1. Introduction
Molecular dynamics (MD) simulations have been performed comprehensively by Rigney and coworkers to show structure formation at the sliding interface of pure crystalline materials [
1,
2,
3,
4]. Although only a two-dimensional (2D) model was applied, the following features were visualized on the atomic scale: (i) mixing of the two first body materials; (ii) evolution of defect potential energy in the vicinity of the interface and (iii) formation of nanocrystalline structures [
1]. Furthermore, it was observed that a highly disordered layer developed in the softer material of the sliding couple and it was suggested that this layer may behave like a liquid [
1]. For a Fe–Cu couple, the whole shear deformation occurring during sliding was concentrated within a superficial amorphous layer at the copper surface [
2]. The width of the amorphous layer increased with the increasing simulation time [
3]. Similar results were obtained for a Fe–Al couple [
4]. A three-dimensional (3D) MD simulation of a nanometric scratch test comprising a diamond tool and a copper single crystal was performed by Zhu et al. [
5]. Again, an amorphous layer was observed at the surface and within the formed chip of the softer material, i.e., copper. Whereas the recrystallization of amorphous copper was observed in [
4], this was not the case in [
5], most likely because a thermostat was incorporated in the model structure.
Morita et al. presented an extended MD model, taking into account possible chemical reactions between different atomic species [
6]. With this model they could show that an amorphous MoS
2 tribolayer bound by iron substrates on both sides may crystallize with alignment of its basal planes parallel to the sliding interface, thus explaining the ultra-low friction of this system. Experimental evidence for this mechanism was provided, e.g., by Theiler et al. [
7].
There are other tribofilms with amorphous structure also providing excellent friction and wear properties, but without evidence of crystallization. Numerous papers report on amorphous carbon coatings which may provide either graphite-like (GLC) or diamond-like (DLC) behavior. Although the beneficial effects on friction and wear reduction are beyond dispute, the reasons are not so obvious. Pastewka et al. performed MD simulations on hydrogenated amorphous carbon (a-C:H) [
8]. They observed two effects which might be responsible for the observed friction reduction. Firstly, there was a local transformation of sp3 to sp2 bonding states at the sliding interface. Furthermore, the passivation of dangling bonds seems to play an important role [
8]. The importance of the local sp3–sp2 transition and the corresponding formation of a soft amorphous layer were corroborated by a further study [
9]. Furthermore, it was demonstrated that the polishing of a diamond crystal with a suspension of diamond particles depends on the formation of an amorphous layer and local sp3–sp2 transition [
10].
A further type of beneficial amorphous tribofilm is formed from the anti-wear oil additive zink dialkyl dithiophosphate (ZDDP). MD sliding simulations of such a film supported by crystalline iron substrates on both sides were performed by Martin et al. [
11]. The study demonstrates that not only the polyphosphate retains its amorphous structure, but it also has the capability to “digest” abrasive iron oxide nanoparticles during the sliding simulation. Furthermore, the incorporation of iron atoms into the zinc polyphosphate glass alters the shear distribution within the tribofilm considerably [
11].
Our own interest in amorphous tribofilms originates from experiences made with hybrid polymer-based nanocomposites which were developed for anti-friction and anti-wear applications [
12,
13]. It has been shown that only if the composites contain both micron-sized carbon fibers and a certain amount of silica nanoparticles, the desired low friction and wear properties are preserved during severe stressing conditions. This was attributed to the formation of a silica-based tribofilm which also was proved experimentally. A prerequisite of silica release from the composite matrix, the epoxy resin, is a local temperature increase (flash temperature) at the primary contact sites, the carbon fibers. A recently developed method of flash temperature assessment yielded a temperature of at least 860 °C for a similar material [
14]. From this we conclude that a temperature of approximately 1100 K has to be taken into account, not only for explaining the degradation of the polymer and the release of silica nanoparticles, but also as the temperature under which sliding takes place. This approach is different compared to all previous MD sliding simulations which were performed at an initial temperature of 273 K.
Österle et al. [
13] explained the anti-friction behavior of the silica-based tribofilm with a nanoscopic model based on movable cellular automata (MCA). Actually, smooth sliding and a rather low coefficient of friction (COF) of 0.2 were predicted if the silica nanoparticles constituting the tribofilm were mixed with either 10% graphite or 20% epoxy (volume fractions). The very low COF of 0.06 observed under the most severe stressing conditions, i.e., if the product of pressure and sliding velocity was equal to 24 MPa·m/s, could not be explained by MCA modeling [
13]. Since both amorphous carbon and the silica-based film were observed in the wear scar, their sliding behavior was the focus of interest. Thus, the objective of the present study was to find out whether MD modeling can provide useful information for obtaining a better understanding of the exceptional tribological properties of the considered hybrid nanocomposite under the most severe stressing conditions. Since the latter lead to high flash temperatures, it was necessary to perform the simulations at elevated temperature.
