1. Introduction
Engine oils play a critical role in friction reduction. Over the last 20 years, advancements in engine oil technology steadily improved fuel economy as the industry moved through GF-1 to GF-5 specifications. These improvements were measured in an industry-accepted engine using a standard dynamometer cycle. The fuel consumption reduction was influenced by changes in base oil chemistry, development of new friction modifiers and their treat levels, and the total additive package consisting of various other components while maintaining and/or improving other performance requirements. Additional fuel economy improvement through engine oil within the same viscosity grade is becoming challenging and therefore, may need to focus on non-traditional base oils.
The importance of base oil type in friction reduction has been demonstrated in the past. Kiovsky et al. [
1] demonstrated that hydrocracked base oil can offer friction advantage over polyalphaolefin and severely hydrogenated mineral oil while keeping the same additive package and same viscosity grade, SAE 5W-30 using laboratory bench tests. Similarly, Igarashi et al. demonstrated 1.2% fuel economy improvement with severely hydrocracked base oil over solvent refined base oil in FTP (Federal Test Procedure) road simulator test with SAE 5W-30 engine oil [
2]. Polyalkylene glycols are classified as API Group V synthetic base stocks and are being explored for engine oil formulation as a step forward for significant fuel consumption reduction. Polyalkylene glycol (PAG) based lubricants are used as fire resistant hydraulic fluids, refrigeration lubricants, compressor lubricants, and gear lubricants. They have also been explored for lubricating two-cycle engines as early as the 1950s [
3]. The advantages of using polyalkylene glycols as base stock for engine oil formulations including lower boundary friction coefficient because of their polar nature, higher oxidative stability, low volatility (5%) for potentially lower oil consumption, etc. [
4]. However, there is very little information available in the literature about its application in modern engines. Using a laboratory bench test rig, Woydt and Kelling [
5], demonstrated a significant reduction in friction coefficient of PAG based lubricants over mineral oil-based engine oils in the temperature range 40–120 °C. In this rig, a section of a ring was pressed against a section of a rotating (against a vertical axis) liner. Motored valvetrain tests showed significant friction reduction at 120 °C oil temperature compared to conventional mineral oil formulation. Cam lobe wear was also reduced by 42% [
6]. Motored bearing friction was also reported to be reduced at 100 °C over a mineral oil base formulation. Similar friction reduction was observed in motored engine tests. The thermo-oxidative stability of PAG oils has also been demonstrated to be superior to mineral based formulation. Greaves et al. [
7] investigated the tribo-film formation characteristics of oil soluble PAG (OSP) as an additive in polyalphaolefin (PAO) base oil and in a PAO base oil with 1% zinc dialkyldithiophosphate (ZDDP) anti-wear additive. OSP addition to PAO reduced friction and wear but no significant tribo-film formed. When ZDDP was added, a tribo-film formed but the rate of film formation appeared slower leading to slight increase in wear.
