Tribofilm Formation As a Result of Complex Interaction at the Tool/Chip Interface during Cutting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermodynamics of Tribofilms
- dSi/dt—the entropy production as a result of the distribution of heat and other flows within a body;
- dSe/dt—the flow of entropy due to external interactions, which could change due to the transition of heat from a frictional surface with a higher temperature into a body (the cutting tool) with a lower temperature, resulting in an entropy increase;
- dSm/dt—the change of entropy due to the formation of chemical compounds on the tool surface due to the entropy of the matter transferred from the contacting frictional body (the workpiece). In the case of the tool-chip interaction, it can be either positive, due to cutting tool-chip interactions with the further formation of seizure zones or build-ups that tend to break away and eventually cause deep surface damage [23], or negative when the material transformed to the tool surface either forms lubricants (MnS for example) or interacts with the environment (tribo-oxidation), forming lubricating tribofilms [1,23];
- dSf/dt—the change of entropy due to tribofilm formation as a result of the surface modification of the cutting tools with further tribo-oxidation;
- dSw/dt—the change of entropy due to the wear process (the “−” sign is used in the Equation 2, because the products of the wear process leave the frictional body with their own entropy). The change of entropy, dSf/dt, could be negative if non-equilibrium processes are occurring on the surface and the entropy of a frictional body is reduced (which is typical for cutting tools [24,25]), or it could be positive if there are equilibrium processes [9]. However, the sum of these terms, i.e., dSi/dt + dSf/dt > 0, is the general entropy production [18]. As follows from (2), lower entropy production corresponds to a lower dSw/dt value. Bearing in mind that entropy is an additive value, we can consider that the lower dSw/dt, the lower the wear rate. Therefore, the entropy production decrease leads to wear rate reduction. Non-equilibrium processes on the surface (dSf/dt < 0) and the formation of lubricating tribofilms due to material transfer from the workpiece with its further tribo-oxidation (dSm/dt < 0), with all other conditions being equal, can also lead to a wear rate decrease.
2.2. Experimental Examples of Tribofilm Formation
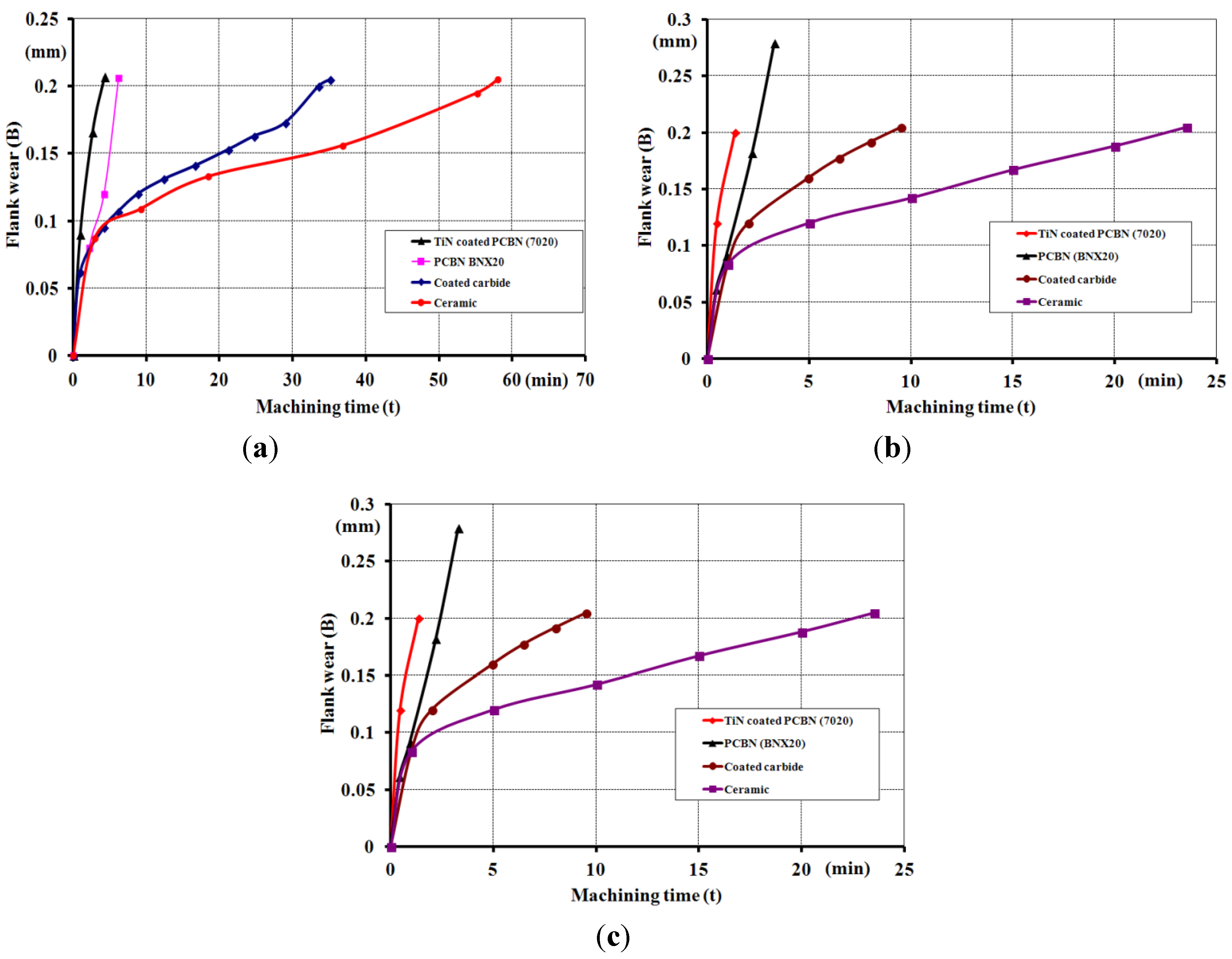
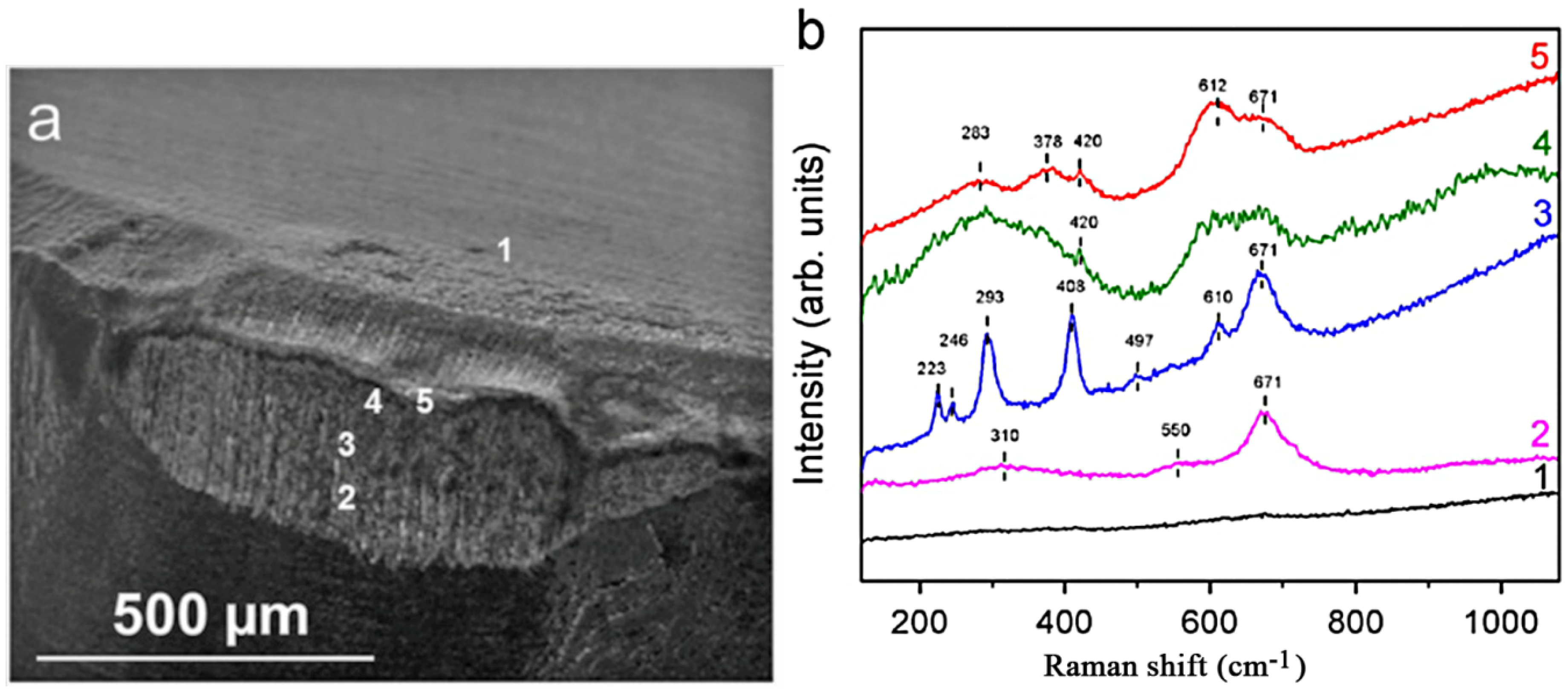
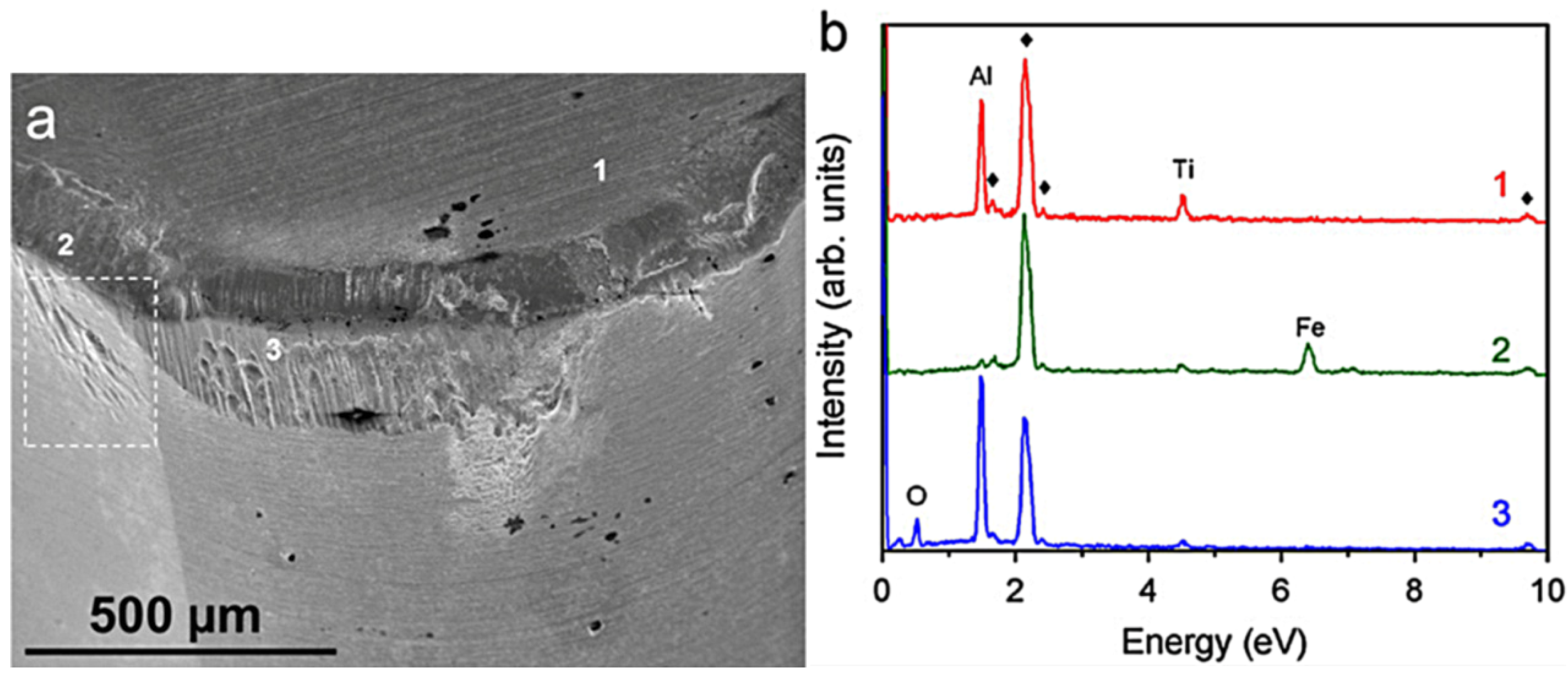


3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacobson, S.; Hogmark, S. Tribofilms—On the crucial importance of tribologically induced surface modification. In Recent Developments in Wear Prevention, Friction and Lubrication; Nikas, G.K., Ed.; Research Signpost: Kerala, India, 2010; p. 19. [Google Scholar]