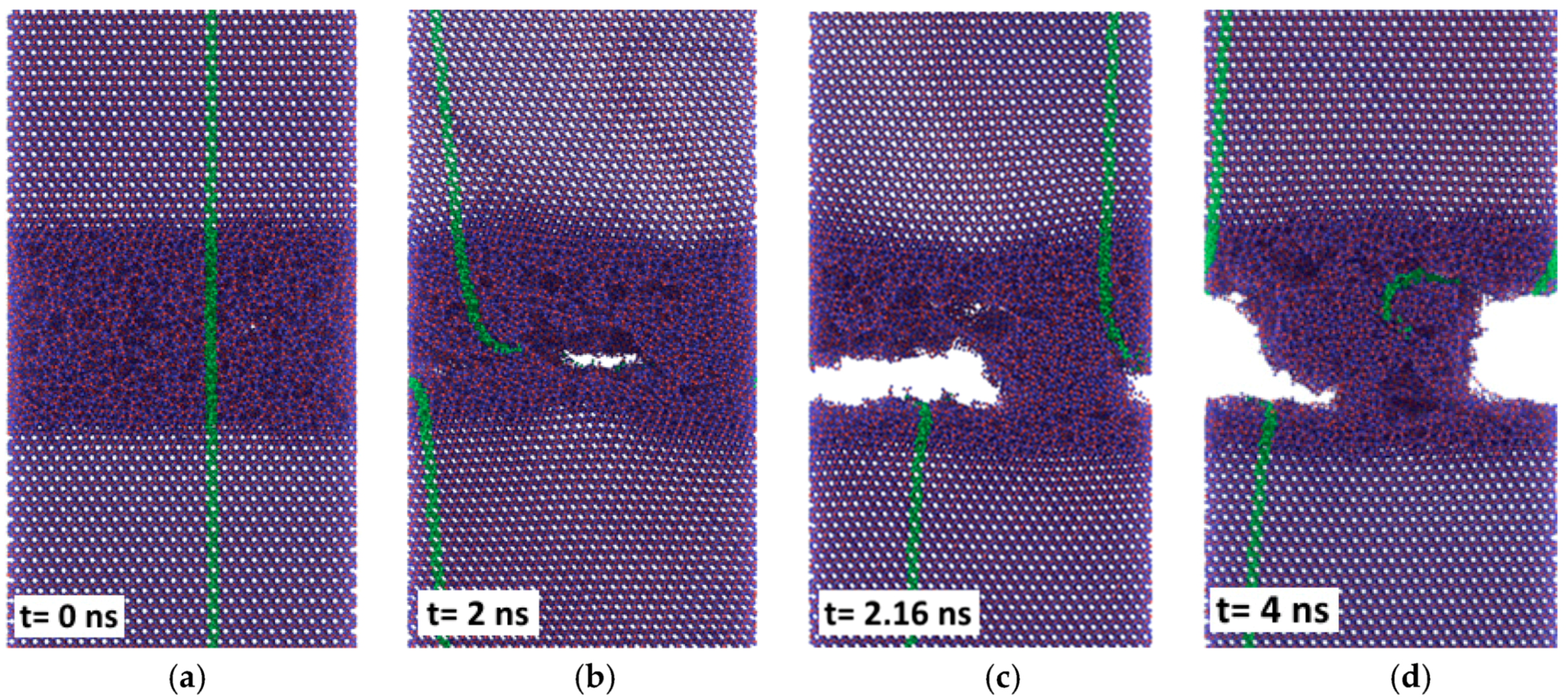
3. Discussion
In our previous experimental work, the beneficial role of silica nanoparticles in a hybrid polymer matrix composite was attributed to the formation of a silica-based tribofilm [
12,
13]. The low friction observed if such a film had formed was attributed to the mixing of the silica with a soft constituent such as graphite or epoxy particles [
13]. An additional experiment performed with pure silica nanoparticles did not provide low friction during sliding at an ambient temperature of approximately 300 K [
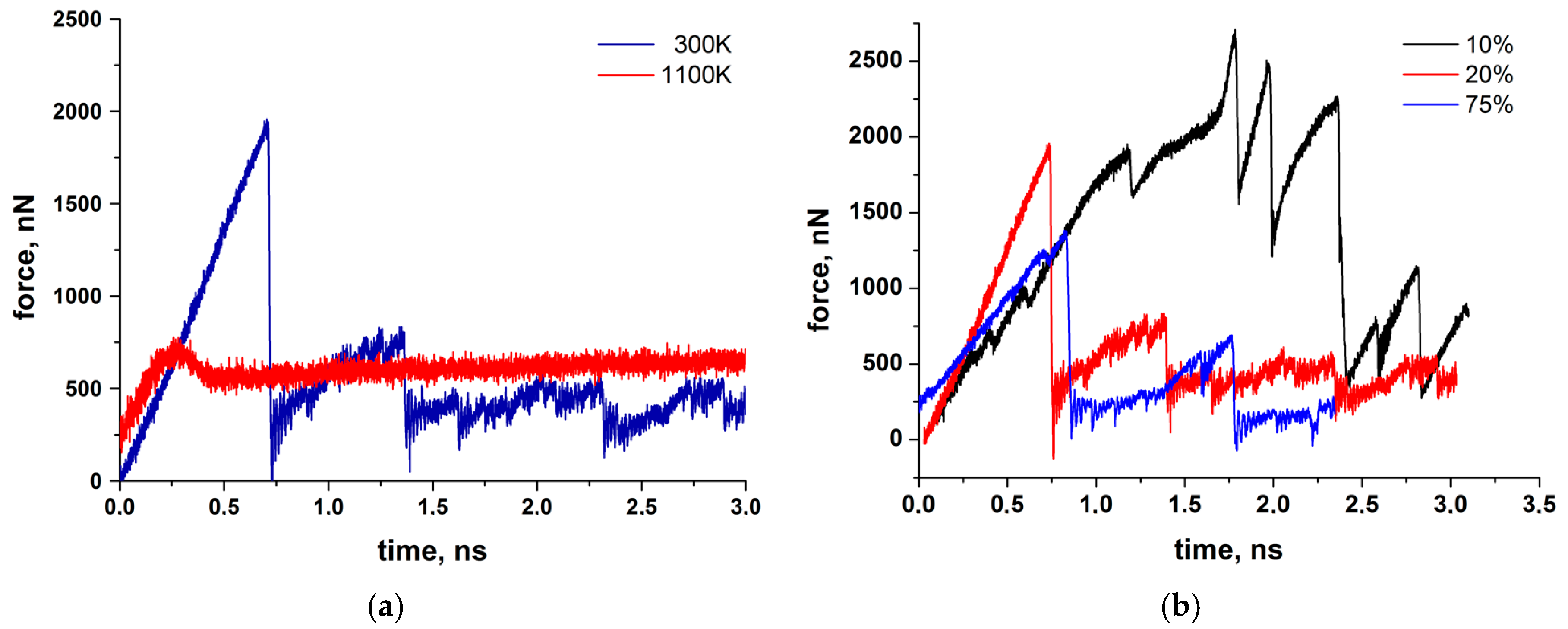
15]. This coincides in principle with results of the MD simulations (