It is quite obvious that PAGs have not been investigated in detail in the past although there have been limited studies on their friction reducing characteristics. However, the friction reduction mechanism has not been explored in detail. An engine oil needs to meet several additional performance attributes—i.e., wear protection capability, oxidation, sludge and varnish protection capability, etc. These have not been investigated in detail either.
The purpose of this paper is to capture opportunities and key challenges in formulating engine oil with PAGs resulting primarily from an extensive research. This provided the team with a lot of insight on the performance of PAG of different chemistries. The research started with various PAG chemistries and evaluating them in simple laboratory bench tests as a screener to understand the effect of chemistry on friction and wear performance, and identifying the friction reduction mechanism. A few formulations were selected and then subjected them to more complex engine component level evaluations and motored and fired engine tests to assess friction, wear, oxidation, and sludge and varnish formation characteristics.
2. Lubricants
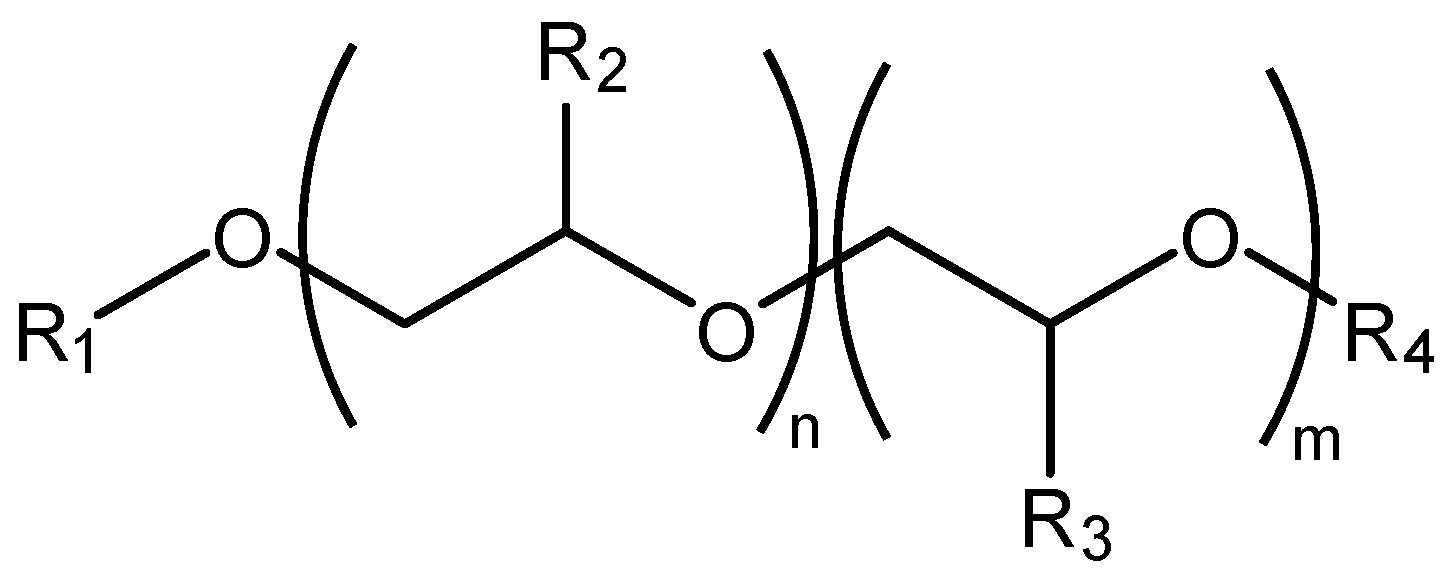
The general structures of the PAGs studied are depicted in
Figure 1. Five different chemistries were created by varying the starting alcohols, oxides, and end groups. R1 is the alcohol initiator. The oxide monomers are either ethylene oxide (–R2 or R3 are H), propylene oxide (R2 or R3 are CH
3), or butylene oxide (R2 or R3 C
2H
5). If the polymer is a homopolymer, –R2 and–R3 are the same oxide monomer; if a copolymer, –R2 and –R3 are different. The end group, –R4, can either be –H or an alkyl group if R4 is an alkyl group, the PAG is ‘capped’, or alternatively a diether. If R4 is hydrogen—i.e., the end group is –OH—the PAG is a mono functional PAG or simply referred to as a ‘PAG’. The five different polymers investigated were capped random copolymer of ethylene oxide and propylene oxide with alcohol 1; capped homopolymer of propylene oxide with alcohol 1; capped homopolymer of propylene oxide and alcohol 2; homopolymer of propylene oxide with alcohol 1; and copolymer of propylene oxide and butylene oxide with alcohol 2. Viscosities of the polymer are determined by their molecular weight, which in turn is determined by the number of moles of oxide added to the starting alcohol (
m and
n in
Figure 1). By adjusting
m and
n a series of chemically similar polymers with different high temperature high shear (HTHS) viscosities can be synthesized. Blending chemically similar polymers with different viscosities make fluids with viscosities between the two polymers. Selected PAG formulations are shown in
Table 1. All fluids except UC-PO-A1-F2-2.6 were formulated with a proprietary additive package [
8,
9] that was designed to be low SAPS, low ash and to have an inherently high viscosity index (VI). The PAGs selected have high viscosity index and therefore, there was no need to add any viscosity index improver. In addition to the proprietary additive package, UC-PO-A1-F2-2.6 included ZDDP for improved anti-wear properties and a molybdenum-based friction modifier.
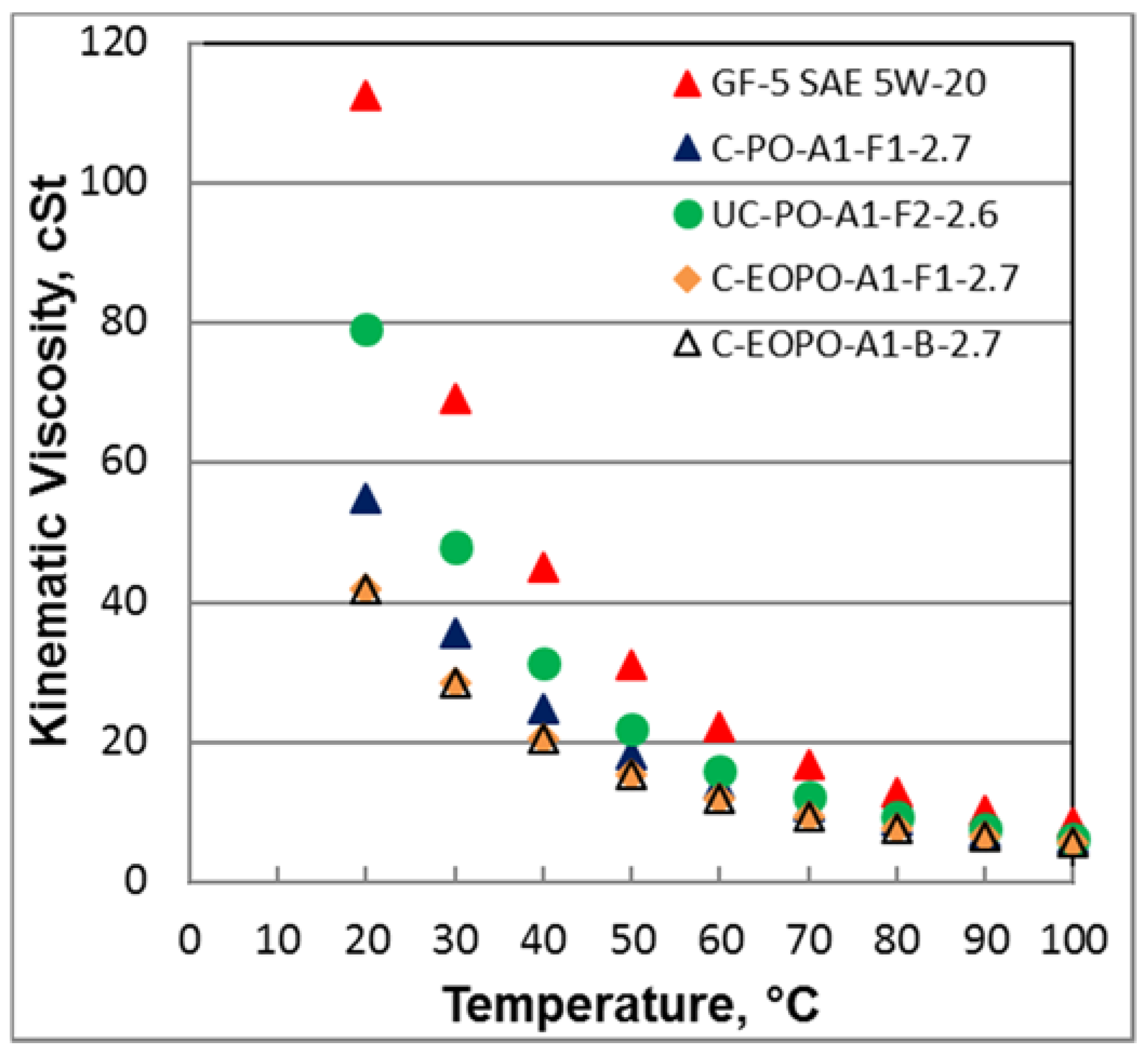
Figure 2 shows kinematic viscosity and temperature relationship for various formulations. It is quite evident some of the PAGs show significantly lower viscosity at lower temperatures than the baseline GF-5 5W-20 while maintaining the 100 °C viscosity at the same level. This is significant because lower viscosity can show improved friction reduction during engine warm up and also during initial phases of fuel economy test where engine oil temperature remains low.
