Pleural Lubrication
Abstract
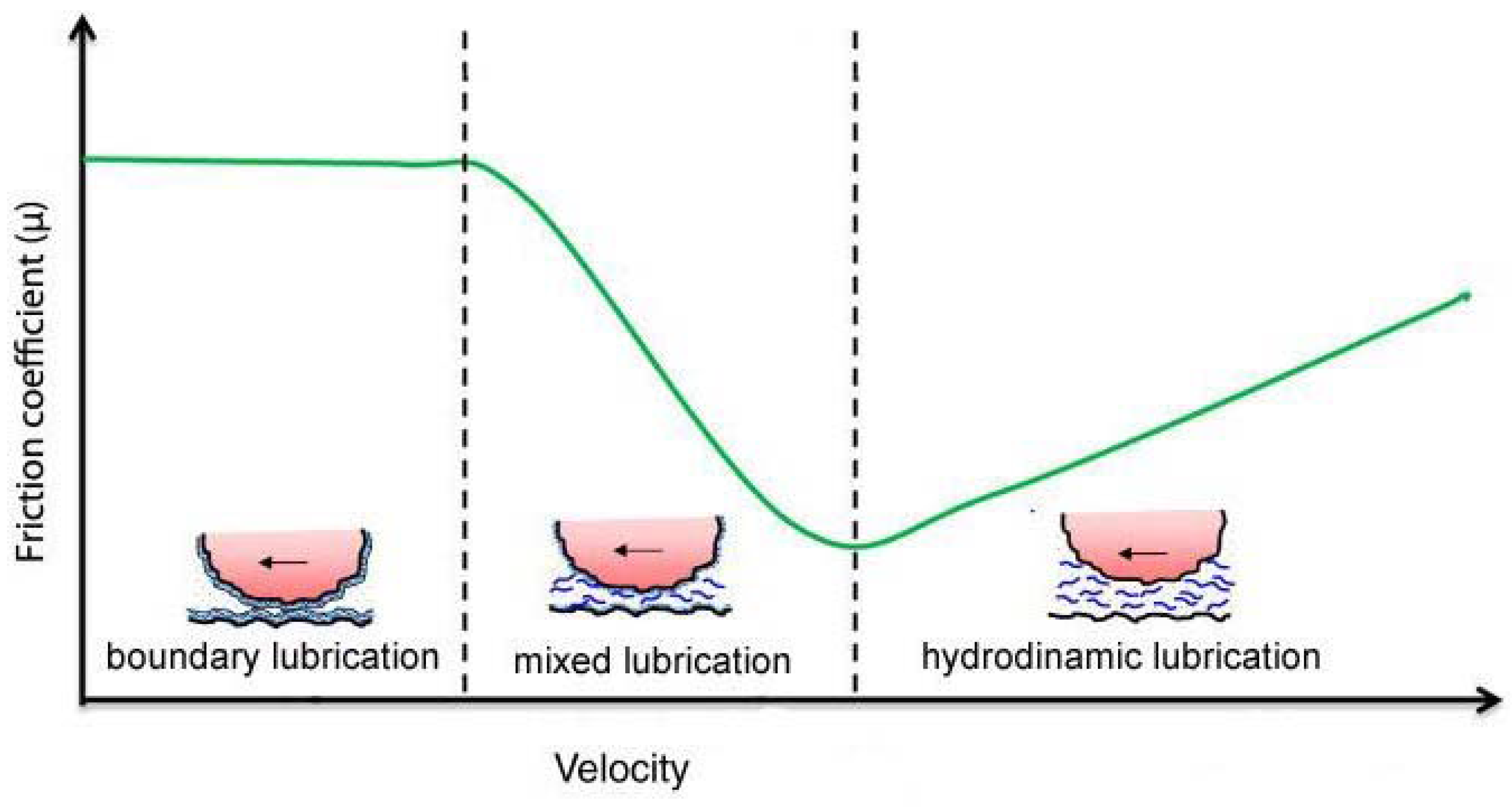
:1. Introduction
2. Boundary Lubricants
2.1. Sialomucin Lubricant Effects
2.2. Hyaluronan Lubricant Effects
2.3. Phospolipids Lubricant Effects
3. Mixed Lubrication in Damaged Mesothelium
4. Hystological Remarks
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mariassy, A.T.; Wheeldon, E.B. The pleura: A combined light microscopic, scanning and trasmission electron microscopic study in the sheep. I. Normal pleura. Exp. Lung Res. 1983, 4, 293–314. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, L. Physiology and pathophysiology of pleural fluid turnover. Eur. Respir. J. 2002, 20, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Lai-Fook, S.J.; Rodarte, J.R. Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J. Appl. Physiol. 1991, 70, 967–978. [Google Scholar] [PubMed]
- Lai, J.; Gouldstone, A.; Butler, J.P.; Federspiel, W.J.; Loring, S.H. Relative motion of the lung and chest wall promotes uniform pleural space thickness. Respir. Physiol. Neurobiol. 2002, 131, 233–243. [Google Scholar] [CrossRef]
- Gouldstone, A.; Brown, R.E.; Butler, J.P.; Loring, S.H. Elastohydrodynamic separation of pleural surfaces during breathing. Respir. Physiol. Neurobiol. 2003, 137, 97–106. [Google Scholar] [CrossRef]
- Agostoni, E.; D’Angelo, E. Pleural liquid pressure. J. Appl. Physiol. 1991, 71, 393–403. [Google Scholar] [PubMed]
- Loring, S.H.; Brown, R.E.; Gouldsone, A.; Butler, J.P. Lubrication regimes in mesothelial sliding. J. Biomech. 2005, 38, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Loring, S.H.; Gioia, M.E.; Pecchiari, M.; Moscheni, C. Friction and lubrication of pleural tissues. Respir. Physiol. Neurobiol. 2004, 142, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Butler, J.P.; Loring, SH. Influence of the softness of the parietal pleura on respiratory sliding mechanisms. Respir. Physiol. Neurobiol. 2011, 177, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.G.; Macklem, P.T. Stress at the pleural surface. Respir. Physiol. 1976, 28, 65–74. [Google Scholar] [CrossRef]
- Andrews, P.M.; Porter, K.R. The ultrastructural morphology and possible functional significance of mesothelial microvilli. Anat. Rec. 1973, 177, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.S. The regional difference of pleural mesothelial cells in rabbits. Am. Rev. Respir. Dis. 1974, 110, 623–633. [Google Scholar] [PubMed]
- Michailova, K.N. Ultrastructural observations on the human visceral pleura. Eur. J. Morphol. 1997, 35, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, E. Mechanics of the pleural space. Physiol. Rev. 1972, 53, 57–128. [Google Scholar]
- Hills, B.A. Graphite-like lubrication of mesothelium by oligolamellar pleural surfactant. J. Appl. Physiol. 1992, 73, 1034–1039. [Google Scholar] [PubMed]
- Wang, P.M.; Lai-Fook, S.J. Effects of ventilation on hyaluronan and protein concentration in pleural liquid in anesthetized and conscious rabbits. Lung 1998, 176, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Ogston, A.G.; Stainer, J.E. The physiological function of hyaluronic acid in synovial fluid; viscous, elastic and lubricant properties. J. Physiol. 1953, 119, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, E.; D’Angelo, E.; Roncoroni, G. The thickness of the pleural liquid. Respir. Physiol. 1968, 5, 1–13. [Google Scholar] [CrossRef]