Figure 7), showing unstable and initially high friction forces at 300 K. Also shown in
Figure 7, performing the simulation at 1100 K (flash temperature) yields much lower reaction forces and smooth sliding behavior. From this we can conclude that the reduction of friction observed experimentally for the silica-based tribofilm at high values of the product of pressure and sliding velocity [
13] may be due to flash temperatures occurring at the carbon fiber contact sites of the composite. Whereas the simulation shown in
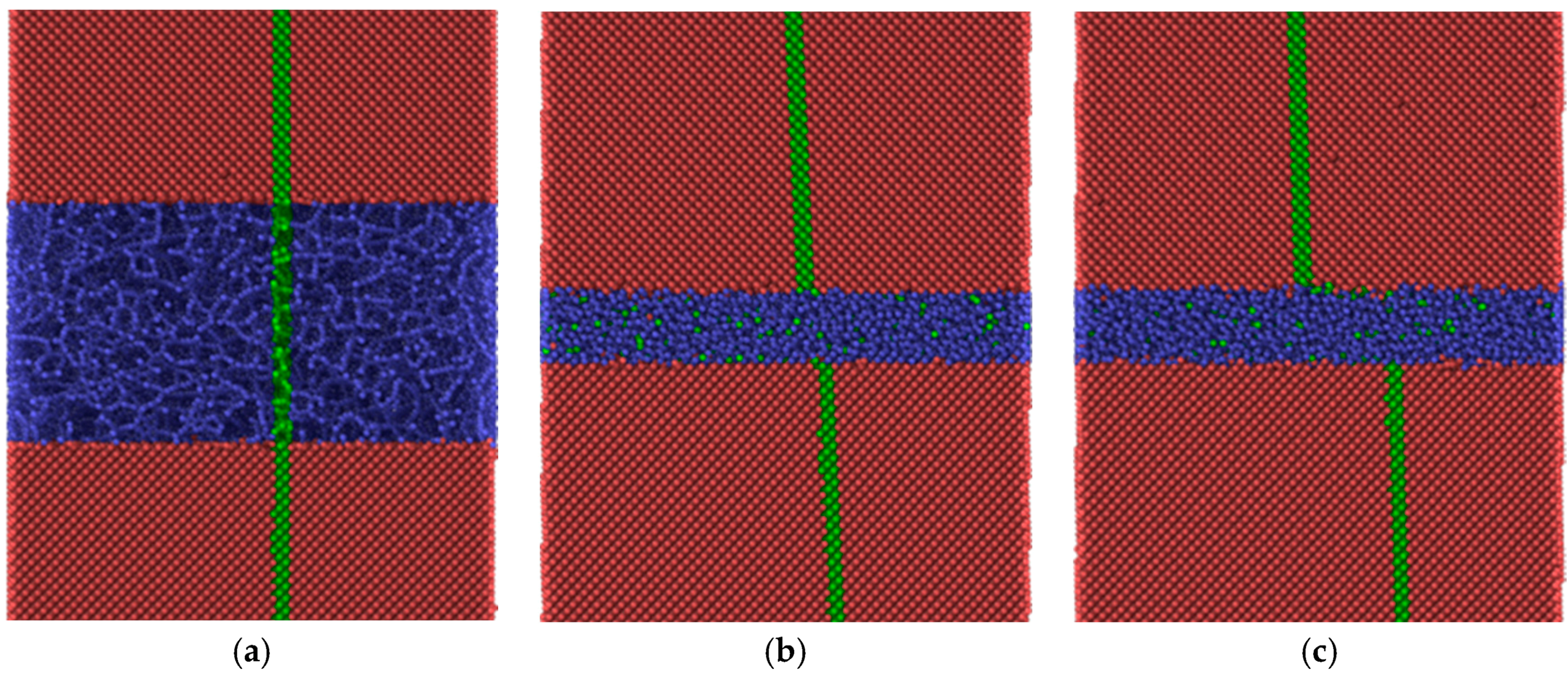
Figure 7a was performed without external pressure, a more realistic simulation result with external pressure is presented in
Figure 11a at 300 K and
Figure 11b at 1100 K.
