Tribological Properties of Double-Network Gels Substituted by Ionic Liquids
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of DN Hydrogel and DN Ion Gel
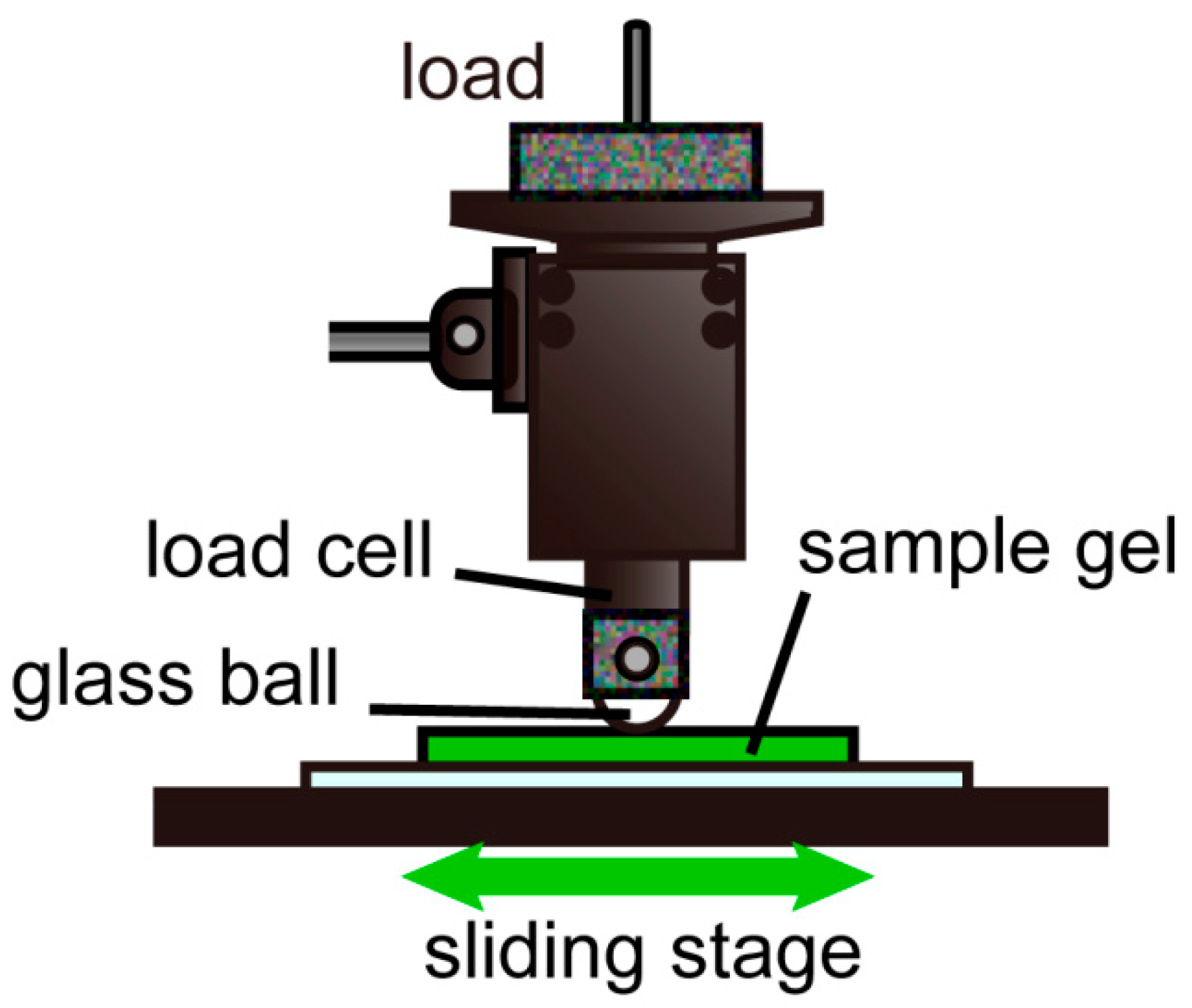
2.2. Friction Test With Ball-on-Plate-Type Tribometer
3. Results and Discussions
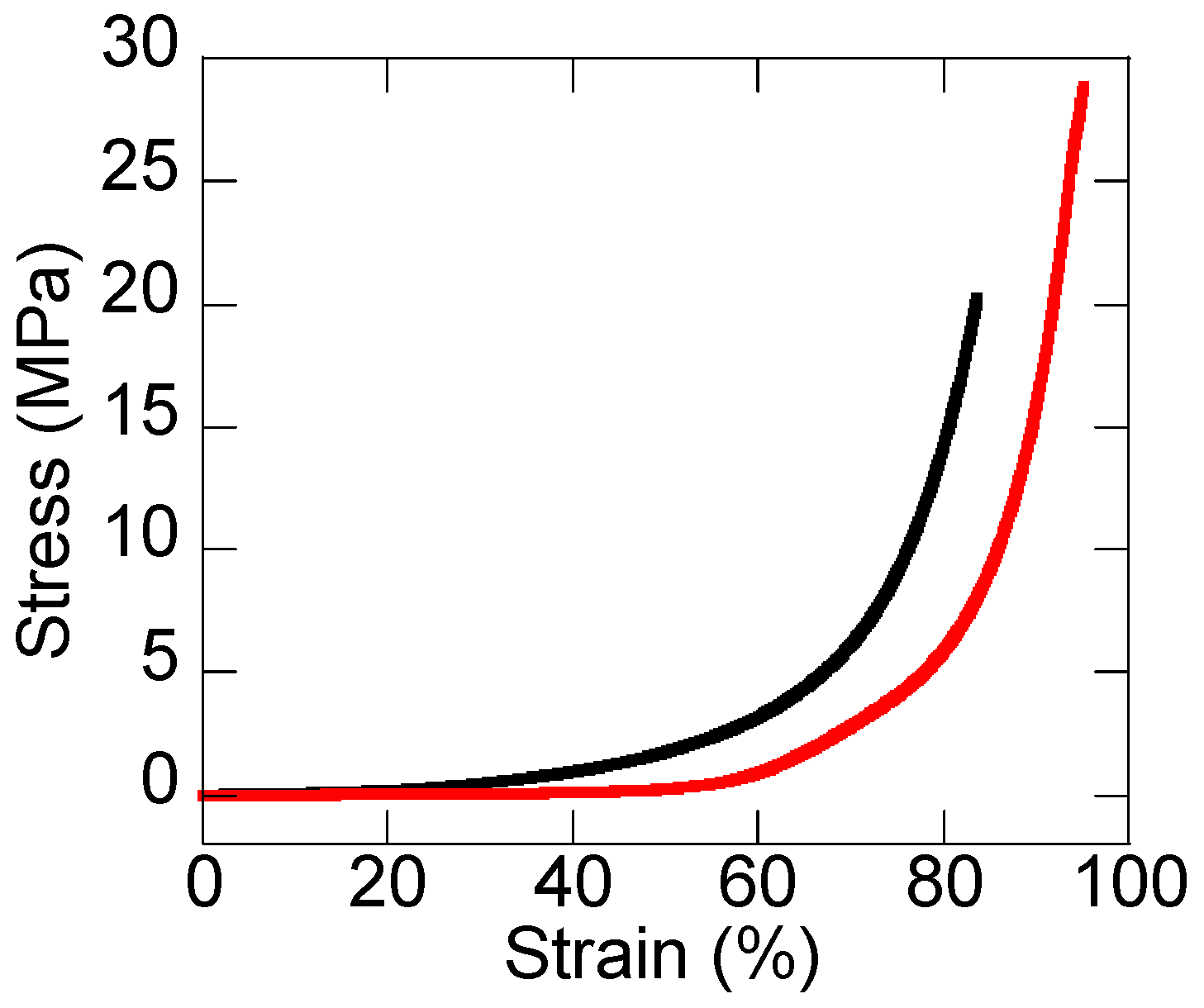
3.1. Mechanical Strength of DN Ion Gel
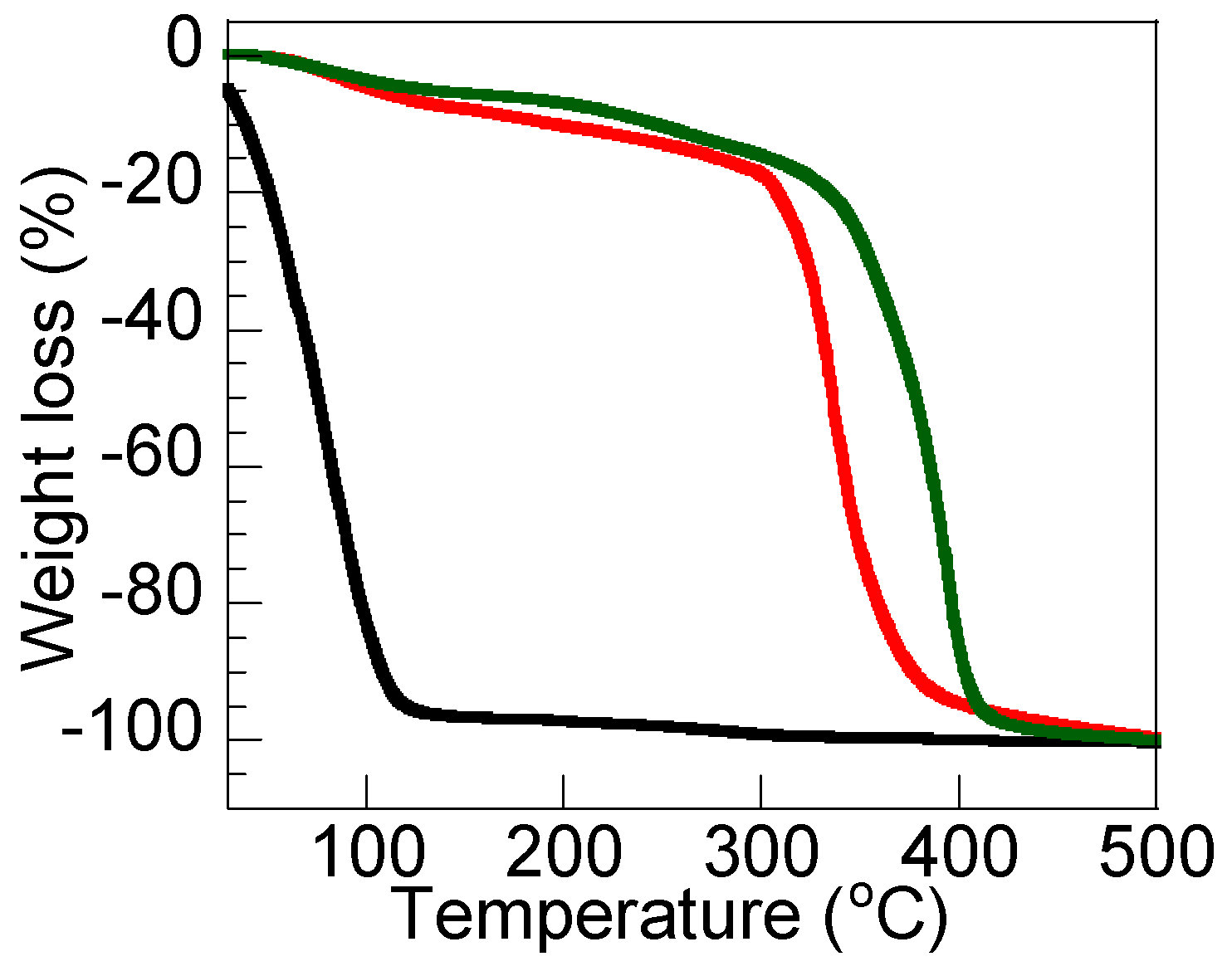
3.2. Thermal Properties of DN Ion Gel
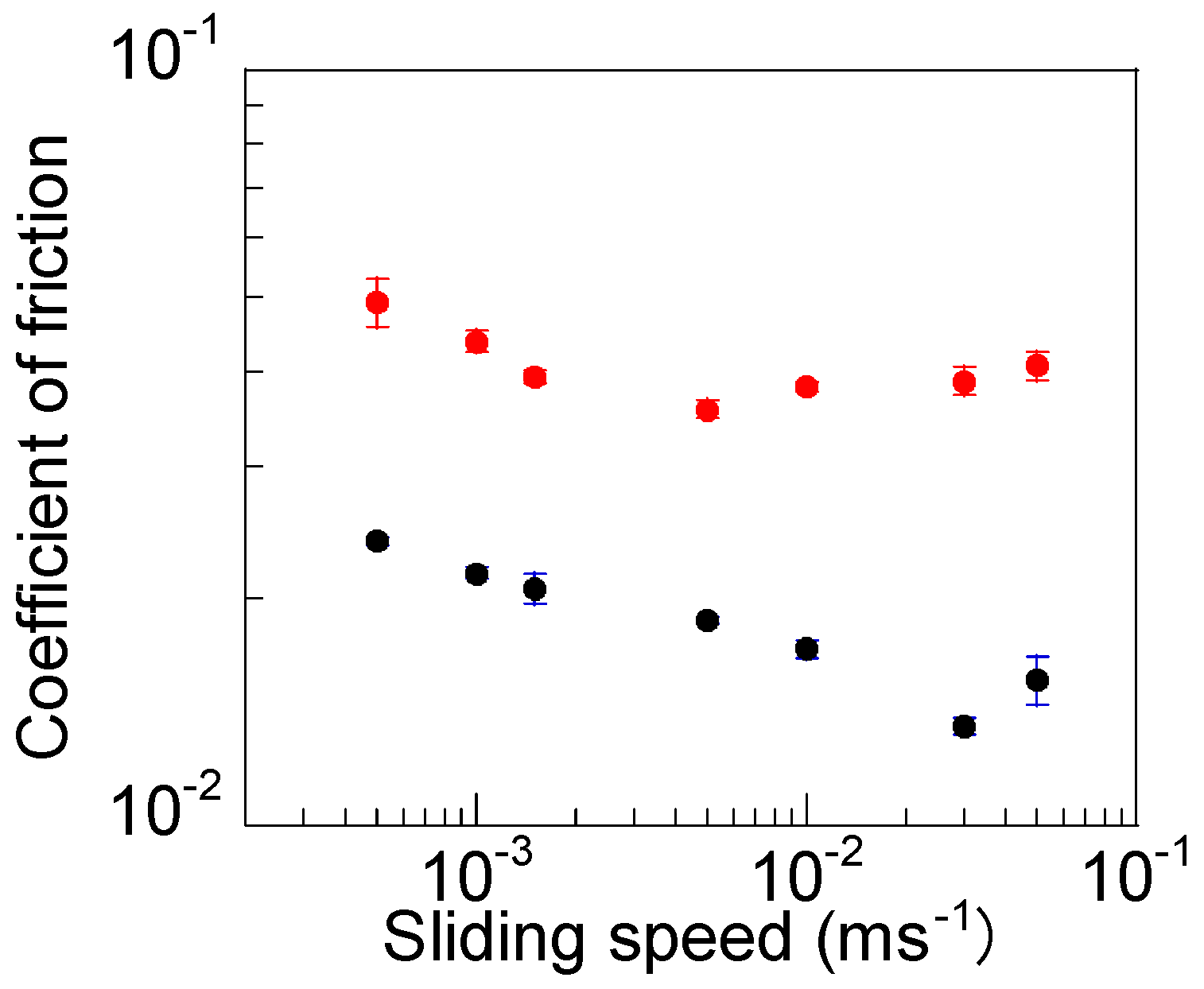
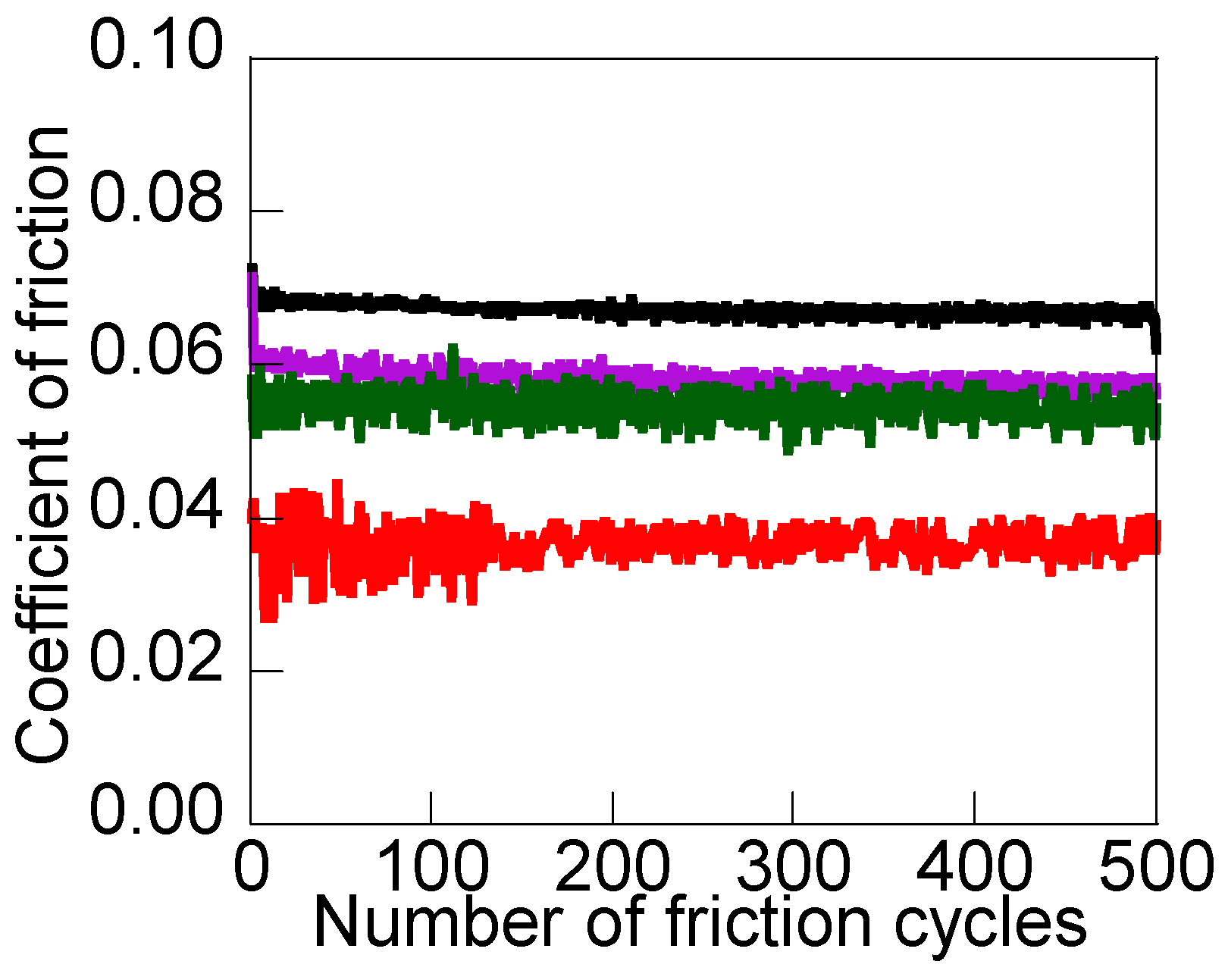
3.3. Tribological Properties of the DN Ion Gel
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Klein, J. Molecular mechanisms of synovial joint lubrication. Proc. Inst. Mech. Eng. 2006, 220, 691–721. [Google Scholar] [CrossRef]
- Gong, J.P.; Kurokawa, T.; Narita, T.; Kagata, G.; Osada, Y.; Nishimura, G.; Kinjo, M. Synthesis of hydrogels with extremely low surface friction. J. Am. Chem. Soc. 2001, 123, 5582–5583. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Gong, J.P. Negatively charged polyelectrolyte gels as bio-tissue model system and for biomedical application. Curr. Opin. Colloid Interface Sci. 2006, 11, 345–350. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Mizukami, M.; Tanabe, T.; Furukawa, H.; Kurihara, K. Friction of polymer hydrogels studied by resonance shear measurements. Soft Matter 2015, 11, 6192–6200. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.Q.Y.; Low, Z.W.K.; Heng, S.J.W.; Chan, S.Y.; Owh, C.; Loh, X.J. Recent Advances in Shape Memory Soft Materials for Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 10070–10087. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Takahashi, T.; Yamaguchi, T.; Ichijo, H. Self-oscillating gel. J. Am. Chem. Soc. 1996, 118, 5134–5135. [Google Scholar] [CrossRef]
- Okumura, Y.; Ito, K. The polyrotaxane gels: A topological gel by figure-of eight cross-links. Adv. Mater. 2001, 13, 485–487. [Google Scholar] [CrossRef]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic-Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-Swelling Properties. Adv. Mater. 2002, 16, 1120–1124. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sakai, T.; Matsunaga, T.; Yamamoto, Y.; Ito, C.; Yoshida, R.; Suzuki, S.; Sasaki, N.; Shibayama, M.; Chung, U. Design and Fabrication of a High-Strength Hydrogel with Ideally Homogeneous Network Structure from Tetrahedron-like Macromonomers. Macromolecules 2008, 41, 5379–5384. [Google Scholar] [CrossRef]
- Wu, Z.L.; Kurokawa, T.; Gong, J.P. Novel Developed Systems and Techniques Based on Double-Network Principle. Bull. Chem. Soc. Jpn. 2011, 84, 1295–1311. [Google Scholar] [CrossRef]
- Minami, I. Ionic liquids in tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590. [Google Scholar] [CrossRef] [PubMed]