One of the challenges in formulating with PAG is the solubility of conventional engine oil additive components. Generally, the anti-wear additive zinc dialkyldithiphosphate (ZDDP), antioxidants, dispersants, antifoam agents, friction modifiers are soluble but not overbased detergents. Even those that are generally soluble, not all chemical variants may be soluble. This puts a huge limitation on formulation space. Therefore, there is opportunity for research for developing effective additive chemistry compatible for PAGs.
3. Opportunities in Friction Performance
The PAG lubricants were evaluated using a variety of techniques to develop a better understanding of the friction performance under different lubrication regimes and the mechanism of friction reduction. This included laboratory bench tests which are very useful for understanding the mechanism of friction reduction by analyzing tribo-films formed on wear surfaces. This is followed by engine component tests, for example, valvetrain friction tests, cranktrain friction tests, and full engine motored tests. Valvetrain test is important to understand friction performance primarily in mixed lubrication condition while the cranktrain test is important to understand friction performance primarily in hydrodynamic lubrication regime. The full engine motored test provides a comprehensive view of friction performance in all lubrication regimes.
3.1. Laboratory Bench Test Evaluations
Friction and wear performance was evaluated under both pure sliding (boundary lubrication) and sliding/rolling conditions (mixed and hydrodynamic conditions) at different temperatures. The effect of PAG chemistry on boundary friction coefficient and wear was quite evident [
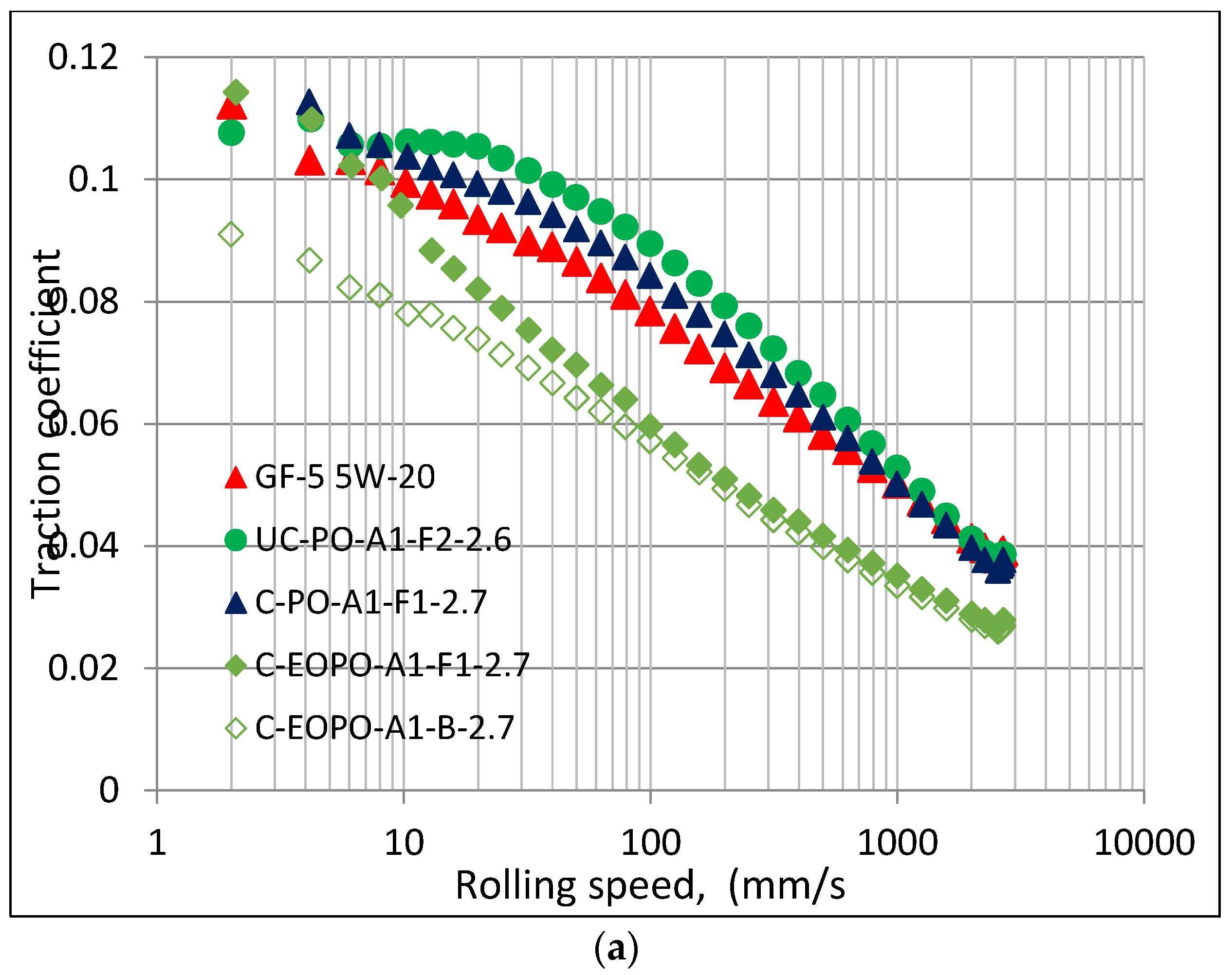
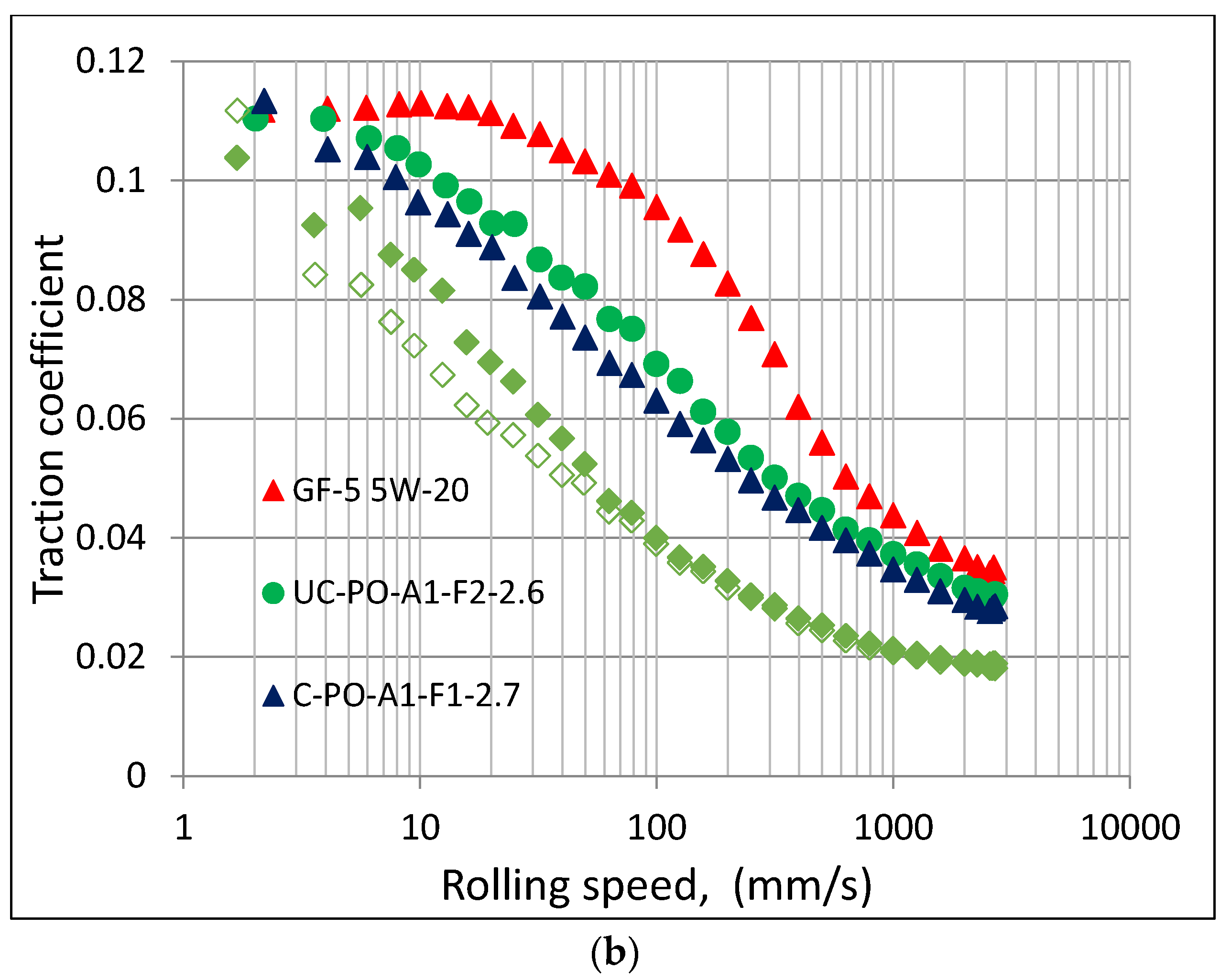