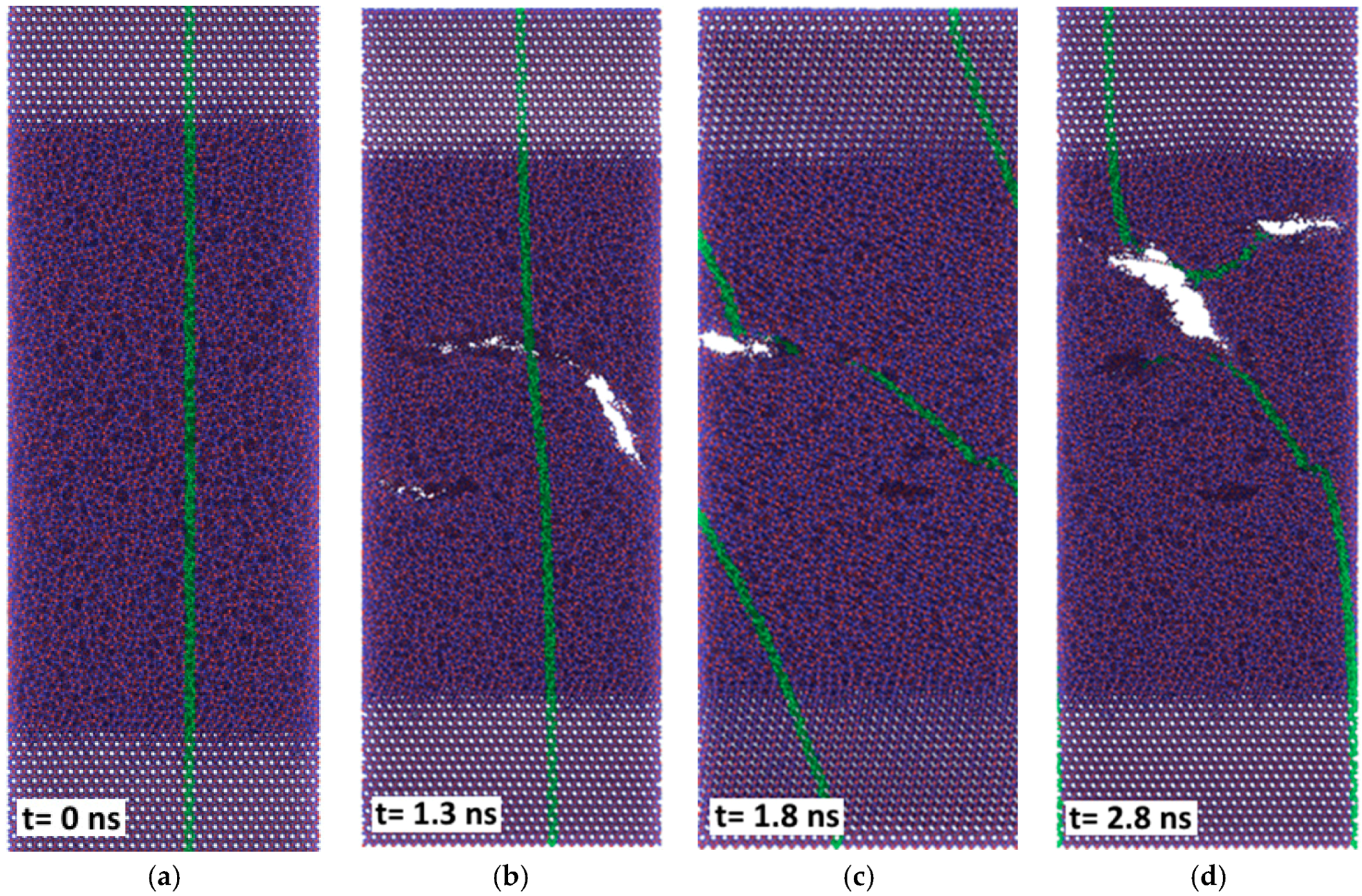
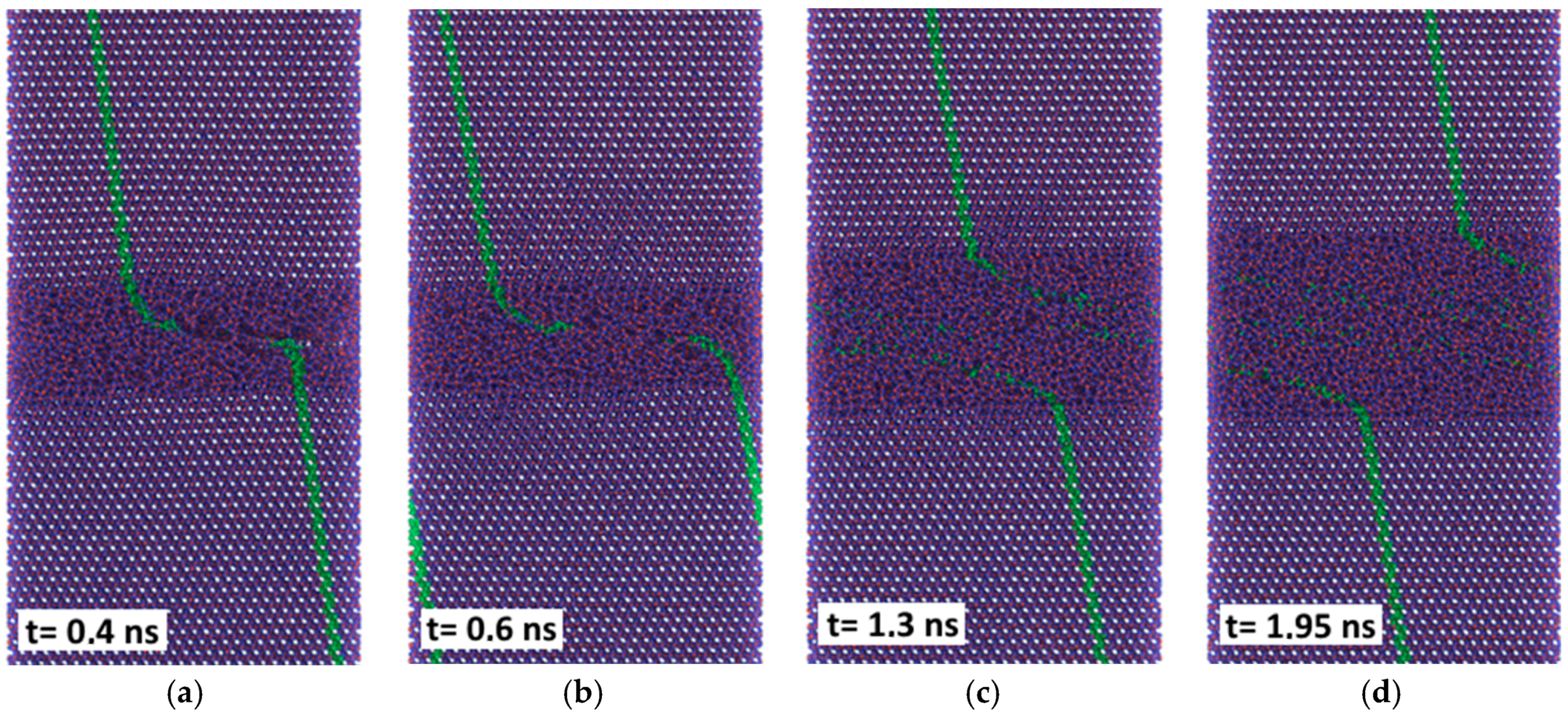
With applied pressure the friction forces of the amorphous silica layer at 300 K are reduced considerably (compare
Figure 11a and
Figure 7), although the sliding remains unstable. The stick-slip type of movement correlates with the formation of the nano-sized rollers which either are linked to the substrates (sticking phase) or can roll along the interface (slipping phase). Thus, under certain conditions, pure silica films may also provide low friction at room temperature.
