Conventional and Highly Crosslinked Polyethylene in Total Knee Arthroplasty—A Design-Independent Wear Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Lubrication of Samples
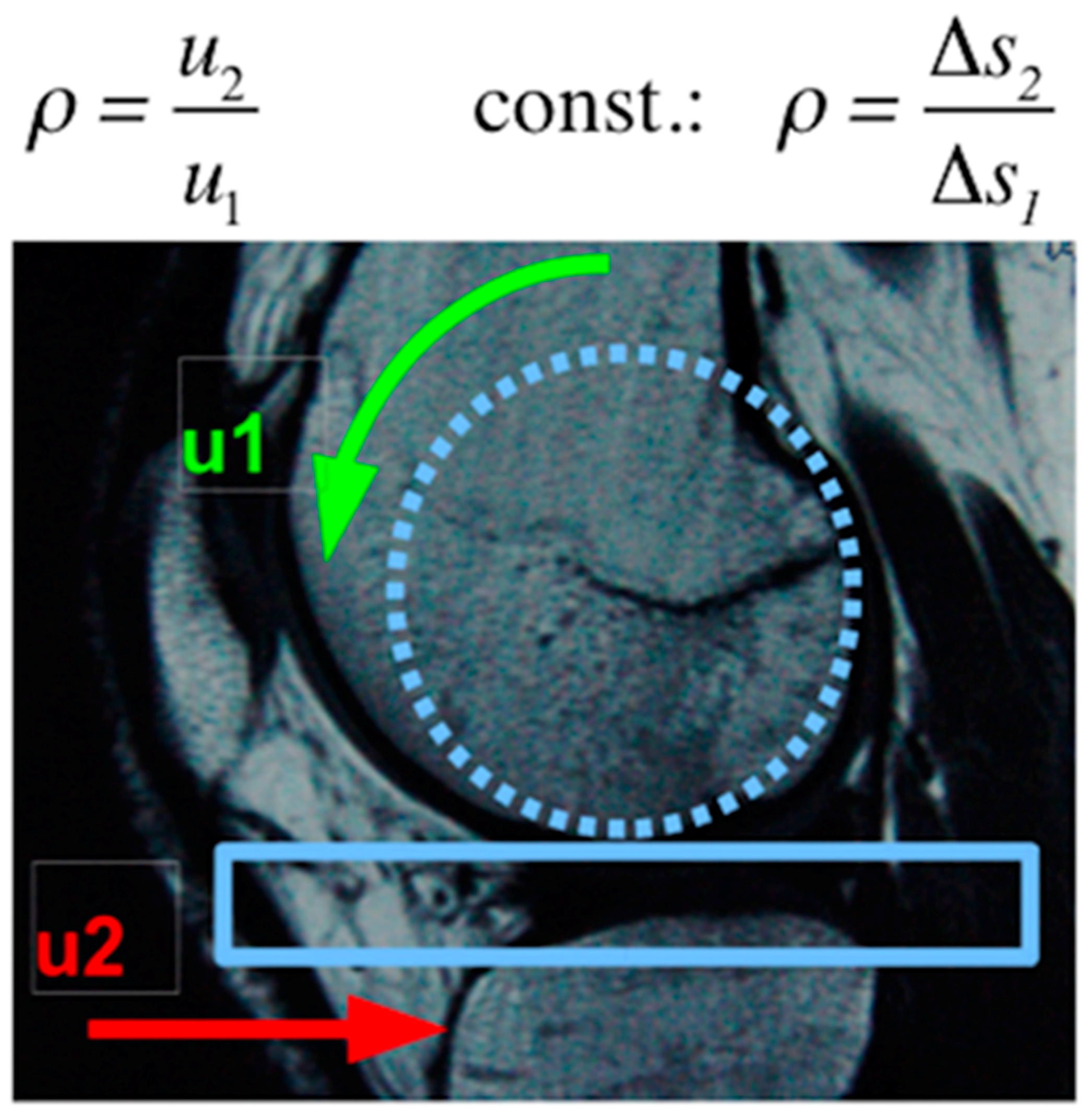
2.2.2. Simulator Station
2.2.3. Gravimetric Measurements
2.2.4. Duration of Testing
2.2.5. Statistical Analysis
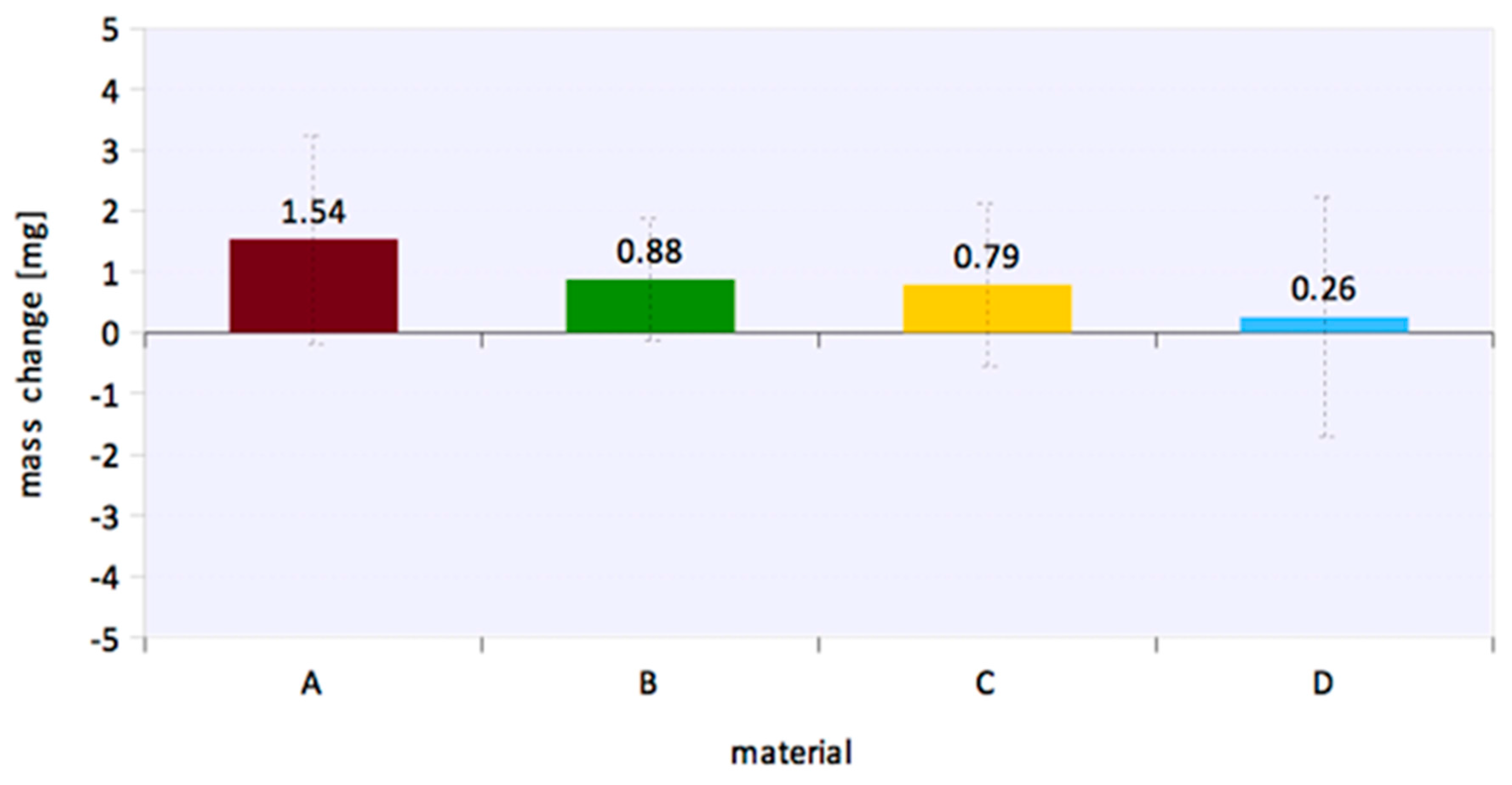
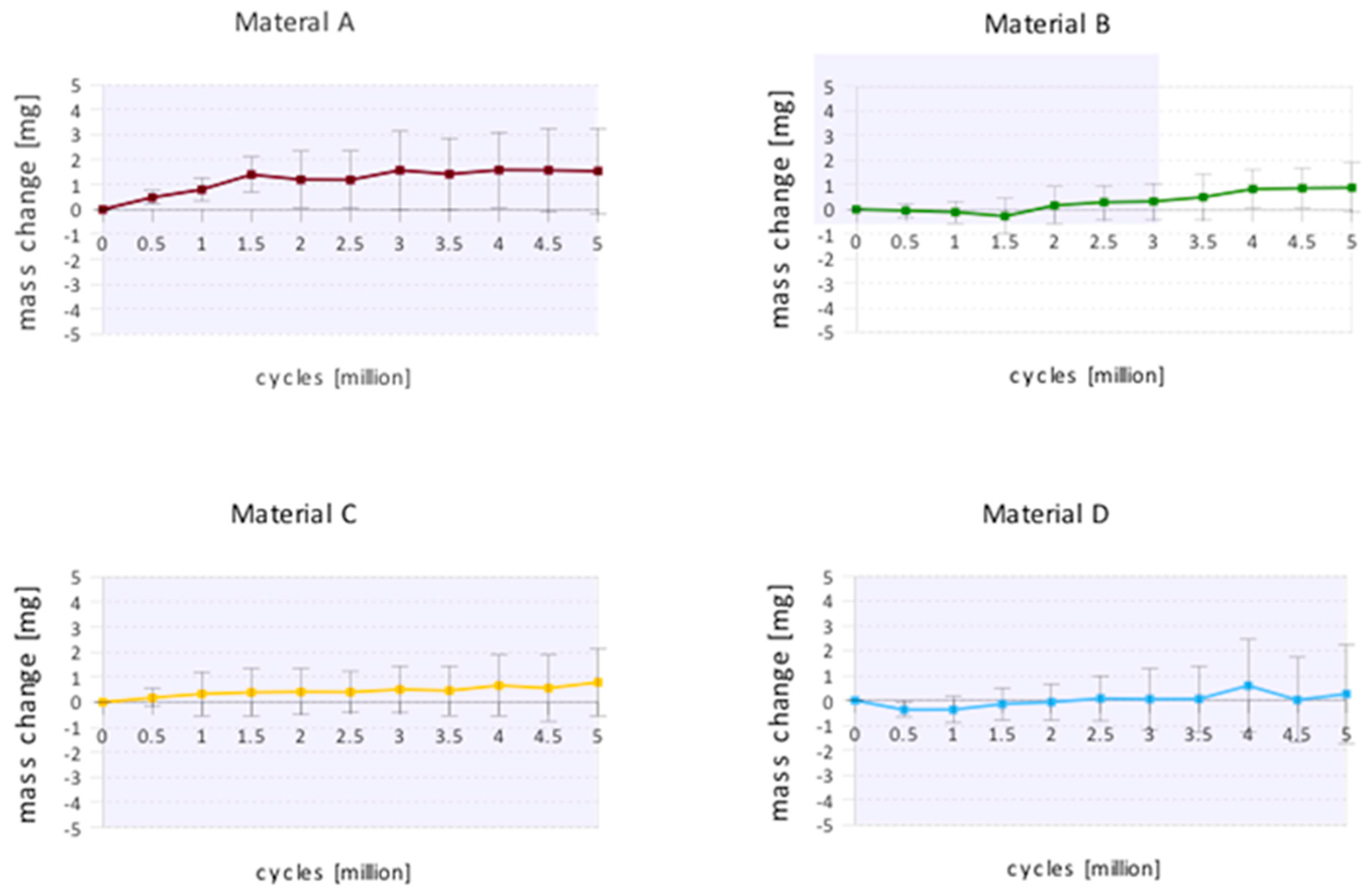
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Purdue, P.E.; Koulouvaris, P.; Potter, H.G.; Nestor, B.J.; Sculco, T.P. The cellular and molecular biology of periprosthetic osteolysis. Clin. Orthop. Relat. Res. 2007, 454, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.; Wright, J.; Coyte, P.; Paul, J.; Dittus, R.; Croxford, R.; Katz, B.; Bombardier, C.; Heck, D.; Freund, D. Health-related quality of life after knee replacement. J. Bone Joint Surg. Am. 1998, 80, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N. Total joint replacement in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2006, 20, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Engh, C.A., Jr.; Parks, N.L.; Engh, G.A. Polyethylene quality affects revision knee liner exchange survivorship. Clin. Orthop. Relat. Res. 2012, 470, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Atwood, S.A.; Van Citters, D.W.; Patten, E.W.; Furmanski, J.; Ries, M.D.; Pruitt, L.A. Tradeoffs amongst fatigue, wear, and oxidation resistance of cross-linked ultra-high molecular weight polyethylene. J. Mech. Behav. Biomed. Mater. 2011, 4, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Brach Del Prever, E.M.; Bistolfi, A.; Bracco, P.; Costa, L. UHMWPE for arthroplasty: Past or future? J. Orthop. Traumatol. 2009, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Gawel, H.A.; Patel, J.D. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin. Orthop. Relat. Res. 2011, 469, 2262–2277. