The Role of Solid Lubricants for Brake Friction Materials
Abstract
:1. Introduction
2. Impact of Solid Lubricant Additions to Brake Pad Formulations
2.1. Why Do We Need Solid Lubricants for Brake Applications?
2.2. Addition of Graphite
2.3. Metal Sulfide Based Solid Lubricants
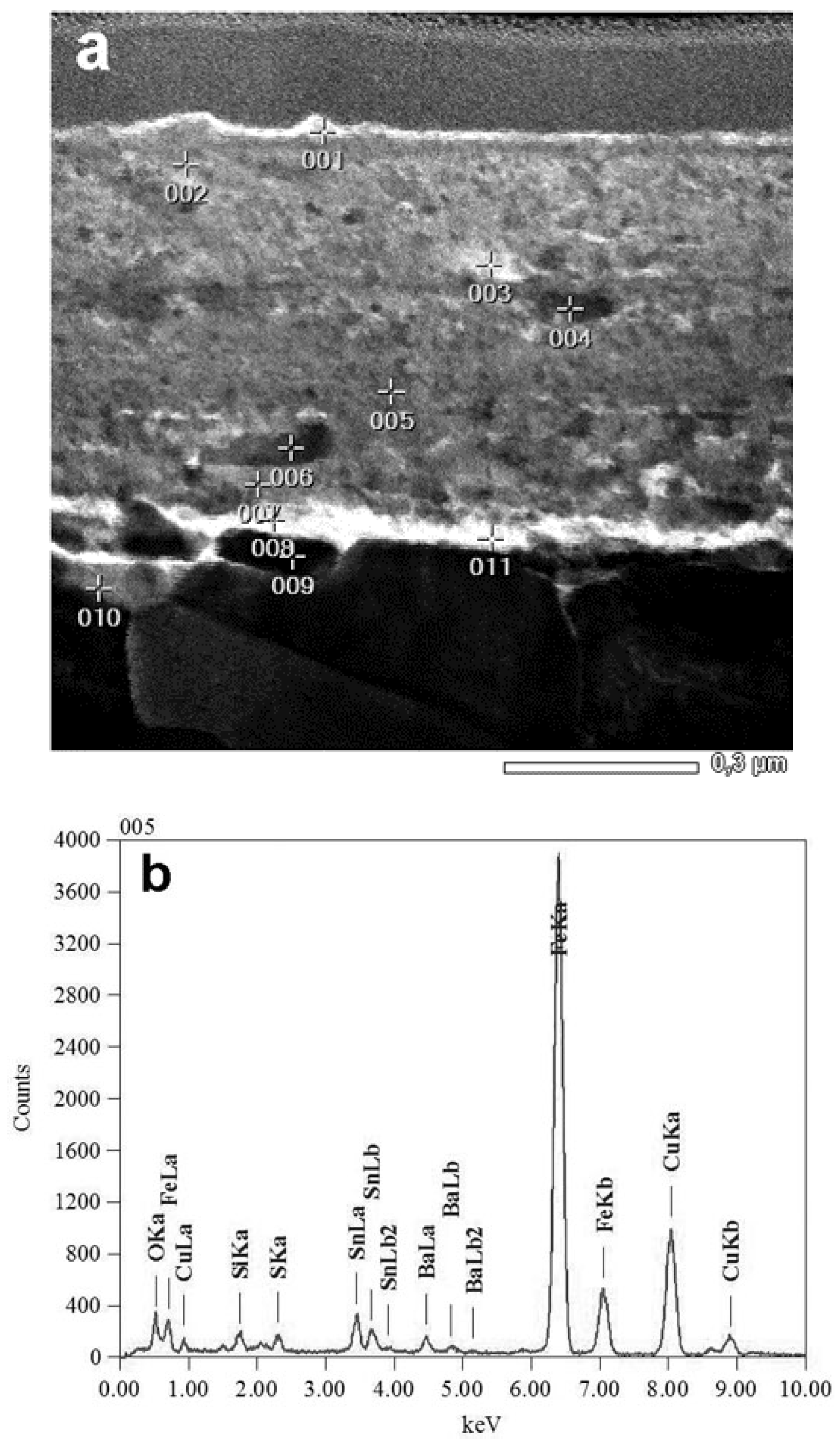
3. Structure and Composition of Tribofilms Formed during Braking
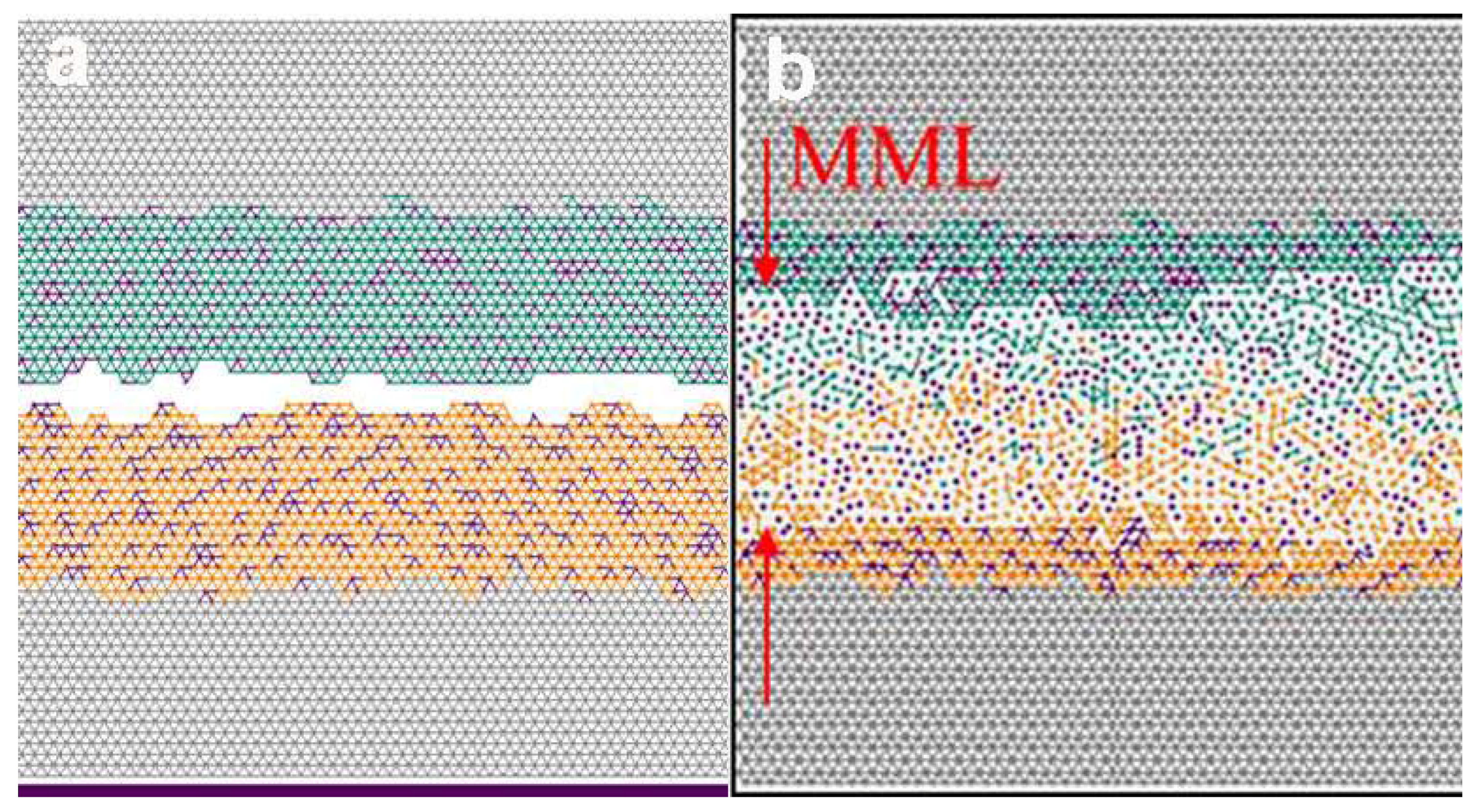
4. Modelling of the Sliding Behavior of Tribofilms Formed during Braking
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhmud, B.; Pasalskiy, B. Nanomaterials in Lubricants: An industrial perspective on current research. Lubricants 2013, 1, 95–101. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Simionesie, D.; Schaske, C. Graphite and hybrid nanomaterials as lubricant additives. Lubricants 2014, 2, 44–65. [Google Scholar] [CrossRef]
- Yazawa, S.; Minami, I.; Prakash, B. Reducing friction and wear of tribological systems through hybrid tribofilm consisting of coating and lubricants. Lubricants 2014, 2, 90–112. [Google Scholar] [CrossRef]
- Braun, D.; Greiner, C.; Schneider, J.; Gumbsch, P. Efficiency of laser surface texturing in the reduction of friction under mixed lubrication. Tribol. Int. 2014, 77, 142–147. [Google Scholar] [CrossRef]
- Alberdi, A.; Hatto, P.; Diaz, B.; Csillag, S. Tribological behavior of nanocomposite coatings based on fullerene-like structures. Vacuum 2011, 85, 1087–1092. [Google Scholar] [CrossRef]
- Thomas, P.; Mansot, J.L.; Molza, A.; Begarin, F.; Dubois, M.; Guerin, K. Friction properties of fluorinated graphitized carbon blacks. Tribol. Lett. 2014, 56, 259–271. [Google Scholar] [CrossRef]
- Steiner, L.; Bouvier, V.; May, U.; Hegadekatte, V.; Huber, N. Modeling of unlubricated oscillating sliding wear of DLC-coatings considereding surface topography, oxidation and graphitization. Wear 2010, 268, 1184–1194. [Google Scholar] [CrossRef]
- Martin, J.M.; Pascal, H.; Donnet, C.; Le Mogne, T.; Loubet, J.L.; Epicier, T. Superlubricity of MoS2: Crystal orientation mechanisms. Surf. Coat. Technol. 1994, 68, 427–432. [Google Scholar] [CrossRef]
- Morita, Y.; Onodera, T.; Suzuki, A.; Sahnoun, R.; Koyama, M.; Tsuboi, H.; Hatakeyama, N.; Endou, A.; Takaba, H.; Kubo, M.; et al. Development of a new molecular dynamics method for tribochemical reaction and its application to formation dynamics of MoS2 tribofilm. Appl. Surf. Sci. 2008, 254, 7618–7621. [Google Scholar] [CrossRef]
- Colas, G.; Saulot, A.; Godeau, C.; Michel, Y.; Berthier, Y. Decrypting third body flows to solve dry lubrication issue-MoS2 case study under ultrahigh vacuum. Wear 2013, 305, 192–204. [Google Scholar] [CrossRef]
- An, V.; Irtegov, Y.; de Izarra, C. Study of tribological properties of nanolamellar WS2 and MoS2 as additives to lubricants. J. Nanomater. 2014. Available online: http://dx.doi.org/10.1155/2014/865839 (accessed on 25 February 2016). [Google Scholar]
- Rapoport, L.; Leshchinsky, V.; Lvovsky, M.; Lapsker, I.; Volovik, Y.; Feldman, Y.; Popovitz-Biro, R.; Tenne, R. Superior tribological properties of powder materials with solid lubricant nanoparticles. Wear 2003, 255, 794–800. [Google Scholar] [CrossRef]
- Martin, J.M.; Onodera, T.; Minfray, C.; Dassenoy, F.; Miyamoto, A. The origin of anti-wear chemistry of ZDDP. Faraday Discuss. 2012, 156, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Kunze, T.; Posselt, M.; Gemming, S.; Seifert, G.; Konicek, R.; Carpick, R.W.; Pastewka, L.; Moseler, M. Wear, plasticity, and rehybridization in tetrahedral amorphous carbon. Tribol. Lett. 2014, 53, 119–126. [Google Scholar] [CrossRef]
- Godet, M. The third body approach: A mechanical view of wear. Wear 1984, 100, 437–452. [Google Scholar] [CrossRef]
- Blau, P.J. Compositions Functions and Testing of Friction Brake Materials and Their Additives; Technical Report 64; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2001. [Google Scholar]
- Goudier, M.; Berthier, Y.; Jacquemard, P.; Rousseau, B.; Bonnamy, S.; Estrade-Szwarckopf, H. Mass spectrometry during C/C composite friction: Carbon oxidation associated with high friction coefficient and high wear rate. Wear 2004, 256, 1082–1087. [Google Scholar] [CrossRef]
- Kasem, H.; Bonnamy, S.; Berthier, Y.; Dufrenoy, P.; Jacquemard, P. Tribological, physicochemical and thermal study of the abrupt friction transition during C/C composite friction. Wear 2009, 267, 846–852. [Google Scholar] [CrossRef]
- Stadler, Z.; Krnel, K.; Kosmac, T. Friction and wear of sintered metallic brake linings on a C/C-SiC composite brake disc. Wear 2008, 265, 278–285. [Google Scholar] [CrossRef]
- Cho, M.H.; Ju, J.; Kim, J.; Jang, H. Tribological properties of solid lubricants (graphite, Sb2S3, MoS2) for automotive brake friction materials. Wear 2006, 260, 855–860. [Google Scholar] [CrossRef]
- Ram-Prabhu, T. Effects of solid lubricants, load, and sliding speed on the tribological behavior of silica reinforced composites using design of experiments. Mater. Des. 2015, 77, 149–160. [Google Scholar] [CrossRef]
- Chen, B.; Bi, Q.; Yang, J.; Xia, Y.; Hao, J. Tribological properties of solid lubricants (graphite, h-BN) for Cu-based P/M friction composites. Tribol. Int. 2008, 41, 1145–1152. [Google Scholar] [CrossRef]
- Su, L.; Gao, F.; Han, X.; Fu, R.; Zhang, E. Tribological behavior of copper-graphite powder third body on copper-based friction materials. Tribol. Lett. 2015, 60, 30. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Xing, J.; Wang, Y.; Fang, L. Wear reduction mechanism of graphite and MoS2 in epoxy composites. Wear 2004, 257, 279–283. [Google Scholar] [CrossRef]