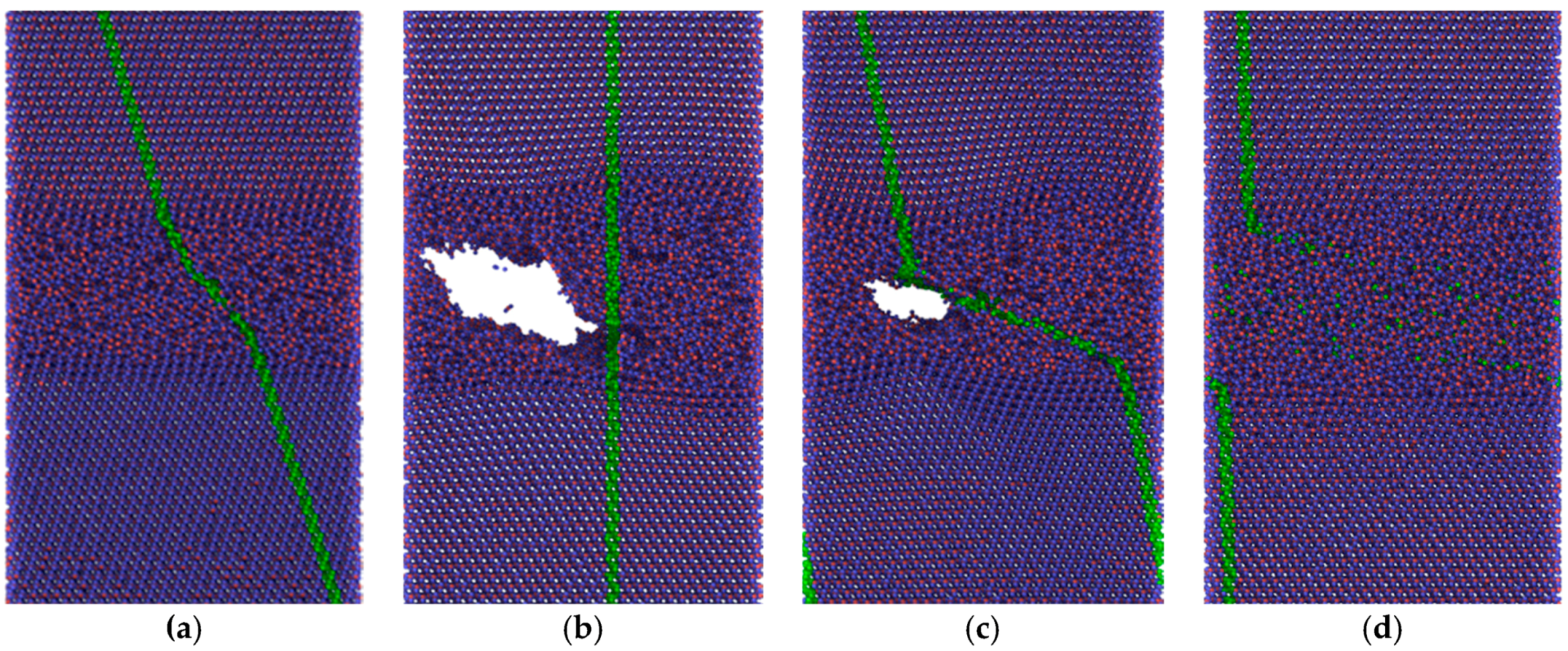
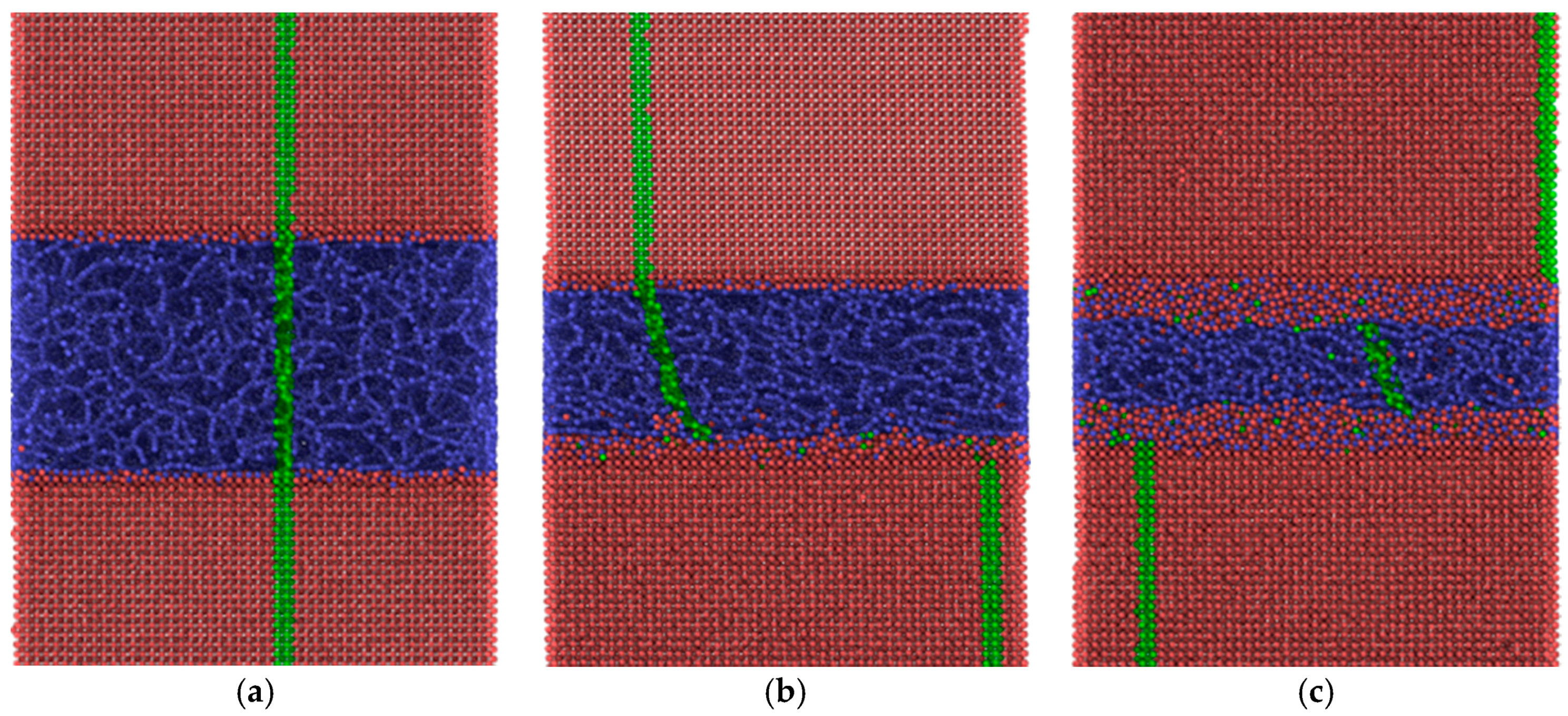
The friction forces obtained by MD simulation for the amorphous carbon film differed considerably with the chosen substrate materials. Strong bonds between carbon atoms both within the amorphous layer and with the diamond substrate are causing high resistance forces, as shown by the red curves in
Figure 11. According to Kunze et al. [
9], low friction of an amorphous carbon layer can only be expected if dangling bonds are passivated by ambient gases. Since such reactions were not considered in the present simulations, the friction forces remained high. The situation was completely different if α-Fe was chosen as the substrate material. Obviously only weak bonds exist between carbon and iron atoms. Thus, carbon atoms with broken bonds to their neighbors can move into the Fe substrate which undergoes amorphization within a certain layer, especially at the higher temperature (
Figure 10c). Such behavior was also observed for other systems at ambient temperature featuring both crystalline [
1,
2,
3,
4,
5] and amorphous [
11] materials.