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.C.; Van Citters, D.W.; Currier, J.H.; Collier, J.P. The effect of radiation dose on the tensile and impact toughness of highly cross-linked and remelted ultrahigh-molecular weight polyethylenes. J. Biomed. Mater. Res. B 2011, 97, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.I.; Ostermeier, S.; Turger, A.; Denkena, B.; Hurschler, C. A rolling-gliding wear simulator for the investigation of tribological material pairings for application in total knee arthroplasty. Biomed. Eng. Online 2010, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). ISO 14243-1:2009: Implants for Surgery—Wear of Total Knee-Joint Prostheses—Part 1: Loading and Displacement Parameters for Wear-Testing Machines with Load Control and Corresponding Environmental Conditions for Test; ISO 14243-1:2009; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Nagerl, H.; Walters, J.; Frosch, K.H.; Dumont, C.; Kubein-Meesenburg, D.; Fanghanel, J.; Wachowski, M.M. Knee motion analysis of the non-loaded and loaded knee: A re-look at rolling and sliding. J. Physiol. Pharmacol. 2009, 60, 69–72. [Google Scholar] [PubMed]
- Schwenke, T.; Borgstede, L.L.; Schneider, E.; Andriacchi, T.P.; Wimmer, M.A. The influence of slip velocity on wear of total knee arthroplasty. Wear 2005, 259, 926–932. [Google Scholar] [CrossRef]
- International ASTMA. ASTM F732-00 Standard Test Method for Wear Testing of Polymeric Materials Used in Total Joint Prostheses; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Haider, H. Tribological Assessment of UHMWPE in the knee. In The UHMWPE Biomaterials Handbook: Ultra High Weight Polyethylene in Total Joint Replacement and Medical Devices; Kurtz, S., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 381–408. [Google Scholar]
- Van Citters, D.W.; Kennedy, F.E.; Collier, J.P. Rolling slidingwear of UHMWPE for knee bearing applications. Wear 2007, 263, 1087–1094. [Google Scholar] [CrossRef]