- Pan, G.; Guo, Q.; Ding, J.; Zhang, W.; Wang, X. Tribological behaviors of graphite/epoxy two phase composite coatings. Tribol. Int. 2010, 43, 1318–1325. [Google Scholar] [CrossRef]
- Melcher, B.; Faullant, P. A Comprehensive Study of Chemical and Physical Properties of Metal Sulfides; SAE Technical Paper 2000-01-2757; SAE: Warrendale, PA, USA, 2000; pp. 39–49. [Google Scholar]
- Gudmand-Hoyer, L.; Bach, A.; Nielsen, G.T.; Morgen, P. Tribological properties of automotive disc brakes with solid lubricants. Wear 1999, 232, 168–175. [Google Scholar] [CrossRef]
- Matejka, V.; Lu, Y.; Matekova, P.; Smetana, B.; Kukutschova, J.; Vaculik, M.; Tomasek, V.; Zla, S.; Fan, Y. Possible stibnite transformation at the friction surface of the semi-metallic friction composites designed for car brake linings. Appl. Surf. Sci. 2011, 258, 1862–1868. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Cho, M.H.; Cho, K.H.; Jang, H. Complementary effects of solid lubricants in the automotive brake lining. Tribol. Int. 2007, 40, 15–20. [Google Scholar] [CrossRef]
- Jang, H.; Kim, S.J. The effects of antimony trisulfide and zirconium silicate in the automotive brake friction material on friction characteristics. Wear 2000, 239, 229–236. [Google Scholar] [CrossRef]
- Holinski, R. Improvement of comfort of friction brakes. In Proceedings of the World Tribology Congress, Vienna, Austria, 3–7 September 2001; Franek, F., Bartz, W.J., Pauschitz, A., Eds.; The Austrian Tribological Society: Vienna, Austria, 2001. [Google Scholar]
- Jang, H.; Lee, J.S.; Fash, J.W. Compositional effects of the brake friction material on creep groan phenomena. Wear 2001, 251, 1477–1483. [Google Scholar] [CrossRef]
- Mutlu, I.; Eldogan, O.; Findik, F. Tribological properties of some phenolic composites suggested for automotive brakes. Tribol. Int. 2006, 39, 317–325. [Google Scholar] [CrossRef]
- Yi, G.; Yan, F. Mechanical and tribological properties of phenolic resin-based friction composites filled with several inorganic fillers. Wear 2007, 262, 121–129. [Google Scholar] [CrossRef]
- Abdel-Rahim, Y.M.; Darwish, S.M. Generalized braking charakteristics of friction pad synthetic graphite composites. Tribol. Int. 2010, 43, 838–843. [Google Scholar] [CrossRef]
- Kolluri, D.K.; Boidin, X.; Desplanques, Y.; Degallaix, G.; Ghosh, A.K.; Kumar, M.; Bijwe, J. Effect of natural graphite particle size in friction materials on thermal localization phenomenon during stop-braking. Wear 2010, 268, 1472–1482. [Google Scholar] [CrossRef]
- Yun, R.; Filip, P.; Lu, Y. Performance and evaluation of eco-friendly brake friction materials. Tribol. Int. 2010, 43, 2010–2019. [Google Scholar] [CrossRef]
- Uexküll, O.; Skerfving, S.; Doyle, R.; Braungart, M. Antimony in brake pads—A carcinogenic component? J. Clean. Prod. 2005, 13, 19–31. [Google Scholar] [CrossRef]
- Morbach, M.; Paul, H.G.; Severit, P. Systematic approach for structured product development of copper free friction materials. In Proceedings of the EuroBrake 2012, Dresden, Germany, 16–18 April 2012.
- Martinez, A.M.; Echeberria, J.; Di Loreto, A.; Zanon, M.; Rampin, I. Joint development of copper-free fow steel brake pads for light vehicles. In Proceedings of the EuroBrake 2014, Lille, France, 16–18 May 2014.
- Martinez, A.M.; Echeberria, J.; Zanon, M.; Di Loreto, A. Characterization of the chemical reactions between solid lubricants and metal powders in low metallic brake pads during braking. In Proceedings of the EuroBrake 2015, Dresden, Germany, 4–6 May 2015.
- Verma, P.C.; Menapace, L.; Bonfanti, A.; Ciudin, R.; Gialanella, S.; Straffelini, G. Braking pad-disc system: Wear mechanisms and formation of wear fragments. Wear 2015, 322–323, 251–258. [Google Scholar] [CrossRef]
- Jacko, M.G.; DuCharme, R.T.; Somers, J.H. Brake and clutch emissions generated during vehicle operation. In Proceedings of the SAE Automobile Engineering Meeting, Detroit, MI, USA, 14–18 May 1973; Society of Automotive Engineers INC: New York, NY, USA, 1973. [Google Scholar]
- Bark, L.S.; Moran, D.; Percival, S.J. Polymer changes during friction material performance. Wear 1977, 41, 309–314. [Google Scholar] [CrossRef]
- Libsch, T.A.; Rhee, S.K. Microstructural changes in semimetallic disc brake pads created by low temperature dynamometer testing. Wear 1978, 46, 203–212. [Google Scholar] [CrossRef]
- Jacko, M.G. Physical and chemical changes of organic disc pads in service. Wear 1978, 46, 163–175. [Google Scholar] [CrossRef]
- Scieszka, S.F. Tribological phenomena in steel-composite brake material friction pairs. Wear 1980, 64, 367–378. [Google Scholar] [CrossRef]
- Jacko, M.G.; Tsang, P.H.S.; Rhee, S.K. Wear debris compaction and friction film formation of polymer composites. Wear 1989, 133, 23–38. [Google Scholar] [CrossRef]
- Wirth, A.; Eggleston, D.; Whitaker, R. A fundamental tribochemical study of the third body layer formed during automotive friction braking. Wear 1994, 179, 75–81. [Google Scholar] [CrossRef]
- Singer, I. Mechanics and chemistry of solids in sliding contact. Langmuir 1996, 12, 4486–4491. [Google Scholar] [CrossRef]
- Filip, P.; Wright, M.A. Characterization of composite materials for automotive braking industry. Prakt. Met. Sonderband 1999, 30, 449–456. [Google Scholar]
- Eriksson, M.; Jacobson, S. Tribological surfaces of organic brake pads. Tribol. Int. 2000, 33, 817–827. [Google Scholar] [CrossRef]
- Garg, B.D.; Cadle, S.H.; Mulawa, P.A.; Groblicki, P.J. Brake wear particulate matter emissions. Environ. Sci. Technol. 2000, 34, 4463–4469. [Google Scholar] [CrossRef]