10]. Similar results were observed under mixed and hydrodynamic lubrication conditions as shown in
Figure 3. At a low temperature of 40 °C, the PAG base oil C-EOPO-A1-B-2.7 showed the lowest traction coefficient in the entire speed range investigated but its formulated version showed an increase in traction coefficient at lower speed range when the lubrication condition becomes more mixed. The increase in traction coefficient with additives has been observed before [
11,
12]. It is known that anti-wear additive zinc dialkyl dithiophosphate, detergent additive, calcium sulfonate form very thin tribo-films of about 80–100 nm. These types of films can be considered as a coating on the substrate. Calculations by Liu and Gangopadhyay [
13] suggest that the compliant coating exhibits a faster increase of asperity contact areas relative to the homogeneous half plane, therefore giving rise to higher traction coefficients. It can also be observed that some of the PAGs exhibited higher and some lower traction coefficients than GF-5 5W-20 oil in mixed lubrication regime. Comparing this result with those in
Figure 2 indicates no direct correlation of traction coefficient (in a mixed lubrication regime) with kinematic viscosity. However, the traction coefficient results were quite different when the temperature was raised to 100 °C when all PAGs showed lower traction coefficient than GF-5 5W-20 oil in both mixed and hydrodynamic lubrication regimes (at the highest speed investigated). However, with the boundary lubrication regime (in a low speed region), the traction coefficients appear to converge. Again, no direct correlation could be observed with traction coefficient in mixed lubrication regime with kinematic viscosity at this temperature. Therefore, the difference in traction coefficients appear to be more related to PAG chemistry.