- Schwiesau, J.; Schilling, C.; Utzschneider, S.; Jansson, V.; Fritz, B.; Blomer, W.; Grupp, T.M. Knee wear simulation under conditions of highly demanding daily activities—influence on an unicompartmental fixed bearing knee design. Med. Eng. Phys. 2013, 35, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, S.; Harrasser, N.; Schroeder, C.; Mazoochian, F.; Jansson, V. Wear of contemporary total knee replacements—A knee simulator study of six current designs. Clin. Biomech. 2009, 24, 583–588. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). ISO 14243-2:2009: Implants for Surgery—Wear of Total Knee-Joint Prostheses—Part 2: Methods of Measurement; ISO 14243-2:2009; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Sobieraj, M.C.; Rimnac, C.M. Ultra high molecular weight polyethylene: Mechanics, morphology, and clinical behavior. J. Mech. Behav. Biomed. Mater. 2009, 2, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Hermida, J.C.; Fischler, A.; Colwell, C.W., Jr.; D’Lima, D.D. The effect of oxidative aging on the wear performance of highly crosslinked polyethylene knee inserts under conditions of severe malalignment. J. Orthop. Res. 2008, 26, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, J.H.; D’Antonio, J.A.; Manley, M.T.; Capello, W.N.; Wang, A. The basis for a second-generation highly cross-linked UHMWPE. Clin. Orthop. Relat. Res. 2006, 453, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Goebel, P.; Zietz, C.; Bieck, R.; Kluess, D.; Bader, R. A novel method for tribological evaluation of bearing materials in total knee replacements. Biomed. Tech. Biomed. Eng. 2012, 57. [Google Scholar] [CrossRef]
- Muratoglu, O.K.; Bragdon, C.R.; Jasty, M.; O’Connor, D.O.; Von Knoch, R.S.; Harris, W.H. Knee-simulator testing of conventional and cross-linked polyethylene tibial inserts. J. Arthroplast. 2004, 19, 887–897. [Google Scholar] [CrossRef]
- Dennis, D.A.; Komistek, R.D.; Walker, S.A.; Cheal, E.J.; Stiehl, J.B. Femoral condylar lift-off in vivo in total knee arthroplasty. J. Bone Joint Surg. Br. 2001, 83, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, K.; Unsworth, A. Is the wear factor in total joint replacements dependent on the nominal contact stress in ultra-high molecular weight polyethylene