- Agostoni, E. Mechanics of the pleural space. In Handbook of Physiology. The Respiratory System. Mechanics of Breathing; Macklem, P.T., Mead, J., Eds.; American Physiological Society: Bethesda, MD, USA, 1986; Volume 3, pp. 531–559. [Google Scholar]
- Agostoni, E.; Zocchi, L. Pleural liquid and its exchange. Respir. Physiol. Neurobiol. 2007, 159, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Lai-Fook, S.J.; Beck, K.C.; Southorn, P.A. Pleural liquid pressure measured by micropipettes in rabbits. J. Appl. Physiol. 1984, 56, 1633–1639. [Google Scholar] [PubMed]
- Agostoni, E.; Agostoni, P.G.; Zocchi, L. Pleural liquid pressure in the zone of apposition and in the lung zone. Respir. Physiol. 1989, 75, 357–370. [Google Scholar] [CrossRef]
- Lai-Fook, S.J.; Wang, P.M. Dynamics of pleural liquid. In Complexity in Structure and Function of the Lung; Hlastala, M.P., Robertson, H.T., Eds.; Dekker: New York, NY, USA, 1998; pp. 123–149. [Google Scholar]
- Lai-Fook, S.J. Pleural mechanics and fluid exchange. Physiol. Rev. 2004, 84, 385–410. [Google Scholar] [CrossRef] [PubMed]
- Von Neergaard, K. Zur Frage des Druckes im Pleuraspalt. Beitr. Klin. Erforsch. Tuberk. Lungenkr. 1927, 65, 476–485. [Google Scholar] [CrossRef]
- Bodega, F.; Pecchiari, M.; Sironi, C.; Porta, C.; Arnaboldi, F.; Barajon, I.; Agostoni, E. Lubricating effect of sialomucin and hyaluronan on pleural mesothelium. Respir. Physiol. Neurobiol. 2012, 180, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Frazer, J.R.; Laurent, T.C. Hyaluronan. In Extracellular Matrix; Comper, W.D., Ed.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1996; Volume 2, pp. 141–169. [Google Scholar]
- Schmidt, T.A.; Gastelum, N.S.; Nguyen, Q.T.; Schumacher, B.L.; Sah, R.L. Boundary lubrication of articular cartilage: Role of synovial fluid constituents. Arthritis Rheum. 2007, 56, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Linn, F.C. Lubrication of animal joints. II. The mechanism. J. Biomech. 1968, 1, 193–205. [Google Scholar] [CrossRef]
- Wang, N.S. Mesothelial cells in situ. In The Pleura in Health and Disease; Chrétien, J., Bignon, J., Hirsh, A., Eds.; Dekker: New York, NY, USA, 1985; pp. 23–42. [Google Scholar]
- Hilkens, J.; Ligtenberg, M.J.L.; Vos, H.L.; Litvinov, S.V. Cell membraneassociated mucins and their adhesion-modulating property. Trends Biochem. Sci. 1992, 17, 359–363. [Google Scholar] [CrossRef]
- Ohtsuka, A.; Yamana, S.; Murakami, T. Localization of membrane-associated sialomucin on the free surface of mesothelial cells of the pleura, pericardium and peritoneum. Histochem. Cell Biol. 1997, 107, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Michailova, K.N. Mesothelial lamellar bodies in norm and experimental conditions. Transmission and scanning electron microscopic observations on the peritoneum, pleura and pericardium. Anat. Embryol. 2004, 208, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Sironi, C.; Bodega, F.; Porta, C.; Agostoni, E. Pleural mesothelium lubrication after hyaluronidase, neuraminidase or pronase treatment. Respir. Physiol. Neurobiol. 2013, 188, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Born, G.V.; Palinski, W. Unusually high concentrations of sialic acids on the surface of vascular endothelia. Br. J. Exp. Pathol. 1985, 66, 543–549. [Google Scholar] [PubMed]