- Eriksson, M.; Lord, J.; Jacobson, S. Wear and contact conditions of brake pads: Dynamical in situ studies of pad on glass. Wear 2001, 249, 272–278. [Google Scholar] [CrossRef]
- Filip, P.; Weiss, Z.; Rafaja, D. On friction layer formation in polymer matrix composite materials for brake applications. Wear 2002, 252, 189–198. [Google Scholar] [CrossRef]
- Kharrazi, Y.H.K.; Österle, W. The microstructure of brake lining materials containing asbestos and possible asbestos substitudes. Prakt. Metallogr. 2002, 39, 542–556. [Google Scholar]
- Ingo, G.M.; Uffizi, M.D.; Falso, G.; Bultrini, G.; Padeletti, G. Thermal and microchemical investigation of automotive brake pad wear residues. Thermochim. Acta 2004, 418, 61–68. [Google Scholar] [CrossRef]
- Mosleh, M.; Blau, P.J.; Dumitrescu, D. Characterisation and morphology of wear particles from laboratory testing of disk brake materials. Wear 2004, 256, 1128–1134. [Google Scholar] [CrossRef]
- Österle, W.; Urban, I. Friction layers and friction films on PMC brake pads. Wear 2004, 257, 215–226. [Google Scholar] [CrossRef]
- Österle, W.; Bettge, D. A comparison of methods for characterizing brake lining surfaces. Prakt. Metallogr. 2004, 41, 494–504. [Google Scholar]
- Cho, M.H.; Cho, K.H.; Kim, S.J.; Kim, D.H.; Jang, H. The role of transfer layers on friction characteristics in the sliding interface between friction materials against gray iron brake disks. Tribol. Lett. 2005, 20, 101–108. [Google Scholar] [CrossRef]
- Österle, W.; Dmitriev, A.I.; Urban, I. Characterization of up to date brake friction materials. In Proceedings of the International Workshop on Advances in Asbestos-Free Friction Compositers—I (IWAAFC-1), New Dehli, India, 5–6 January 2006; Bijwe, J., Ed.; ITMMEC, Indian Institute of Technology: Dehli, India, 2006; pp. 21–35. [Google Scholar]
- Österle, W.; Urban, I. Third body formation on brake pads and rotors. Tribol. Int. 2006, 39, 401–408. [Google Scholar] [CrossRef]
- Cristol-Bulthe, A.L.; Desplanques, Y.; Degallaix, G.; Berthier, Y. Mechanical and chemical investigation of the temperature influence on the tribological mechanisms occurring in OMC/cast iron friction contact. Wear 2008, 264, 815–825. [Google Scholar] [CrossRef]
- Österle, W.; Kloß, H.; Urban, I.; Dmitriev, A.I. Towards a better understanding of brake friction materials. Wear 2007, 263, 1189–1201. [Google Scholar] [CrossRef]
- Roubicek, V.; Raclavska, H.; Juchelkova, D.; Filip, P. Wear and environmental aspects of composite materials for automotive braking industry. Wear 2008, 265, 167–175. [Google Scholar] [CrossRef]
- Österle, W.; Dörfel, I.; Prietzel, C.; Rooch, H.; Cristol-Bulte, A.L.; Degallaix, G.; Desplanques, Y. A comprehensive study of third body formation at the interface between a brake pad and brake disc during the final stage of a pin-on-disc test. Wear 2009, 267, 781–788. [Google Scholar] [CrossRef]
- Österle, W.; Bresch, H.; Dörfel, I.; Prietzel, C.; Seeger, S.; Fink, C.; Giese, A.; Walter, J. Surface film formation and dust generation during brake performance tests. In Proceedings of the Conference on Braking 2009, York, UK, 9–10 June 2009; Institution of Mechanical Engineers, Chandos Publishing: Oxford, UK, 2009; pp. 29–38. [Google Scholar]
- EL-Tayeb, N.S.M.; Liew, K.W. On dry and wet sliding performance of potentially new frictional brake pad materials for automotive industry. Wear 2009, 266, 275–287. [Google Scholar] [CrossRef]
- Kukutschova, J.; Roubicek, V.; Maslan, M.; Jancik, D.; Slovak, V.; Malachova, K.; Pavlickova, Z.; Filip, P. Wear performance and wear debris of semimetallic automotive brake materials. Wear 2010, 268, 86–93. [Google Scholar] [CrossRef]
- Österle, W.; Prietzel, C.; Dmitriev, A.I. Investigation of surface film nanostructure and assessment of its impact on friction force stabilization during automotive braking. Int. J. Mater. Res. 2010, 101, 669–675. [Google Scholar] [CrossRef]
- Österle, W.; Bresch, H.; Dörfel, I.; Fink, C.; Giese, A.; Prietzel, C.; Seeger, S.; Walter, J. Examination of airborne brake dust. In Proceedings of the 6th European Conference on Braking JEF 2010, Lille, France, 24–25 November 2010; pp. 55–60.
- Österle, W.; Prietzel, C.; Kloß, H.; Dmitriev, A.I. On the role of copper in brake friction materials. Tribol. Int. 2010, 43, 2317–2326. [Google Scholar] [CrossRef]
- Hinrichs, R.; Vasconcellos, M.A.Z.; Österle, W.; Prietzel, C. A TEM snapshot of magnetite formation in brakes: The role of the disc’s cast iron graphite lamellae in third body formation. Wear 2011, 270, 365–370. [Google Scholar] [CrossRef]
- Österle, W.; Dmitriev, A.I. Functionality of conventional brake friction materials—Preceptions from findings observed at different length scales. Wear 2011, 271, 2198–2207. [Google Scholar] [CrossRef]
- Österle, W.; Dmitriev, A.I.; Kloß, H. Does ultra-mild wear play any role for dry friction applications, such as automotive braking? Faraday Discuss. 2012, 156, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.P.; Haertel, W.; Zanotto, P.S.; Sinatora, A. Inluence of mild and severe wear condition in the formation and stability of friction film in clutch system. Wear 2013, 302, 1384–1391. [Google Scholar] [CrossRef]
- Österle, W.; Dmitriev, A.I. Some considerations on the role of third bodies during automotive braking. SAE Int. J. Passeng. Cars Mech. Syst. 2014, 7, 1287–1294. [Google Scholar] [CrossRef]
