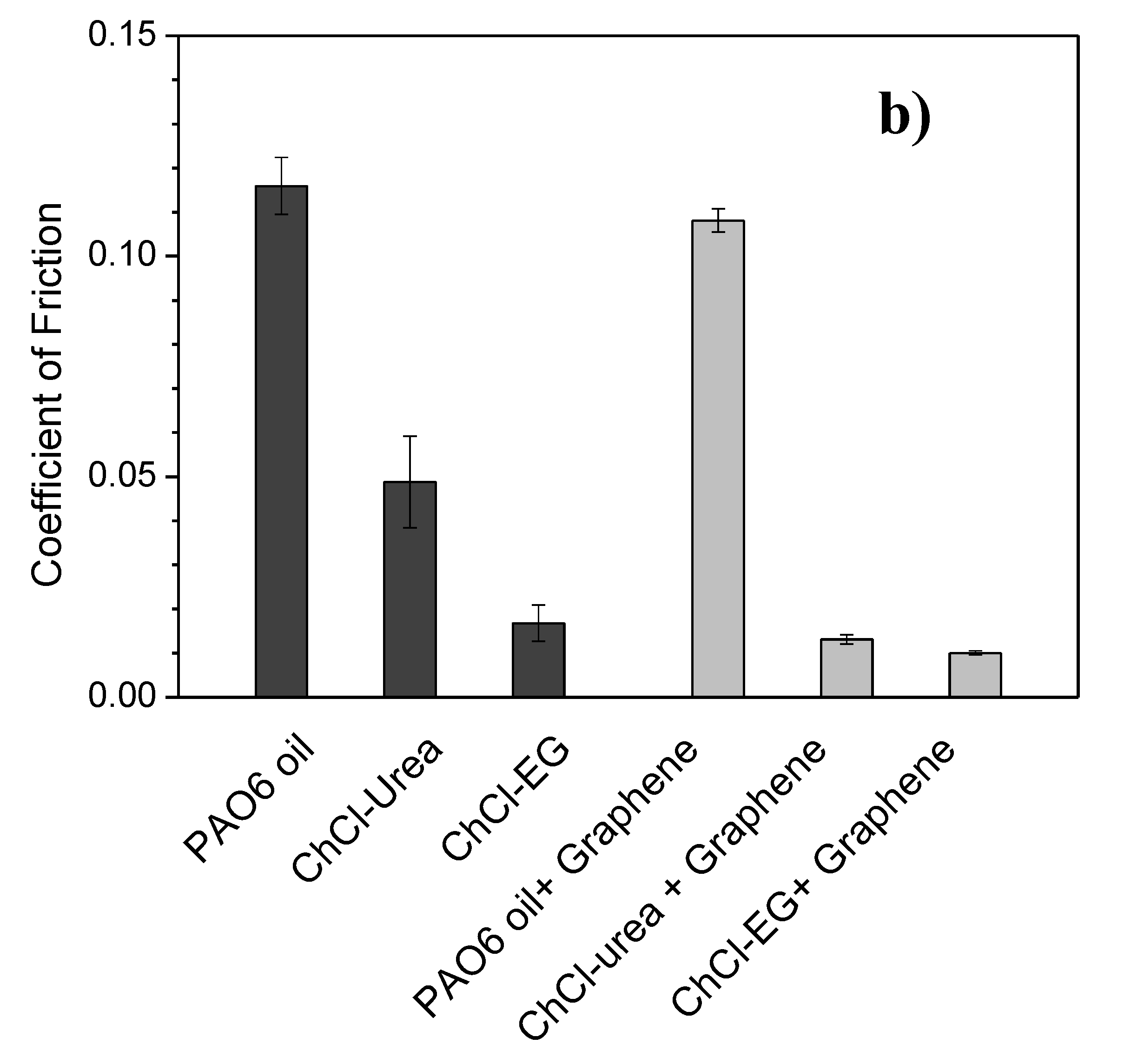
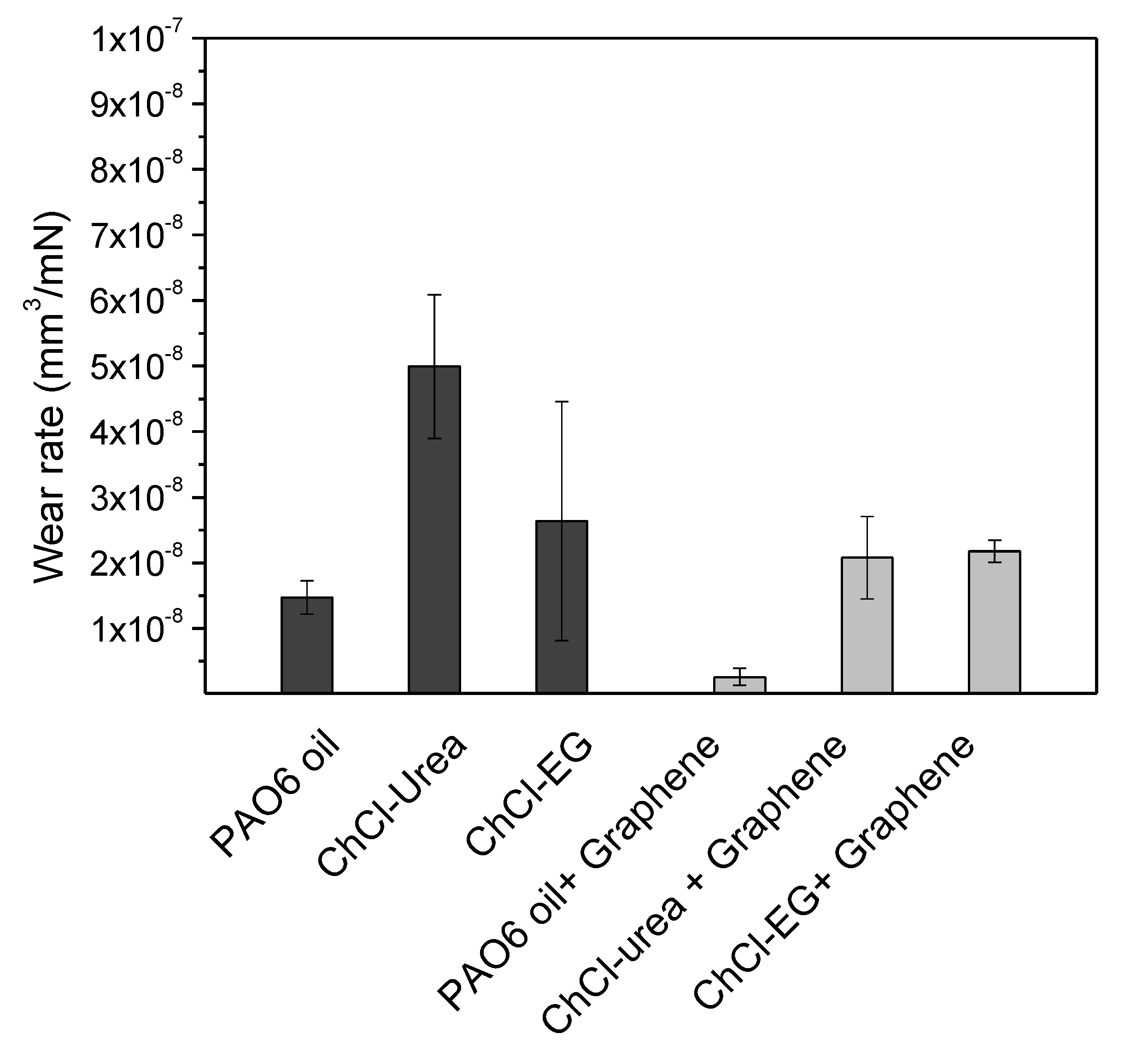
| Lubricant | Wear Rate (10−8 mm3/mN) | SD (10−8 mm3/mN) | COF | SD |
|---|---|---|---|---|
| PAO6 | 1.5 | 0.2 | 0.116 | 0.006 |
| ChCl-urea | 4.9 | 1.1 | 0.05 | 0.01 |
| ChCl-malic acid | 1.3 | 0.9 | 0.024 | 0.004 |
| ChCl-ethylene glycol | 2.6 | 1.8 | 0.017 | 0.004 |
| PAO6 + graphene | 0.2 | 0.1 | 0.108 | 0.003 |
| ChCl-urea + graphene | 2.1 | 0.6 | 0.013 | 0.001 |
| ChCl-malic acid + graphene | 1.1 | 1.1 | 0.043 | 0.009 |
| ChCl-ethylene glycol + graphene | 2.2 | 0.2 | 0.010 | 0.005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, I.; Guerra, S.; de Damborenea, J.; Conde, A. Reduction of the Coefficient of Friction of Steel-Steel Tribological Contacts by Novel Graphene-Deep Eutectic Solvents (DESs) Lubricants. Lubricants 2019, 7, 37. https://doi.org/10.3390/lubricants7040037
Garcia I, Guerra S, de Damborenea J, Conde A. Reduction of the Coefficient of Friction of Steel-Steel Tribological Contacts by Novel Graphene-Deep Eutectic Solvents (DESs) Lubricants. Lubricants. 2019; 7(4):37. https://doi.org/10.3390/lubricants7040037
Chicago/Turabian StyleGarcia, Ignacio, Silvia Guerra, Juan de Damborenea, and Ana Conde. 2019. "Reduction of the Coefficient of Friction of Steel-Steel Tribological Contacts by Novel Graphene-Deep Eutectic Solvents (DESs) Lubricants" Lubricants 7, no. 4: 37. https://doi.org/10.3390/lubricants7040037
APA StyleGarcia, I., Guerra, S., de Damborenea, J., & Conde, A. (2019). Reduction of the Coefficient of Friction of Steel-Steel Tribological Contacts by Novel Graphene-Deep Eutectic Solvents (DESs) Lubricants. Lubricants, 7(4), 37. https://doi.org/10.3390/lubricants7040037