contacts? Proc. Inst. Mech. Eng. H 2004, 218, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, T.; Kaddick, C.; Schneider, E.; Wimmer, M.A.; International standards organization. Fluid composition impacts standardized testing protocols in ultrahigh molecular weight polyethylene knee wear testing. Proc. Inst. Mech. Eng. H 2005, 219, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Delport, H.P.; Sloten, J.V.; Bellemans, J. Comparative gravimetric wear analysis in mobile versus fixed-bearing posterior stabilized total knee prostheses. Acta Orthop. Belg. 2010, 76, 367–373. [Google Scholar] [PubMed]
- Scholes, S.C.; Unsworth, A. The effects of proteins on the friction and lubrication of artificial joints. Proc. Inst. Mech. Eng. H 2006, 220, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Tipper, J.L.; Ingham, E.; Stone, M.H.; Fisher, J. The influence of phospholipid concentration in protein-containing lubricants on the wear of ultra-high molecular weight polyethylene in artificial hip joints. Proc. Inst. Mech. Eng. H 2001, 215, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.M.; Charron, K.D.; Zhao, L.; MacDonald, S.J.; Medley, J.B. Commissioning of a displacement-controlled knee wear simulator and exploration of some issues related to the lubricant. Proc. Inst. Mech. Eng. H 2011, 225, 736–752. [Google Scholar] [CrossRef] [PubMed]
- Bruck, A.L.; Karuppiah, K.S.; Sundararajan, S.; Wang, J.; Lin, Z. Friction and wear behavior of ultrahigh molecular weight polyethylene as a function of crystallinity in the presence of the phospholipid dipalmitoyl phosphatidylcholine. J. Biomed. Mater. Res. B 2010, 93, 351–358. [Google Scholar] [CrossRef] [PubMed]

| Material | A | B | C | D |
|---|---|---|---|---|
| Aesculap—Experimental | Zimmer Prolong™ | Zimmer Durasul™ | Stryker X3™ | |
| Type of Polyethylene | Conventional | Highly Crosslinked | Highly Crosslinked | Highly Crosslinked |
| Production Resin | GUR 1020 | GUR 1050 | GUR 1050 | GUR 1020 |