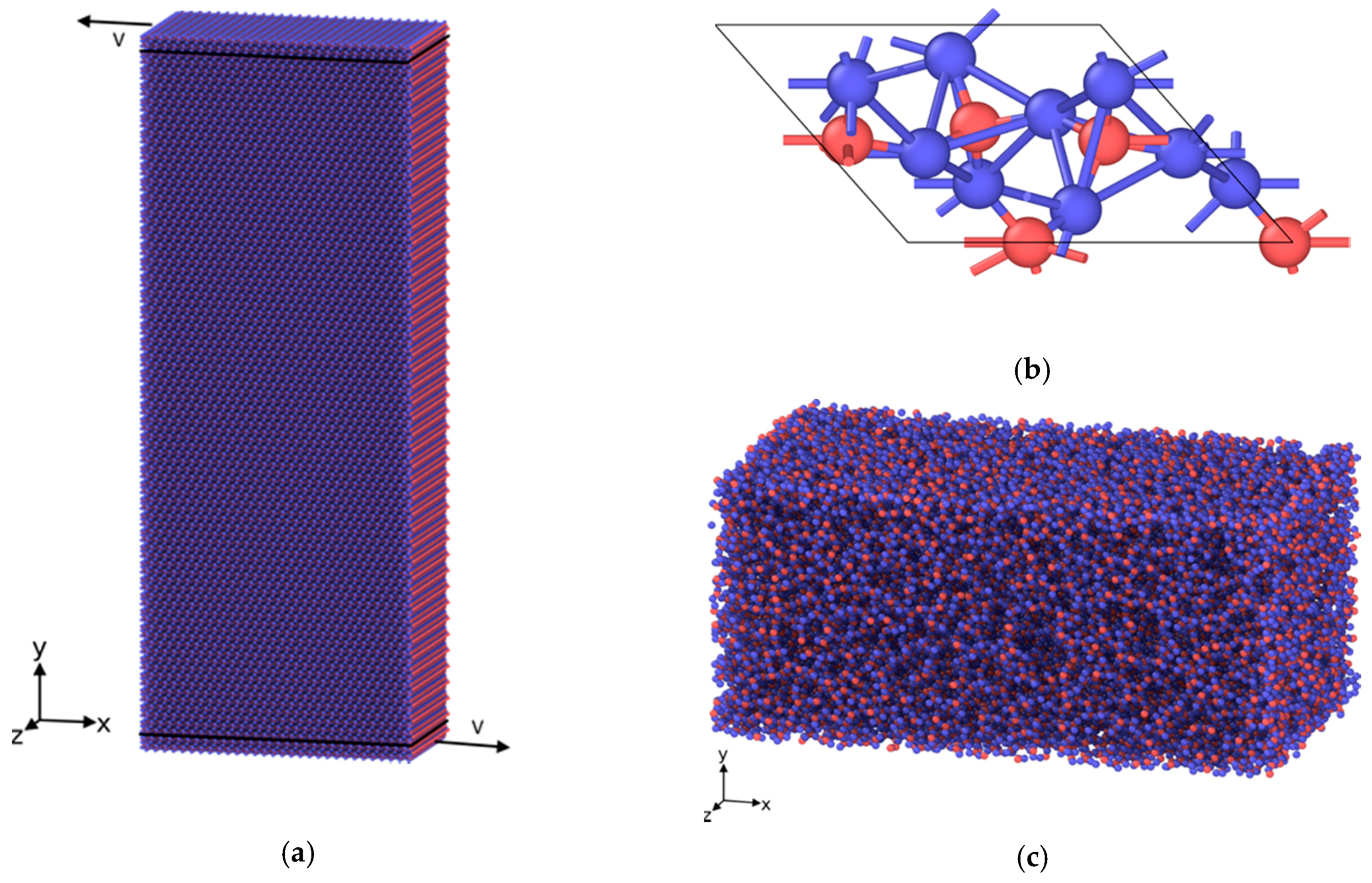
4. Materials and Methods
As briefly sketched in
Section 2.1, the material systems considered within this study were modeled by a bottom-up approach using atomic potentials found in the literature. The calculations were performed on a supercomputer resource of Tomsk State University using free access software package LAMMPS [
16]. LAMMPS is a classical molecular dynamics code from Sandia National Laboratory, and an acronym for Large-scale Atomic/Molecular Massively Parallel Simulator. It integrates Newton’s equations of motion for an ensemble of atoms, or molecules that interact via short- or long-range forces with a variety of initial and boundary conditions. Interatomic interaction in case of iron and diamond crystallites with carbon interlayers was described by the modified embedded atom method (MEAM) [
17]. The results were compared with experimental data as well as with ab-initio calculations, such as: Structural and elastic properties of cementite, energy of interstitial C atoms in Fe lattice, and the heat of Fe–C alloy formation in L12 and B1 structures. Furthermore, the properties of pure materials within this MEAM potential (C and Fe) were also tested. To study the behavior of silica layers the three-body interatomic interaction suggested by Tersoff [
18] was used.
Modified Embedded-Atom Method (MEAM) potentials [
18] are an extension to the original Embedded-Atom Method (EAM) potentials by adding angular forces. It is thus suitable for modeling metals and alloys with fcc, bcc, hcp and diamond cubic structures, as well as covalently bonded materials such as silica and carbon. In the MEAM formulation, the total energy
E of a system of atoms is given by:
where
F is the embedding energy which is a function of the atomic electron density ρ, and ϕ is a pair potential interaction. The pair interaction is summed over all neighbors
j of atom
i within the cutoff distance. As with EAM, the multi-body nature of the MEAM potential is a result of the embedding energy term.
The Tersoff approach computes a three-body Tersoff potential for the energy
E of a system of atoms as:
In Equation (3):
where
fR is a two-body term and
fA includes three-body interactions.
R and
D are outer and inner cutoff radii of the order of 2.5 and 0.15 nm, respectively. The values change a bit according to the nature of the three atoms under consideration, as compiled in a table of the original paper [
18]. The summations in the formula are over all neighbors
j and
k of atom
i within a cutoff distance =
R +
D. This empirical interatomic potential permits the calculation of structural properties and energetics of complex systems. A new approach for constructing such potentials, by explicitly incorporating the dependence of bond order on local environment, permits an improved description of covalent materials.