Tribo-Film Analysis
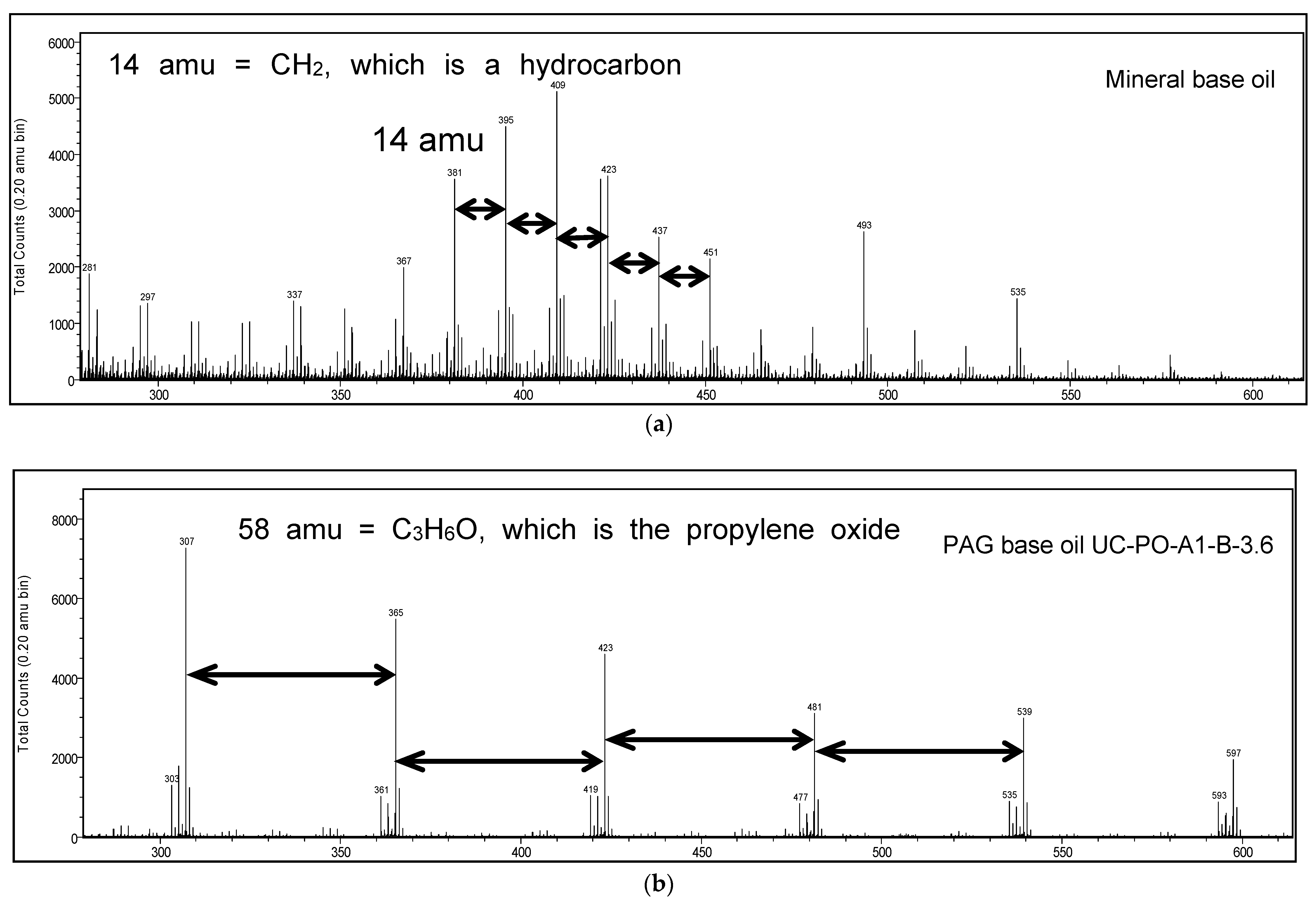
To investigate this further, the disks following traction evaluation were analyzed using ToF-SIMS (Time-of-Flight secondary ion mass spectroscopy). Disks following tests with mineral base oil and PAG (both with no additives) were examined. ToF-SIMS is a very surface sensitive technique enabling molecular fragment (chemistry) information from the top 1 nm layer of the surface. Regions inside and outside the wear scar were examined and both positive and negative ion spectra were obtained for analysis. An area measuring 100 µm × 100 μm was analyzed for each acquisition. In order to ensure surface composition was not altered by the ion bombardment process (static SIMS conditions), data acquisition times were limited to five minutes. The disk wear surface following test with mineral base oil showed presence of 80 nm iron oxide film while that with PAG base oil showed much thinner oxide film, about 5 nm (based on Auger and X-ray photoelectron spectroscopy data). Time-of-flight secondary ion mass spectra obtained from wear surfaces generated with mineral base oil and PAG base oil UC-PO-A1-B-3.6 are shown in
Figure 4. Classic hydrocarbon fragmentation pattern dominates the spectrum of the mineral base oil (
Figure 4a) as expected. A pattern of peaks 14 amu apart can be observed on the spectrum, which is the mass of a CH
2 hydrocarbon fragment, the basic building block of a hydrocarbon molecule. In contrast, spectrum (
Figure 4b) is significantly different for the PAG base oil, which shows a pattern of peaks that are 58 amu apart. This is mass of a single propylene oxide monomer. Both XPS (not shown in here) and ToF-SIMS results indicate that a thin film of the respective base oil has formed on the rubbing surface. Similar results were obtained when disks following tests with fully formulated GF-5 5W-20 and PAG oil were examined. However, the wear surface contained tribo-films consisting of sulfates and phosphates [
14].
The observation that PAG molecules are adsorbed on the surface is not surprising considering their polar nature. A decrease in friction coefficient has been hypothesized in past research [
15,
16] due to adsorption of polar end groups like carboxyl, ester, amine etc. on the metal surface. But recently ToF-SIMS analysis of tribo-films formed on wear surfaces provided direct evidence of the adsorption of a polar ester structure found in a polymethacylate, a viscosity index improver, leading to the conclusion that the adsorbed species reduced the friction coefficient [
17]. A few other researchers also showed similar evidence of adsorption of glycerol monoleate friction modifier on a wear surface using ToF-SIMS [
18,
19]. It is believed in the present investigation the presence of PAG molecules at the contact surface contributes to the observed friction reduction.