| Irradiation Method | - | β- (Electron Beam-) Irradiation 65 kGy | β- (Electron Beam-) Irradiation 95 kGy | γ -Irradiation Sequential Irradiation 3 × 30 kGy |
| Accumulated Irradiation Dose | 25–40 kGy | 65 kGy | 95 kGy | 90 kGy |
| Post Irradiation Aftertreatment | No | Remelting | Remelting | Sequential Annealing (3× Annealed—one following each radiation step) |
| Sterilization Method | β- (Electron Beam-) Irradiation 25–40 kGy in nitrogen atmosphere | Gasplasma | Ethylenoxide (EtO) | Gasplasma |
| Knee Simulator | Rolling–sliding Testing Rig |
|---|---|
| Femoral condyles | Cylinder, CoCr-Alloy, diameter: 40 mm (width: 50 mm) |
| Tibial insert | Plane polymer sample |
| Knee Simulator | Rolling–sliding Test Station |
|---|---|
| ISO 14243-1 movement profile | Rolling–sliding, cyclic sinus shaped anterior-posterior-movement at a constant rolling–sliding ratio of ρ = 1:2 |
| Frequency 1 Hz | Frequency 2 Hz |
| ISO 14243-1 load profile | Constant vertical load of 2.5 kN |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulus, A.C.; Woicinzski, M.; Jansson, V.; Utzschneider, S. Conventional and Highly Crosslinked Polyethylene in Total Knee Arthroplasty—A Design-Independent Wear Investigation. Lubricants 2017, 5, 25. https://doi.org/10.3390/lubricants5030025
Paulus AC, Woicinzski M, Jansson V, Utzschneider S. Conventional and Highly Crosslinked Polyethylene in Total Knee Arthroplasty—A Design-Independent Wear Investigation. Lubricants. 2017; 5(3):25. https://doi.org/10.3390/lubricants5030025
Chicago/Turabian StylePaulus, Alexander C., Matthias Woicinzski, Volkmar Jansson, and Sandra Utzschneider. 2017. "Conventional and Highly Crosslinked Polyethylene in Total Knee Arthroplasty—A Design-Independent Wear Investigation" Lubricants 5, no. 3: 25. https://doi.org/10.3390/lubricants5030025
APA StylePaulus, A. C., Woicinzski, M., Jansson, V., & Utzschneider, S. (2017). Conventional and Highly Crosslinked Polyethylene in Total Knee Arthroplasty—A Design-Independent Wear Investigation. Lubricants, 5(3), 25. https://doi.org/10.3390/lubricants5030025