- Simionescu, M.; Simionescu, N.; Silbert, J.E.; Palade, G.E. Differentiated microdomains on the luminal surface of the capillary endothelium. II. Partial characterization of their anionic sites. J. Cell Biol. 1981, 90, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, M.; Simionescu, N.; Santoro, F.; Palade, G.E. Differentiated microdomains of the luminal plasmalemma of murine muscle capillaries: Segmental variations in young and old animals. J. Cell Biol. 1985, 100, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Bodega, F.; Sironi, C.; Porta, C.; Zocchi, L.; Agostoni, E. Pleural mesothelium lubrication after phospholipase treatment. Respir. Physiol. Neurobiol. 2014, 194, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Ohashi, Y.; Mori, Y. Hyaluronic acid in rabbit pericardial fluid and its production by pericardium. FEBS Lett. 1986, 203, 273–278. [Google Scholar] [CrossRef]
- Bodega, F.; Sironi, C.; Porta, C.; Pecchiari, M.; Zocchi, L.; Agostoni, E. Mixed lubrication after rewetting blotted mesothelium. Respir. Physiol. Neurobiol. 2013, 185, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Castor, C.W.; Prince, R.K.; Hazelton, M.J. Hyaluronic acid in human synovial effusions; A sensitive indicator of altered connective tissue cell function during inflammation. Arthritis Rheum. 1966, 9, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Pecchiari, M.; Sartori, P.; Conte, V.; D’Angelo, E.; Moscheni, C. Friction and morphology of pleural mesothelia. Respir Physiol Neurobiol. 2016, 220, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, T.; Fröseth, B.; Riska, H.; Klockars, M. Concentration of hyaluronic acid in pleural fluid as a diagnostic aid for malignant mesothelioma. Chest 1988, 94, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Hills, B.A.; Butler, B.D.; Barrow, R.E. Boundary lubrication imparted by pleural surfactants and their identification. J. Appl. Physiol. 1982, 53, 463–469. [Google Scholar] [PubMed]
- Hills, B.A.; Butler, B.D. Phospholipids identified on the pericardium and their ability to impart boundary lubrication. Ann. Biomed. Eng. 1985, 13, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.C.; Chen, Y.; Hills, Y.C.; Hills, B.A. Differences in surfactant lipids collected from pleural and pulmonary lining fluids. Pharm. Res. 2005, 22, 1926–1930. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.C.; Chen, Y.; Hills, Y.C.; Hills, B.A. Comparison of surfactant lipids between pleural and pulmonary lining fluids. Pulm. Pharmacol. Ther. 2006, 19, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.S. Isolation and assay of dipalmitoyl lecithin in lung extracts. Am. J. Physiol. 1964, 207, 402–406. [Google Scholar] [PubMed]