3.2. Motored Valvetrain Friction Evaluation
Following laboratory bench tests, evaluations were extended to engine components to explore if similar friction reductions could be demonstrated. In this test, one camshaft in the cylinder head (from a production engine) was rotated using an electric motor and the torque to turn was recorded from in-line torque meter. A direct acting valvetrain architecture was used where the cam slides against a flat bucket tappet. The camshaft was rotated from 350 to 2000 RPM and the contact operated mostly in mixed lubrication regime. The details of the test could be found elsewhere [
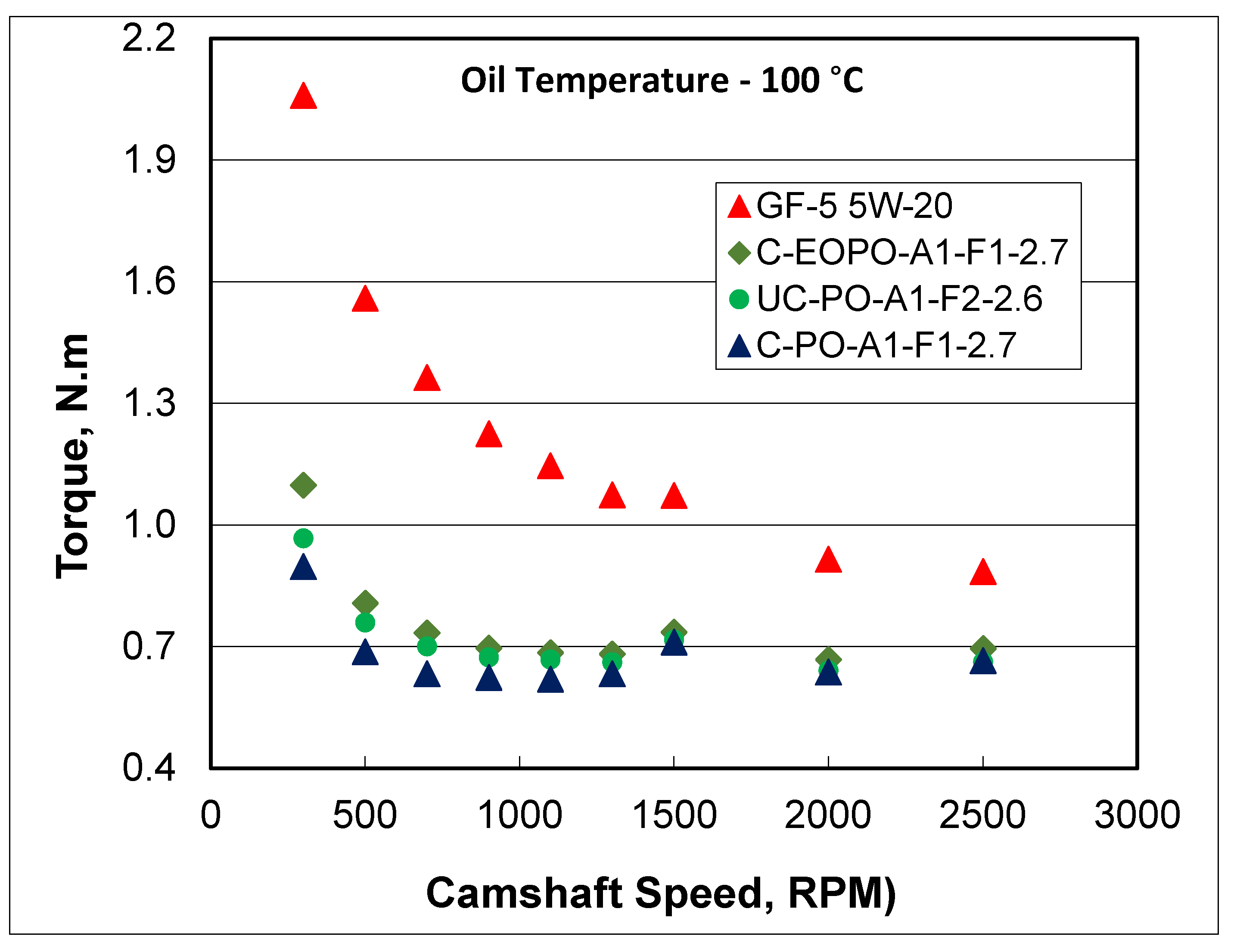
10]. Results in
Figure 5 showed about 50% friction reduction by PAG formulations compared to baseline oil GF-5 5W-20 depending on camshaft speed. Also, the extent of friction reduction depended on PAG formulations. These results are in line with those obtained from sliding/rolling (MTM2) tests. The valvetrain friction evaluations were conducted at other temperatures as well. When the friction response of various PAG formulations was compared at the same viscosity (by looking into friction data at different temperatures), it became obvious that PAG chemistry was primarily responsible for friction reduction. When the bucket tappets were analyzed using ToF-SIMS technique, again the presence of PAG molecules was observed on tappet surfaces in addition to tribo-films consisting of phosphates and sulfates, similar to that typically found with conventional additive package [
10].
Sander et al. [
20] evaluated friction in a diesel engine motored under motored and pressurized motored conditions and compared results between a fully synthetic 0W-20 and a PAG (ethylene oxide-propylene oxide based) oil at the same high temperature high shear (HTHS) viscosity of 2.6 mPa·s. At 90 °C oil temperature, PAG oil offered about 2–10% friction improvement under motoring conditions. However, under motored pressurized conditions, even higher (up to 25% depending on pressurization level) friction reduction was observed. In addition, main bearing temperature was also reduced by several degrees.
3.3. Motored Cranktrain Friction Evaluation
One of the PAG formulations, C-EOPO-A1-F1-2.7, was evaluated in a motored cranktrain friction rig where the crankshaft was rotated by a motor with connecting rod attached to the piston. The hardware represents a current production in line four-cylinder engine block. Therefore, this test accounts for the majority of the friction elements in an engine, i.e., main and connecting rod bearing, piston skirt, and piston rings.
Figure 6 shows the experimental set up. The crankshaft is rotated by an electric motor with an in-line torque meter. Engine oil is circulated in the block using an external electric pump and an external oil sump, which is electrically heated. Water-glycol mixture was also circulated in the block through an external pump and an external sump, which is electrically heated to bring the temperature of an equilibrium quicker. The block was broken-in by running the rig 600–4000 RPM for various length of time until friction readings have stabilized. The results in
Figure 7 shows substantial friction reduction (6–30% depending on speed) at 100 °C oil temperature compared to GF-5 SAE 5W-20 oil. Similar results were observed at other temperatures.