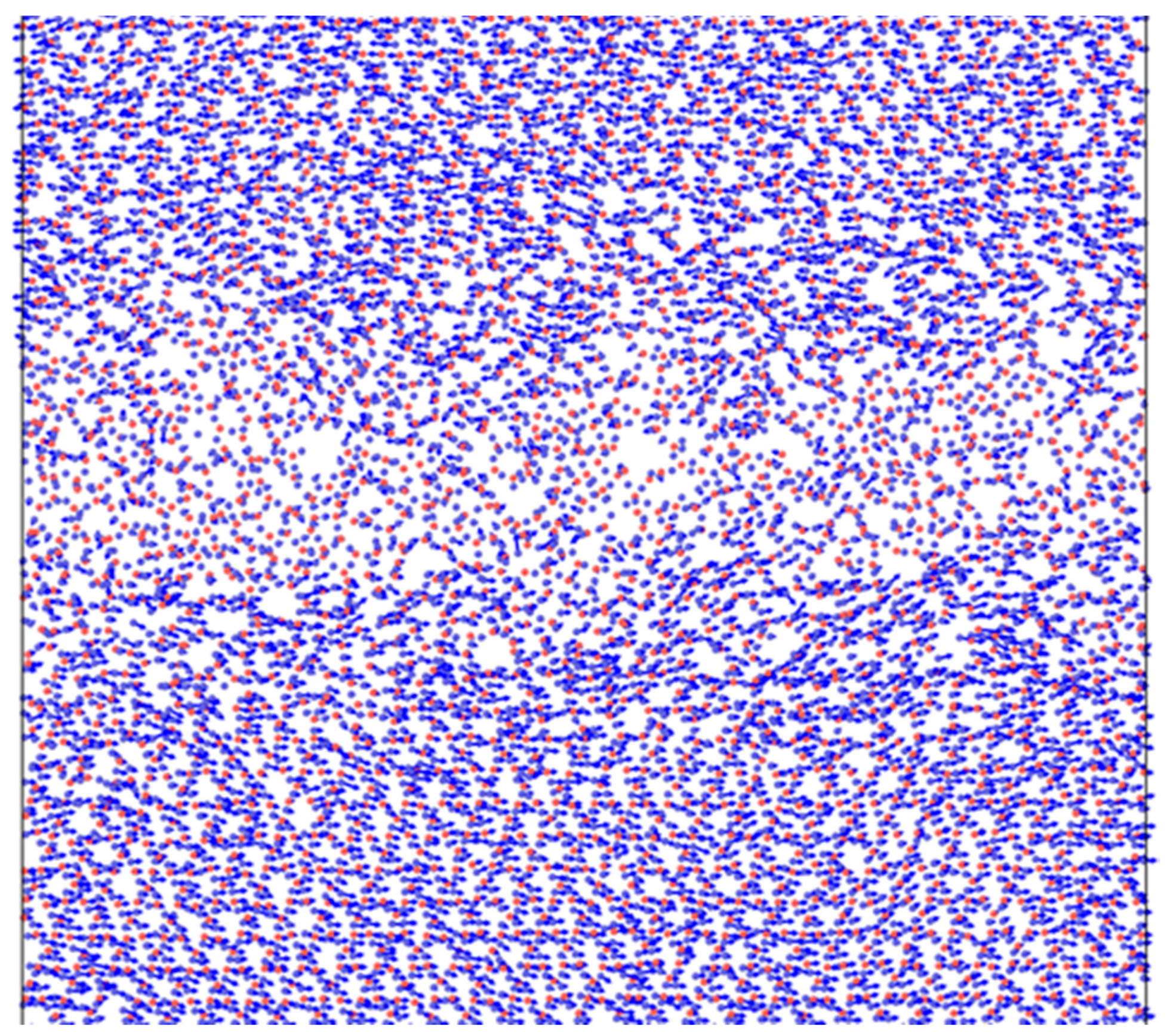
To produce the silica amorphous layer atoms belonging to the central part of the considered material volume were instantly heated up to 6000 K and then quenched to the desired temperature. In both cases the procedure of velocity rescaling was applied. In contrast to silica, the carbon amorphous layer was created directly through the procedure of random distribution of carbon atoms. After that, some of them were deleted according to the critical distance between nearest atoms, and finally the system was relaxed to the final equilibrium state.
5. Conclusions
The following fundamental knowledge supporting previous experimental findings was obtained from MD-based sliding simulations:
Amorphous tribofilms have the potential to provide low friction forces if certain prerequisites are fulfilled.
Amorphous silica films are prone to stick-slip sliding at room temperature, but provide smooth sliding at elevated temperature. This correlates with decreasing friction forces and wear rates under severe stressing conditions [
13].
Amorphous carbon films can provide either high or low friction irrespective of temperature depending on the supporting substrate materials. If bound to ferritic steel, sliding is restricted to the interface between steel and amorphous carbon, and the tangential reaction forces are low.
For the latter mechanism of friction reduction, neither passivation of dangling bonds nor hybridization has to be taken into account.
Although the modeled structures are simpler than real structures, the prediction of smooth sliding of both films under conditions leading to a high flash temperature correlate well with experimental results, while the sliding behavior at moderate stressing conditions can be described better by MCA modeling.