- Österle, W.; Orts-Gil, G.; Gross, T.; Deutsch, C.; Hinrichs, R.; Vasconcellos, M.A.Z.; Zoz, H.; Yigit, D.; Sun, X. Impact of high energy ball milling on the nanostructure of magnetite-graphite and magnetite-graphite-molybdenum disulphide blends. Mater. Charact. 2013, 86, 28–38. [Google Scholar] [CrossRef]
- Österle, W.; Deutsch, C.; Gradt, T.; Orts-Gil, G.; Schneider, T.; Dmitriev, A.I. Tribological screening tests for the selection of raw materials for automotive brake pad formulations. Tribol. Int. 2014, 73, 148–155. [Google Scholar] [CrossRef]
- Häusler, I.; Dörfel, I.; Peplinski, B.; Dietrich, P.M.; Unger, W.E.S.; Österle, W. Comprehensive characterization of a tribofilm produced by ball milling of a model tribo system. Mater. Charact. 2016, 111, 183–192. [Google Scholar] [CrossRef]
- Söderberg, A.; Andersson, S. Simulation of wear and contact pressure distribution at the pad-to-rotor interface in a disc brake using general purpose finite element analysis software. Wear 2009, 267, 2243–2251. [Google Scholar] [CrossRef]
- Dick, T.; Cailletaud, G. Analytic and FE based estimations pf the coefficient of friction of composite surfaces. Wear 2006, 260, 1305–1316. [Google Scholar] [CrossRef]
- Lee, W.G.; Cho, K.H.; Jang, H. Molecular dynamics simulation of rolling friction using nanosize spheres. Tribol. Lett. 2009, 33, 37–43. [Google Scholar] [CrossRef]
- Ostermeyer, G.P.; Müller, M. New insights into the tribology of brake systems. Automob. Eng. 2008, 222, 1167–1200. [Google Scholar] [CrossRef]
- Wahlström, J. A Study of Airborne Wear Particles from Automotive Disc Brakes. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2011. [Google Scholar]
- Fillot, N.; Iordanoff, I.; Berthier, Y. Wear modelling and the third body concept. Wear 2007, 262, 949–957. [Google Scholar] [CrossRef]
- Fillot, N.; Iordanoff, I.; Berthier, Y. Modelling third body flows with a discrete element method—A tool for understanding wear with adhesive particles. Tribol. Int. 2007, 40, 973–981. [Google Scholar] [CrossRef]
- Psakhie, S.G.; Horie, Y.; Ostermeyer, G.P.; Korostelev, S.Y.; Smolin, A.Y.; Shilko, E.V.; Dmitriev, A.I.; Blatnik, S.; Špegel, M.; Zavšek, S. Movable cellular automata method for simulating materials with mesostructured. Theor. Appl. Fract. Mech. 2001, 37, 311–334. [Google Scholar] [CrossRef]
- Dmitriev, A.I.; Smolin, A.Y.; Psakhie, S.G.; Österle, W.; Kloss, H.; Popov, V.L. Computer modeling of local tribological contacts by the example of the automotive brake friction pair. Phys. Mesomech. 2008, 11, 73–84. [Google Scholar] [CrossRef]
- Dmitriev, A.I.; Österle, W.; Wetzel, B.; Zhang, G. Mesoscale modeling of the mechanical and tribological behavior of a polymer matrix composite based on epoxy and 6 vol % silica nanoparticles. Comput. Mater. Sci. 2015, 110, 204–214. [Google Scholar] [CrossRef]
- Dmitriev, A.I.; Österle, W. Modelling the sliding behaviour of tribofilms forming during automotive braking: Impact of loading parameters and property range of constituents. Tribol. Lett. 2014, 53, 337–351. [Google Scholar] [CrossRef]
- Österle, W.; Dmitriev, A.I.; Kloß, H. Prerequisites for smooth sliding behaviour derived by modelling on the nanometre scale. In Proceedings of the 3rd European Conference on Tribology, Vienna, Austria, 7–9 June 2011; pp. 869–870.
- Dmitriev, A.I.; Oesterle, W.; Kloss, H. Nano-scale modelling of automotive pad-disc interface. The influence of structure & composition. In Proceedings of the International Conference on BALTRIB 2011, Kaunas, Lithuania, 17–19 November 2011; pp. 236–242.
- Österle, W.; Dmitriev, A.I.; Kloß, H. Possible impacts of third body nanostructure on friction performance during dry sliding determined by computer simulation based on the method of movable cellular automata. Tribol. Int. 2012, 48, 128–136. [Google Scholar] [CrossRef]
- Österle, W.; Kloß, H.; Dmitriev, A.I. Modeling of friction evolution and assessment of impacts on vibration excitation at the pad-disc interface. In Proceedings of the 26th Annual Brake Colloquium, San Antonio, TX, USA, 12–15 October 2008; pp. 303–311.
- Dmitriev, A.I.; Österle, W.; Kloß, H. Numerical simulation of mechanically mixed layer formation at local contacts of an automotive brake system. Trib. Trans. 2008, 51, 810–816. [Google Scholar] [CrossRef]
- Dmitriev, A.I.; Österle, W. Modeling of brake pad-disc interface with emphasis to dynamics and deformation of structures. Tribol. Int. 2010, 43, 719–727. [Google Scholar] [CrossRef]
- Dmitriev, A.I.; Österle, W.; Kloß, H.; Orts-Gil, G. A study of third body behaviour under dry sliding conditions. Comparison of nanoscale modelling with experiment. Estonian J. Eng. 2012, 18, 270–278. [Google Scholar]
- Österle, W.; Dmitriev, A.I.; Orts-Gil, G.; Schneider, T.; Ren, H.; Sun, X. Verification of nanometre-scale modelling of tribofilm sliding behaviour. Tribol. Int. 2013, 62, 155–162. [Google Scholar] [CrossRef]
- Österle, W.; Dmitriev, A.I.; Kloß, H. Assessment of sliding friction of a nanostructured solid lubricant film by numerical simulation with the method of movable cellular automata (MCA). Tribol. Lett. 2014, 54, 257–262. [Google Scholar]
- Wahlström, J.; Dmitriev, A.I.; Kloß, H. A comparison of measured and simulated friction, wear and particle emission of disc brakes. Tribol. Int. 2015, 92, 503–511. [Google Scholar]
| Friction Material and Rotor Material If Other than Cast Iron | Tested Variable | Observations/Ref. | COF1 COF2 |
|---|---|---|---|
| vol %: fibers 23, organics 23, sulfides 8 | Metal content: 0%, 9%, 14% | Metal-free: highest wear; no systematic impact of sulfides, but Sb2S3 and PbS better than Cu2S [27] | 0.4 0.4 |