3.4. Motored Engine Friction Evaluation
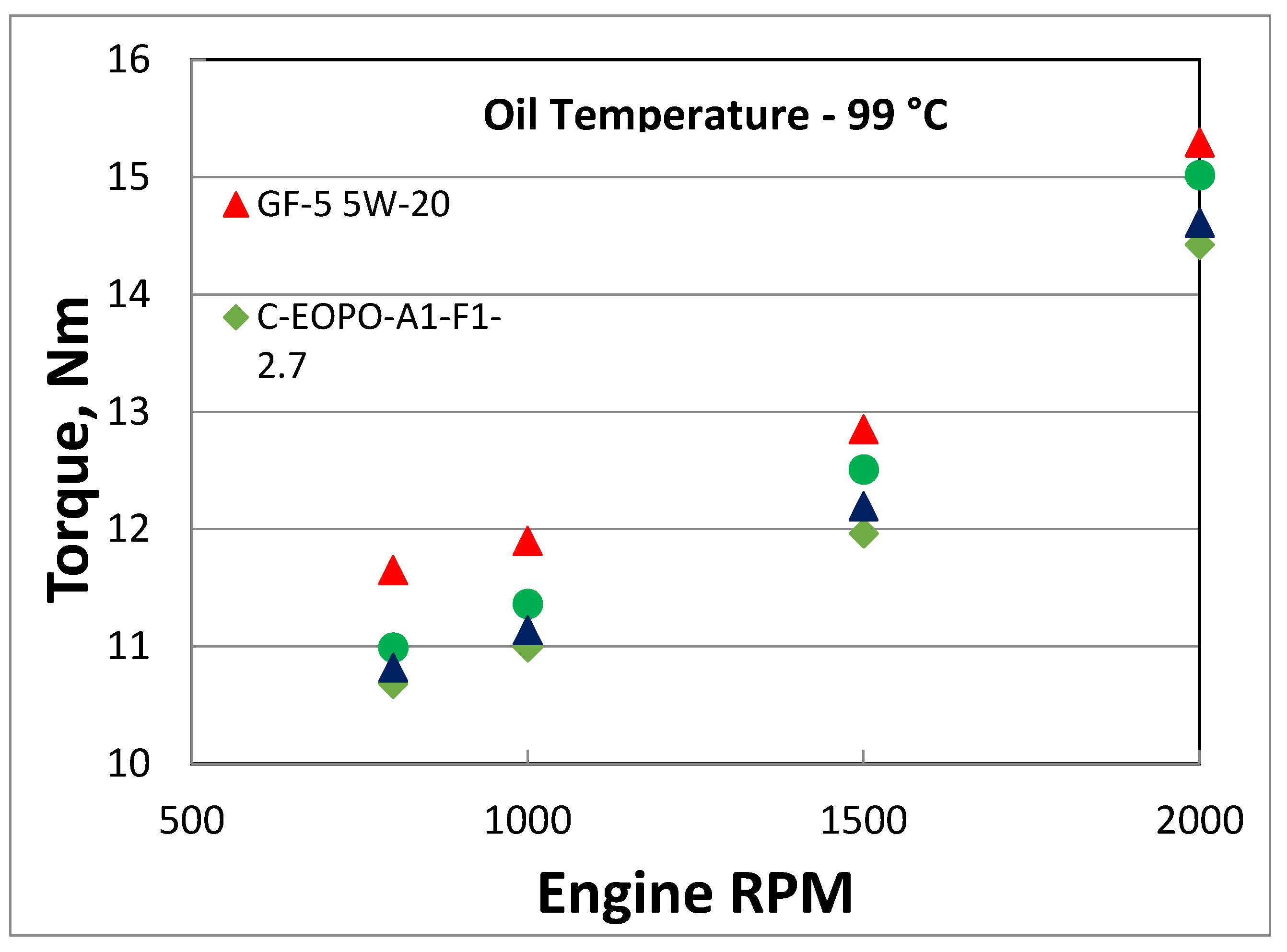
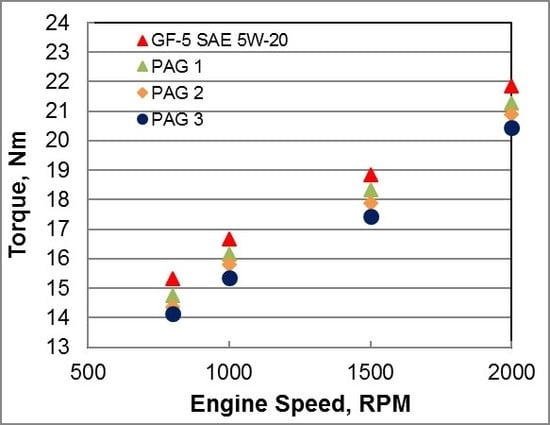
An in-line four-cylinder engine with direct acting mechanical bucket tappet type valvetrain architecture was used for friction evaluation. Prior to friction measurements the engine was broken-in. Friction data was collected from 800 to 5000 RPM engine speed and oil was supplied to the engine using an external sump. At each oil temperature, three tests were run and the average of the three tests is reported here. Tests were conducted at engine oil temperatures at 39, 79, and 99 °C. All three PAG oils showed lower friction torque than GF-5 5W-20 oil at 99 °C oil temperature as shown in
Figure 8. The friction improvement was higher at lower speeds near mixed lubrication regime where asperity interactions exists. The range of friction improvement was in the range of 0.4% to 8% depending on PAG oil and engine speed.
3.5. Chassis Roll Fuel Economy Evaluation
Chassis roll fuel economy was measured in a vehicle with an in-line four-cylinder engine and manual transmission under metro-highway cycles. A robot driver was used to reduce test to test variation.
Figure 9 shows fuel economy data within 95% confidence interval and PAG oil C-EOPO-A1-F1-2.7 showed about 1% improvement over GF-5 5W-20 oil. The improvement could be related to lower oil temperature during these test cycles. The low oil temperature pushes critical tribological contacts in the engine to operate in more mixed to hydrodynamic lubrication condition where lower viscosity of PAG oil reduces frictional losses. Adsorption of PAG molecules may also contribute to lower frictional losses because under transient conditions, the contacts (cam and tappets, piston rings and liner, etc.) experience boundary lubrication regimes. Also, PAG oil C-EOPO-A1-F1-2.7 has lower viscosity at lower temperatures (see
Figure 2) because of its higher VI index compared to GF-5 5W-20 oil.
The results presented above clearly demonstrated that PAG oils can offer lower friction under mixed lubrication conditions and may be under boundary lubrication conditions as well. This translated into lower frictional losses in cam and tappet contacts in valvetrain, cranktrain, engine, and also in chassis roll evaluations.
4. Durability Considerations
Improving fuel economy is certainly one of the primary goals for next generation engine oils but engine oil needs to meet several other performance requirements i.e., wear, sludge and varnish formation, oxidation, etc. These are all evaluated by engine dynamometer tests for prolonged duration to ensure adequate performance. The performance of PAG oil was evaluated in these tests as described below.