- Dobbie, J.W.; Anderson, J.D. Ultrastructure, distribution, and density of lamellar bodies in human peritoneum. Perit. Dial. Int. 1996, 16, 482–487. [Google Scholar] [PubMed]
- Hills, B.A. The Biology of Surfactant; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Hills, B.A. Oligolamellar lubrication of joints by surface active phospholipid. J. Rheumatol. 1989, 16, 82–91. [Google Scholar] [PubMed]
- Bernhard, W.; Postle, A.D.; Rau, G.A.; Freihorst, J. Pulmonary and gastric surfactants. A comparison of the effect of surface requirements on function and phospholipid composition. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 129, 173–182. [Google Scholar] [CrossRef]
- Lu, K.W.; Goerke, J.; Clements, J.A.; Taeusch, H.W. Hyaluronan decreases surfactant inactivation in vitro. Pediat. Res. 2005, 57, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, D.W.; Nitzan, U.; Dan, P.; Yedgar, S. The role of hyaluronic acid in protecting surface-active phospholipids from lysis by exogenous phospholipase A(2). Rheumatology 2001, 40, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Iwanicki, J.L.; Lu, K.W.; Taeusch, H.W. Reductions of phospholipase A(2) inhibition of pulmonary surfactant with hyaluronan. Exp. Lung Res. 2010, 36, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Pasquali-Ronchetti, I.; Quaglino, D.; Mori, G.; Bacchelli, B.; Ghosh, P. Hyaluronan-phospholipid interactions. J. Struct. Biol. 1997, 120, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Abe, T.; Matsuura, A. Prevention of postoperative intrapleural adhesion of the thoracotomy incision by a bioresorbable membrane in the rat adhesion model. Ann. Thorac. Cardiovasc. Surg. 2000, 6, 151–160. [Google Scholar] [PubMed]
- Getman, V.; Devyatko, E.; Wolner, E.; Aharinejad, S.; Mueller, M.R. Fleece bound sealing prevents pleural adhesions. Interact. Cardiovasc. Thorac. Surg. 2006, 5, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Karacam, V.; Onen, A.; Sanli, A.; Gurel, D.; Kargi, A.; Karapolat, S.; Ozdemir, N. Prevention of pleural adhesions using a membrane containing polyethylene glycol in rats. Int. J. Med. Sci. 2011, 8, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Ar’Rajab, A.; Snoj, M.; Larsson, K.; Bengmark, S. Exogenous phospholipid reduces postoperative peritoneal adhesions in rat. Eur. J. Surg. 1995, 161, 341–344. [Google Scholar] [PubMed]
- Bhandarkar, D.S.; Nathanson, L.K.; Hills, B.A. Spray of phospholipid powder reduces peritoneal adhesions in rabbits. Aust. N. Z. J. Surg. 1999, 69, 388–390. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porta, C.; Sironi, C.; Bodega, F.; Agostoni, E. Pleural Lubrication. Lubricants 2016, 4, 15. https://doi.org/10.3390/lubricants4020015
Porta C, Sironi C, Bodega F, Agostoni E. Pleural Lubrication. Lubricants. 2016; 4(2):15. https://doi.org/10.3390/lubricants4020015
Chicago/Turabian StylePorta, Cristina, Chiara Sironi, Francesca Bodega, and Emilio Agostoni. 2016. "Pleural Lubrication" Lubricants 4, no. 2: 15. https://doi.org/10.3390/lubricants4020015
APA StylePorta, C., Sironi, C., Bodega, F., & Agostoni, E. (2016). Pleural Lubrication. Lubricants, 4(2), 15. https://doi.org/10.3390/lubricants4020015