| vol %: fibers 12, organics 33, solid lubs. 10–14, abrasives2–6 | Sb2S3: 2–6 ZrSiO4: 6–2 | Formation of Sb2O3 stabilizes COF; ZrSiO4 prevents fade [30] | 0.3 0.4 |
| Non-asbestos organic pads (no details on base composition) | 13 different sulfide additions 6 wt % each | SnS/SnS2 blend recommended as substitute for Sb2S3 [26] | 0.4–0.5 0.35 |
| vol %: fibers 22, organics 28, solid lubs. 13, abrasives 1.5/3 | Graphite: 10/11 Sb2S3: 3/3.5 ZrSiO4: 3/1.5 | Optimum performance for combination of 11% graphite, 3.5% Sb2S3 and 1.5% ZrSiO4 [32] | 0.65 no data |
| wt %: no fibers but Cu particles 15–25, organics 25, graphite 5 | Cu particle size | Anomalous COF: increasing with temperature [33] | 0.2–0.3 0.4–0.5 |
| vol %: fibers 13 (aramid + ceramic), resin 18, solid lubs. 10 | Graphite/Sb2S3/MoS2 different ratios | 7 Graphite + 3 Sb2S3 best fade resistance and lowest wear [20] | 0.45 0.5 |
| vol %: fibers 17, organics 40, solid lubs. 18, abrasives 4 | Graphite: 0–9 Sb2S3: 9–0 | Both solid lubs. needed. Best performance: graphite 6, Sb2S3 3 [29] | 0.35 0.42 |
| vol %: fibers 21, resin 35, solid lubs. 0–25, abrasives 4 | 0%, 5%, 10%, 15%, 20%, 25% of either talcum, h-BN or petroleum coke | Best performance properties with 10 vol % h-BN [34] | 0.45 0.45 |
| P/M pad against C/C-SiC disc wt %: metal particles 84–100, graphite 0–15, abrasives 0–5 | Metal 100 Metal 85/graphite 15 Metal 95/SiC5 | Effect of graphite : increased COF (0.6) and wear [19] | 0.4–0.5 0.3–0.6 |
| P/M pad against steel disc wt %: metal particles (Cu) 90 Solid lubs. 10, abrasives 6 (SiC) | Graphite/h-BN ratio | With increasing graphite content COF/wear decreased. Graphite + h-BN stabilized COF [22] | 0.4–0.5 no data |
| wt %: fibers 33, resin 10, graphite 10, abrasives? | Graphite particle size | COF decreased with increasing particle size [35] | 0.45 no data |
| wt %: fibers 33, resin 10, graphite 10, abrasives not specified | Graphite particle size | Best performance for lowest particle size (21 µm) [36] | no data 0.3–0.4 |
| vol %: fibers 6–11, organics 45, solid lubs. 10, coke 14–17 abrasives 2–5 | Towards eco-friendly: Cu-free, Sb2S3-free, no ceramic whiskers | Combination of 14 coke, 10 graphite and 0.4 MoS2 provided good brake performance [37] | 0.4–0.5 0.3–0.4 |
| vol %: fibers 50, organics 35, solid lubs. 4.8 (Sb2S3 only), abrasives 0/5.6 | Al2O3 content: 0% and 5.6% | Overall higher COF with Al2O3. Unexpected fade ascribed to Sb instead of Sb2O3-formation [28] | 0.3/0.5 0.2/0.4 |
| P/M pad against cat iron disc vol %: metal particles 80 (Cu), solid lubs. 10, abrasives 10 | Type of solid lub. either graphite, h-BN or MoS2 | Ranking for best brake performance: MoS2 > graphite > h-BN [21] | 0.2–0.3 |
| Reference | Methods | Observations | Authors’ Interpretation | Comments |
|---|---|---|---|---|
| [43] 1973 | LM TEM | Much less coarse and fine asbestos fibers observed, as expected. | Most asbestos converted to olivine and stored at surface as tribofilm. | Olivine mixed with other wear products has formed a tribofilm. |
| [44] 1973 | EA TGA PGC | Thermal degradation of phenolic resin during braking. | Formation of a residual polymer which is aromatic hydrocarbon in nature. | Formation of a soft solid state wear product which is mixed with other wear debris. |
| [45] 1978 | SEM EDS XRD | Mix of debris from pad and rotor forming tribofilms. Reduction of Fe and graphite grain size (XRD). Enrichment of inorganic species near pad surface. | No oxide observed at low temperature attributed to continuous removal. Plastically deformed layer and transferfilm at disc surface identified. | Almost all interpretations consistent with most recent findings. Exception: no iron oxide observed at low temp. This may be due to very thin tribofilm. |
| [46] 1978 | X-LM TGA XRD XFA | Changes of tribo-affected zone with increasing temperature: More inorganic, less asbestos, less polymer species. | Increasing wear with increasing temperature, complex material changes. Black tribofilm interpreted as carbonaceous material | Black tribofilm at elevated temperatures consists of magnetite mixed with carbonaceous material. |
| [47] 1980 | LM SEM EDS MH | Wear particles consisting of iron and iron oxide. Cu transfer to disc. | Formation of metallic iron and copper was attributed to hydrogen evolution during resin degradation. | Observations confirmed by later studies. Role of hydrogen not confirmed. |
| [48] 1989 | LM SEM PGC | Incompletely oxidized metal and graphites. 430 °C wear debris contained degraded resin. | Tribofilm continuously formed by compaction of wear debris, and sheared on both counterbodies. | This interpretation is up to date. |
| [49] 1994 | XPS EDS | MoS2 and Ba SO4 not stable but transformed to MoO3 and BaO, respectively. | Although, transferfilm chemistry depends on abrasive addition, little effect on friction and wear. | MoOx confirmed [50] BaO not confirmed by additional studies. |
| [51] 1999 | LM SEM EDS TEM-ER | Tribofilm from wear debris. 3rd body differs from original materials. Aramid-based tribofilm. | Good coverage of surface with tribofilm formed from wear debris provides wear protection. | The general conclusion is correct. The role of aramid for tribofilm formation not confirmed by further studies. |
| [27] 1999 | SEM EDS AES | Occasional transfer of pad material to disc. FeO and C at the surface (AES). | No tribofilm formed at low temperature, only at high temperature (SEM/EDS). | Thin tribofilms not detected by SEM/EDS. FeO not confirmed. State of the art: Fe3O4. |