- Morina, A.; Neville, A. Tribofilms: Aspects of formation, stability and removal. J. Phys. D Appl. Phys. 2007, 40, 5476–5487. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S. Adaptive hard coatings design based on the concept of self-organization during friction. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.-W., Eds.; Springer: London, UK, 2013; pp. 16–23. [Google Scholar]
- Bushe, N.; Gershman, I. Tribological compatibility and nanotribological characteristics. In Self-Organization during Friction: Advance Surface Engineered Materials and Systems Design; Fox-Rabinovich, G.S., Totten, G., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, NW, USA, 2006. [Google Scholar]
- Fox-Rabinovich, G.S. Principles of friction control for the surface engineered materials. In Self-organization during Friction: Advanced Surface Engineered Materials and Systems Design; Fox-Rabinovich, G.S., Totten, G., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, NW, USA, 2006; pp. 3–13. [Google Scholar]
- Kostetsky, B.I. Structural-energetic adaptation of materials at friction. Frict. Wear 1985, 6, 201–212. [Google Scholar]
- Kostetsky, B.I. An evolution of the materials’ structure and phase composition and the mechanisms of the self-organizing phenomenon at external friction. Frict. Wear 1993, 14, 773–783. [Google Scholar]
- Bershadskiy, L.I. On self-organizing and concept of tribosystem self-organizing. Frict. Wear 1992, 8, 1077–1094. [Google Scholar]