- Somers, A.; Howlett, P.; MacFarlane, D.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Kamijo, T.; Arafune, H.; Morinaga, T.; Honma, S.; Sato, T.; Hino, M.; Mizukami, M.; Kurihara, K. Lubrication Properties of Ammonium-Based Ionic Liquids Confined between Silica Surfaces Using Resonance Shear Measurements. Langmuir 2015, 31, 13265–13270. [Google Scholar] [CrossRef] [PubMed]
- Arafune, H.; Kamijo, T.; Morinaga, T.; Honma, S.; Sato, T.; Tsujii, Y. A Robust Lubrication System Using an Ionic Liquid Polymer Brush. Adv. Mater. Interfaces 2015, 2, 1500187. [Google Scholar] [CrossRef]
- Arafune, H.; Honma, S.; Morinaga, T.; Kamijo, T.; Miura, M.; Furukawa, H.; Sato, T. Highly Robust and Low Frictional Double-Network Ion Gel. Adv. Mater. Interfaces 2017, 4. [Google Scholar] [CrossRef]
- Moghadam, F.; Kamio, E.; Yoshizumi, A.; Matsuyama, H. An amino acid ionic liquid-based tough ion gel membrane for CO2 capture. Chem. Commun. 2015, 51, 13658–13661. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Hamaya, T.; Okada, T. New highly proton-conducting membrane poly(vinylpyrrolidone)(PVP) modified poly(vinyl alcohol)/2-acrylamido-2-methyl-1-propanesulfonic acid (PVA–PAMPS) for low temperature direct methanol fuel cells (DMFCs). Polymer 2005, 46, 10809–10816. [Google Scholar] [CrossRef]
- Raviv, U.; Giasson, S.; Kampf, N.; Gohy, J.-F.; Jérôme, R.; Klein, J. Normal and frictional forces between surfaces bearing polyelectrolyte brushes. Langmuir 2008, 24, 8678–8687. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, I.E.; Briscoe, W.H.; Titmuss, S.; Jacobs, R.M.J.; Osborne, V.L.; Edmondson, S.; Huck, W.T.S.; Klein, J. Direct Measurement of Normal and Shear Forces between Surface-Grown Polyelectrolyte Layers. J. Phys. Chem. B 2009, 113, 3947–3956. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Tominaga, T.; Katsuyama, Y.; Kuwabara, R.; Furukawa, H.; Osada, Y.; Gong, J.P. Elastic-hydrodynamic transition of gel friction. Langmuir 2005, 21, 8643–8648. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arafune, H.; Muto, F.; Kamijo, T.; Honma, S.; Morinaga, T.; Sato, T. Tribological Properties of Double-Network Gels Substituted by Ionic Liquids. Lubricants 2018, 6, 89. https://doi.org/10.3390/lubricants6040089
Arafune H, Muto F, Kamijo T, Honma S, Morinaga T, Sato T. Tribological Properties of Double-Network Gels Substituted by Ionic Liquids. Lubricants. 2018; 6(4):89. https://doi.org/10.3390/lubricants6040089
Chicago/Turabian StyleArafune, Hiroyuki, Fumiya Muto, Toshio Kamijo, Saika Honma, Takashi Morinaga, and Takaya Sato. 2018. "Tribological Properties of Double-Network Gels Substituted by Ionic Liquids" Lubricants 6, no. 4: 89. https://doi.org/10.3390/lubricants6040089
APA StyleArafune, H., Muto, F., Kamijo, T., Honma, S., Morinaga, T., & Sato, T. (2018). Tribological Properties of Double-Network Gels Substituted by Ionic Liquids. Lubricants, 6(4), 89. https://doi.org/10.3390/lubricants6040089