| [52] 2000 | SEM OP IIT | Primary and secondary contact plateaus. Ultra fine-grained top layer. High hardness of this layer. | Wear particle flow between protruding plateaus. Compaction and tribo-sintering of particles. FeO and/or Fe3O4 (EDS). No signs of C in tribofilm. | State of the art description of the contact situation on the µm-scale Misinterpretation of nanoscale features (appl. methods not appropriate). |
| [53] 2000 | PSF ELPI EAA PIXE | Significant fraction of airborne wear particles has diameters <100 nm. Fe was major element of sampled particles. | “Fiber emissions are not a concern”, because of decomposition. Hydrocarbons may be ejected at higher temperatures. | Since O was not measured by PIXE, Fe3O4 nanoparticles are not only the major species of tribofilms, but also of airborne dust. |
| [54] 2001 | LM at glass disc | Particle flow visualized. | In-situ observation of secondary contact creation. | Previous hypotheses confirmed |
| [55] 2001 | SEM EDS X-TEM SAED XPS | Major species of tribofilm: Nano-barite mixed with other pad constituents (alloyed barite). | Hard ingredients like quartz served as primary plateaus. Barite-based 3rd body material forms secondary plateaus. | The unconventional pad material contained 48% barite. Barite-based tribofilm is not typical for commercial pad materials. |
| [55] 2002 | LM GA-XRD X-SEM TEM-ER EDS | Heterogeneous tribofilm containing fragments of all pad constituents and reaction products in a matrix of Fe- and Cu-oxides. TEM revealed crystalline carbonaceous film (<10 nm thick). | Tribolayer consisting of fragments of pad ingredients in Fe/Cu-oxide matrix covered with carbonaceous tribofilm. | Fe-oxide based tribolayers confirmed by many other studies. Cu-oxide rarely observed, but rather metallic copper particles. Nanocrystalline carbonaceous film not confirmed. Could have been confused with nc magnetite-based film. |
| [56] 2002 | TEM | Particles scratched from pad surface after braking tests. Nanocrystalline iron oxide (Fe2O3) identified. | Tribooxidation of disc and steel fibers and transformation of asbestos to olivine has occurred during braking. | Fe2O3 was not confirmed as major phase during subsequent studies, but rather Fe3O4. |
| [57] 2004 | SEM EDS TGA | Wear particles of real brake sampled on filter show all pad constituents as well as Fe or Fe-oxide. | Wear product coinsists of fragmented constituents (pad), Fe-oxide from disc and degraded resin. | SEM/EDS revealed the coarse fraction of the wear debris and Fe-containing agglomerates of the fine fraction. |
| [58] 2004 | DLS SEM EDS | Bimodal size distribution (nano/micro) of wear particles deposited near the friction couple. Nano fraction: high concentration of Fe, O, C. | Sub-micron particles: wear product of disc. Micron-sized particles: wear product of pads. | Agglomerates of fully processed third body consist mainly of iron oxide. Micron-sized particles represent early stages of pad wear. |
| [59] 2004 | LM SEM/EDS FIB/SIM X-SEM X-TEM | Areas covered by nc-tribofilm on pad revealed in bright contrast in SIM. Tribofilm and wear particles mainly consist of iron oxides; Cu-oxides rarely observed; Cu and Zn-transfer to disc. | Tribooxidation of metallic constituents provides the major part of wear debris and tribofilm. More Cu- and less Fe-oxide at pad surface; the other way round for disc. Zn provides film adhesion. | Fe-oxides confirmed. Role of Cu-oxides not clear. Role of Zn transfer not confirmed, most likely over-estimated. |
| [60] 2004 | LSM SIM | Contact areas identified by SIM are smooth areas, but not clearly identified as protruding plateaus. | Not only the protruding plateaus, but also deeper areas may have been contacting areas. | This interpretation did not consider flow of 3rd body particles and the possibility of particle trapping in troughs. |
| [61] 2005 | X-SEM ERM | Transfer layers in a thickness range 10–50 µm were obtained by drag tests at elevated temperatures. | Solid lubricants exert great impact on transfer layer thickness. No effect on COF but smoother sliding. | The formation of such thick transfer layers is rarely observed in practice. |
| [62] 2006 | SEM EDS XFA | Examples of binding solid lubricants to meso-scale pad constituents observed for commercial pad materials. | Distribution and retention of solid lubricants at the pad surface is an important issue. | Ingredients must be available everywhere at the rubbing interfaces for being incorporated into the 3rd body. |
| [63] 2006 | FIB/TEM GDOS RS | Fe3O4-based tribofilm also contains amorphous and graphite-like C (RS). Ca-, S- and Cu-transfer to disc (GDOS). | Tribooxidation of Fe- constituents and mixing with graphite and other solid lubricants occurs on the nanometer scale. | Ca-enrichment at the disc surface not reported by other studies, except one [34]. |
| [64] 2007 | TGA MS SEM | Oxidation of resin into volatile species at 300–600 °C. Mechanical activation lowers temperature range. | Good friction performance even at high temperatures attributed to 3rd body layers formed during degradation of resin. | Obviously, secondary plateaus were retained at elevated temperatures (SEM). |
| [34] 2007 | SEM EDS | EDS-maps suggest more material transfer from pad to disc for formulations with addition of sol. lubs. | The observed material transfer stabilized the COF and led to reduced wear. | Obviously an unconventional CaCO3-based film has formed, because of high CaCO3-content of the pad. |