4.1. Sequence IVA Valvetrain Test
This test is designed to represent extended idling conditions and low temperature wear protection capability. Oil temperatures are maintained at 50 and 60 °C and are reflective of typical taxi cab driving conditions [
21]. The test duration is 100 h and is a combination of two drive cycles that are repeated 100 times: the first consists of running the engine at 800 RPM at 50 °C for 50 min followed by the second at 1500 RPM at 60 °C for 10 min. The cam nose wear is reported at the end of test. PAG oil UC-PO-A1-F2-2.6 was evaluated. The average camshaft wear was reported as 1037.36 μm, which was well in excess of the 90 μm maximum allowed in the GF-5 specification. The wear was equally severe on both intake and exhaust camshaft lobes. Oil samples taken every 25 h showed zinc and molybdenum concentrations were <50% of their initial values, which corresponds with increasing levels of iron found in the fluid. This indicates that the anti-wear additives were depleted or reduced below an effective concentration at ~50 h, leading to increased wear of the camshaft.
4.2. Sequence VG Test
Sequence VG was run to evaluate the capability of PAG oils in controlling deposit formation. This is a 216 h test designed to simulate moderate temperature taxi and delivery services. The test is run in three stages; with Stage 1 running at 1200 RPM for 120 min at 68 °C oil temperature, Stage 2 running at 2900 RPM at 100 °C oil temperature for 75 min, and Stage 3 running at 700 RPM at 45 °C oil temperature for 45 min. At the end of test, sludge deposits on rocker arm covers, cam baffles, timing chain cover, oil pan baffle, oil pan, and valve decks are rated. Varnish deposits are also rated on piston skirts (thrust) and cam baffles. The results are shown in
Table 2. PAG oil UC-PO-A1-F2-2.6 performed well on sludge but not varnish formation. None of the PAG oils studied contained a conventional overbased detergent. High TBN overbased detergents were insoluble in PAG fluids. A proprietary acid scavenger was used to neutralize acidic materials that formed in the fluid during use [
8,
9]. Bench tests and some engine test data suggested that the non-varnishing properties of PAG oils would be sufficient to ensure engine cleanliness [
22,
23].
4.3. Sequence IIIG Oxidation Test
The test measures engine oil oxidation (through viscosity increase), piston deposit, and valvetrain (cam and follower) wear. The engine is run at oil temperature of 150 °C, load of 94 kW at 3600 RPM. Once the engine is brought to the final steady state conditions, it is run for 100 h. Oil samples are collected at every 25 h to monitor viscosity increase. A Sequence IIIG test was not performed on any of the PAG-based oils evaluated in the Department of Energy study. However, a formulation using the same PAG base stocks as UC-PO-A1-F2-2.6 with a modified anti-oxidant package was evaluated as part of another study, results in
Table 3. The 33% increase in 40 °C viscosity of the oil was well below the 150% maximum allowed by the GF-5 specification. Oil consumption at 100 h was also relatively low at 1.1 L. Cam and lobe wear was excessive and piston deposits were unacceptable, though there were no hot stuck rings. The wear and deposit results from this test are in agreement with wear and deposit results from the Sequence IVA and VG suggesting that an overbased detergent and improved anti-wear package is needed.
5. Challenges
The various engine dyno tests run with PAG oils revealed following challenges.
Wear—extensive laboratory bench tests showed some of the PAG oils exhibited equal or better performance than GF-5 5W-20 oil under sliding conditions [
13]. However, in engine dyno tests, the wear level far exceeded the passing limit. Obviously, the bench test did not represent engine operating conditions. Among many differences between bench and engine test conditions—such as sample metallurgy, load, speed etc.—the effect of oil aging during engine test was not captured in bench tests. Therefore, efforts should be focused on this for bench test development. These tests also highlighted the need for a suitable anti-wear additive to be developed for PAG oil. A recent paper [
24] addressed this issue and developed an anti-wear additive with improved wear performance based on pin-on-vee tests. It remains to be seen how this additive will perform in engine dyno test.
Oxidation—anti-oxidant packages for PAG-based oils can be optimized to improve oxidation performance in engine dynamometer tests, as demonstrated in the Sequence IIIG test. The impact of anti-oxidants on other additives, such as anti-wear additives and deposits, should be included in any experimental design.
Sludge and varnish—the PAG-based fluids evaluated did not contain any dispersants or overbased detergents. An optimized anti-oxidant package will likely reduce varnish formation. Polyisobutylene succinimides (PIBSA) based dispersants are not compatible with PAG-based oils. Other dispersant chemistries, such as those based on epoxy amine alkoxylates [
25] or other chemistries, should be evaluated to improve sludge and soot dispersion.
Additive solubility/compatibility—the insolubility of overbased detergents in PAG-based oils remains a challenge in the formulation of these fluids. Overbased detergents provide alkalinity for the neutralization of acidic degradation and combustion compounds as well preventing the formation of deposits by suspending neutralized salts and other polar moieties in the oil. Control of acidic degradation compounds in PAG-based fluids can be accomplished by other means, such as the proprietary acid scavenger found in the fluids studied in this report. The acid scavenger, however, does little to prevent the formation of deposits, especially in the ring and land areas of pistons. It is not obvious that traditional detergents, designed to suspend polar compounds in relatively non-polar oils, are even appropriate for PAG-based oils.
Miscibilty with mineral oils—PAG oils are not fully miscible (about 80%) with mineral oil at room temperature resulting in formation of two layers; the PAG layer being heavier rests below the mineral oil layer. This could be an issue during oil change when an existing mineral oil-based formulation is replaced by PAG based formulation because existing oil cannot be fully drained even with filter change. The additive components in oil remaining in the engine could interact negatively with PAG formulation.
6. Alternate Solutions
An alternative solution could be the use of oil soluble PAG (OSP) in formulation, however, many of the same additive solubility issues would remain. The friction performance of OSP has been investigated, but not in detail. Friction results in motored valvetrain and motored engine tests were encouraging, but were inferior to those observed for other PAG chemistries. Further work is needed. Another potential solution is co-basing OSP with mineral oil. This strategy could potentially address many challenges including additive solubility, miscibility, and seal compatibility. Preliminary work [
23] suggests that conventional additive packages are compatible with Group III + OSP blends while improving the frictional performance over Group III only oils. Obviously, there is a need to define the optimum OSP concentration and optimize additive packages to take advantage of the potential benefits of OSP.
This investigation established that PAG based engine oils can offer encouraging fuel economy benefits as demonstrated by different evaluation methods. This investigation also identified significant challenges related to wear, oxidation, and varnish formation requiring further study to identify appropriate additive components or developing new ones.