| [65] 2007 | FIB/SIM TEM/EDS | Plastically deformed layer below tribofilm at disc surface. Nc Fe3O4-based tribofilm mixed with small amount of pad constituents. | Mixing of Fe3O4 with soft nanoparticles from pad ingredients is a prerequisite for obtaining smooth sliding conditions. | This finding defined the basic structure for modelling the sliding behaviour of tribofilms, as discussed in Chapter 3. |
| [66] 2008 | X-LM X-SEM GA-XRD | Zone of severe plastic deformation. Discontinuous tribofilm 3rd body mainly Fe-oxide Transfer of reaction products to disc surface. | Since nanocrystalline wear particles were observed, their potential risks have to be considered in the future. | Identification of reaction products at disc surface by GA-XRD not confirmed by further studies. |
| [67] 2009 | LM X-SEM EDS FIB EF-TEM | Cross-section with pad still pressed against disc showed continuous 3rd body layer. Nasnoscale elemental mapping revealed C and Cu nanoparticles and submicron-sized Al2O3. | More 3rd body present at the interface while the pad is still pressed against the disc than usually observed during post mortem studies. | Complicated 3rd body structure with different submicron- or even micron-sized particles (depending on the thickness of the tribofilm) embedded in multi-phase nc-matrix. |
| [68] 2009 | SMPS TEM EF-TEM HR-TEM | Nanoparticle emissions correlated with fading cycles. Nanostructure of collected dust particles. | Most pad constituents and Fe3O4 observed in a single nanoparticle of approximately 300 nm diameter. | Final state of 3rd body formation leads to mixing of wear products on the nanometer scale |
| [69] 2009 | SEM | Cavities formed around steel fibers which then were filled with wear debris under dry conditions and with water under wet conditions. | COF-increase with pressure attributed to increase of real contact area. COF-decrease under wet conditions attributed to wear debris removal and mixed lubrication. | The study shows that mechanisms taking place at the meso- and micro-scale have to be kept in mind. |
| [70] 2010 | PSS MöS TEM FTIR TGA | Submicron-sized wear particles detected. Fe2+, Fe3+ and Fe0 Core-shell Fe-Fe3O4-particles in matrix of amorphous carbon. Resin degradation observed. Resin degradation confirmed. | C-based ingredients (graphite, resin, coke …) all transformed to amorphous C, thus explaining the high fraction of amorphous carbon in the wear product. | The high amount of amorphous carbon in the wear product was not corroborated by other studies. Actually the investigated formulation contained much C. On the other hand, TEM always considers only very small volumes, and thus uncloses the danger of over-interpretation. |
| [37] 2010 | SEM EDS | Different pad materials all covered with Fe-oxide based tribofilm. | Cu- and Sb-free pad forms similar tribofilm as conventional materials. | |
| [71] 2010 | TEM/EDS EF-TEM HR-TEM | Nc- and multiphase-structure of Fe3O4-based tribofilm revealed. | Smooth sliding attributed to tribofilm structure, as shown by modelling. | Contrary to [70], it was observed that Fe3O4 is the matrix which contains C inclusions. |
| [72] 2010 | EEPS CI EF-TEM STEM EDS | Airborne particles down to diameters of some few nanometers detected. Collected particles are agglomerates of nanoparticles of pad ingredients and Fe3O4. | Differences of pad formulations mirrored in differences of composition of nanoparticle-agglomerates. | Nanostructure and composition of airborne dust particles provide information of tribofilm structure. |
| [73] 2010 | FIB TEM EF-TEM | Cu- and barite-nanoparticles (100 nm) embedded in Fe3O4-matrix. | Similar COF-stabilization effect of Cu-nanoparticles as graphite nanoparticles shown by modelling. | |
| [74] 2011 | GA-XRD RS X-FIB EF-TEM HR-TEM | Fe3O4 on rubbed disc, disordered graphite, graphite lamella, exfoliated nano-graphite and graphite embedded in Fe3O4 observed. | Shear-induced crack at graphite-pearlite interface provides access of O thus enabling Fe3O4-formation, graphite nanosheet exfoliation and embedding. | This is only one mechanism of formation of graphite-like species. |
| [75] 2011 | STEM/EDS HR-TEM X-TEM | Further example of Fe3O4-based tribofilm. Thick film consisting of 3 layers, formed during fading cycle. | Soft nanoinclusions in brittle Fe3O4 matrix provide smooth sliding. | |
| [28] 2011 | SEM/EDS GA-XRD | Besides Fe3O4-based 3rd body, also Sb-sulfide and Sb-Fe particles were observed at pad surface. | The results suggest reduction of Sb2S3 to metallic Sb and alloying with Fe. | Unusual finding, not confirmed by other studies. |
| [76] 2012 | EF-TEM HR-TEM | Extension of [73]. Cu, barite and zirconia nano-particles embedded in Fe3O4. Magnetite and graphite nanocrystals shown by lattice fringes. | Not only soft, but also hard nanoinclusions (ZrO2) identified in a tribofilm formed by dynamometer testing. | |
| [77] 2013 | SEM EDS | Transfer of barite, Cu and Zn from pad to disc. | The film consists of milled and compacted wear debris of the pad. | Most likely, the film consists mainly of Fe3O4; separation from strong Fe-peak of substrate not possible by SEM/EDS. |
| [78] 2014 | FIB/SIM ATB-KT | Example of film built from powder particles. COF of Fe3O4 + 15% graphite: 0.25 and Fe3O4 + 15% graphite + 5% SiC: 0.35. | Artificial third bodies are useful for systematic studies on pad formulations and for verification of modelling results. | |
| [40] 2014 | SEM/EDS FIB/X-SEM | Fe, C, O, S, Mg, Ba, Zn, Sn, Si, Al all observed at rubbed pad surface. | Reaction of metal powders with solid lubricants considered as beneficial. | Reaction products could not be identified unambiguously with the applied methods. |
| [42] 2015 | SEM X-SEM EDS XRD | Fe-Zr mixed oxides in films and wear debris. XRD: mainly Fe2O3, also metallic copper. | Released and fragmented hard particles from pad mixed with Fe-oxide from disc. Cu needed for binding constituents of the tribofilm together. | In principle in accord with previous findings, but hematite instead of magnetite is unlikely, because of the black colour of brake dust. |
| Acronym | Method |
|---|---|
| AES | Auger Electron Spectroscopy |
| ATB-KT | Artificial Third Body-Kato Test |
| CI | Cascade Impactor |
| COF | Coefficient of Friction |
| DLS | Dynamic Light Scattering |
| EA | Elemental Analysis |
| EAA | Electrical Aerosol Analyser |
| EDS | Energy Dispersive X-ray Spectroscopy |
| EEPS | Engine Exhaust Particle Sizer |
| EF-TEM | Energy-filtered TEM |
| ELPI | Electrical Low Pressure Impactor |
| ERM | Electrical Resistance Measurement |
| GDOES | Glow Discharge Optical Emission Spectroscopy |
| GA-XRD | Grazing Angle X-ray Diffraction |
| FIB | Focused Ion Beam |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HR-TEM | High Resolution Transmission Electron Microscopy |
| IIT | Instrumented Indentation Testing (Nanoindentation) |
| LM | Light Microscopy |
| LSM | Laser Scanning Microscopy |
| MH | Micro Hardness |
| MS | Mass Spectroscopy |
| MöS | Mössbauer Spectroscopy |
| Nc | Nanocrystalline |
| OP | Optical Profilometry |
| PGC | Pyrolisis Gas Chromatography |
| PIXE | Proton Induced X-ray Emission |
| PSF | Particle Sampling on Filter |
| PSS | Particle Sampling by Sweeping the test chamber |
| RS | Raman Spectroscopy |
| SEM | Scanning Electron Microscopy |
| SIM | Scanning Ion Microscopy |
| SMPS | Scanning Mobility Particle Sizer |
| STEM | Scanning Transmission Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| TEM-ER | Transmission Electron Microscopy of Extraction Replica |
| TGA | Thermogravimetric Analysis |
| XFA | X-ray Fluorescence Analysis |
| X-LM | Cross-sectional LM |
| X-SEM | Cross-sectional SEM |
| X-TEM | Cross-sectional TEM |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
| Reference | Modeled Structures (vol %) M: Magnetite, C: Graphite | Results (beyond Already Known) | Comments |
|---|---|---|---|
| [65] 2007 |
|
| 1., 4.: High COF fluctuations. Modeling parameters not yet optimized. |
| [91] 2008 | M + xC/M + xC, C = 5–27 Pressure variation: 15–53 MPa | C = 5: no MML, unstable sliding, COF = 0.5 C = 27: MML, smooth sliding, COF = 0.3. Decreasing COF with increasing pressure correlated with MML-thickness | COF = f(p) can explain lower COF compared to [65]. |
| [96] 2008 | M + 5C/M + 5C M + 27C/M + 27C | Some more graphite distributions tested. Same quantitative results as [91] | No difference of mean COF. |
| [90] 2008 |
|
| p-range: 20–230 MPa. Different for 1., 2., 3. And 4. |
| [97] 2008 | M/M at different pressures | COF = 0.6 at 30 MPa drops to COF = 0.4 at 50 MPa, although no MML is formed | Similar result for M + 5C. |
| [98] 2010 | Impact of graphite concentration (0%–30%) and pressure (15–55 MPa) | Data showing pressure dependency of COF for graphite contents: 0%, 5.5%, 13%, 17.5%, 27% in Fe3O4-based tribofilms | Visualization of particle velocity vectors. |
| [73] 2010 |
|
| Prerequisite for similar behavior as graphite: Recrystallized Cu, assumed to form at high T. |
| [93] 2011 | M + 5.5C + 5.5Cu-clusters/M + 5.5C + 5.5Cu-clusters | Similar behavior as M + 11C, i.e., MML formed, Cu-clusters dissolved within MML | Cu-clusters observed in [73]. |
| [94] 2011 |
|
| Good correlation with experiments. |
| [99] 2012 | M + 13C agglomerates sliding against each other, p = 30 MPa | Similar COF as compact films | [94] confirmed. |
| [95] 2012 |
|
| Beneficial effect of Cu-clusters predicted. |
| [76] 2012 |
| Visualization of particle movement (velocity vectors) for the two structures. | Review of other structures. |
| [100] 2013 | Pressure dependencies for:
|
| |
| [101] 2014 |
| Only slight impact of SiC content on COF as function of p (range 0.35–0.2). More pronounced effect on frictional energy. | Hypothetical study, not related to brakes. |
| [92] 2014 | M + 13C/M + 13C Hypothetic considerations how elevated temperatures may affect mechanical properties of constituents and sliding behavior. | If the temperatures are high enough to make the oxide ductile, the model predicts a change of the tribological behavior: Increasing COF-pressure dependency predicted with increasing temperature. COF may drop from 0.35 to 0.2 at p = 40 MPa. | Velocity reduction from 10 to 1 m/s: no difference of final structure and COF. |
| [78] 2014 | Summary of previous findings | The increase of COF during a stop braking event can be explained by linking MCA-results with patch dynamics [85,86,102] | Loose wear particles play an important role. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Österle, W.; Dmitriev, A.I. The Role of Solid Lubricants for Brake Friction Materials. Lubricants 2016, 4, 5. https://doi.org/10.3390/lubricants4010005
Österle W, Dmitriev AI. The Role of Solid Lubricants for Brake Friction Materials. Lubricants. 2016; 4(1):5. https://doi.org/10.3390/lubricants4010005
Chicago/Turabian StyleÖsterle, Werner, and Andrey I. Dmitriev. 2016. "The Role of Solid Lubricants for Brake Friction Materials" Lubricants 4, no. 1: 5. https://doi.org/10.3390/lubricants4010005
APA StyleÖsterle, W., & Dmitriev, A. I. (2016). The Role of Solid Lubricants for Brake Friction Materials. Lubricants, 4(1), 5. https://doi.org/10.3390/lubricants4010005