- Gershman, J.S.; Bushe, N.A. Thin films and self-organization during friction under the current collection conditions. Surf. Coat. Technol. 2004, 186, 405–411. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Yamamoto, K.; Beake, B.D.; Gershman, I.S.; Kovalev, A.I.; Aguirre, M.H.; Veldhuis, S.C.; Dosbaeva, G.; Endrino, J.L. Hierarchical adaptive nano-structured PVD coatings for extreme tribological applications: The quest for non-equilibrium states and emergent behavior. Sci. Technol. Adv. Mater. (STAM) 2012, 13. [Google Scholar] [CrossRef]
- Bushe, N.A.; Kopitko, V.V. Compatibility of Rubbing Surfaces; Nauka: Moscow, Russia, 1981. [Google Scholar]
- Kostetsky, B.I. Friction, Lubrication and Wear in Machines; Technika: Kiev, Ukraine, 1970. [Google Scholar]
- Ivanova, V.S.; Bushe, N.A.; Gershman, I.S. Structure adaptation at friction as a process of self-organization. Frict. Wear 1997, 18, 74–79. [Google Scholar]
- Kostetskaya, N.B. Structure and Energetic Criteria of Materials and Mechanisms Wear-Resistance Evaluation. Ph.D. Thesis, Kiev State University, Kiev, Ukraine, 1985. [Google Scholar]
- Kostetskaya, N.B. Mechanisms of deformation, fracture and wear particles forming during the mechanical-chemical friction. Frict. Wear 1990, 16, 108–115. [Google Scholar]
- Ivanova, V.S. Fracture synergetic and mechanical properties. In Synergetic and Fatigue Fracture of Metals; Nauka: Moscow, Russia, 1989; pp. 6–27. [Google Scholar]
- Kabaldin, Y.G.; Kojevnikov, N.V.; Kravchuk, K.V. HSS cutting tool wear resistance study. Frict. Wear 1990, 11, 130–135. [Google Scholar]
- Gershman, I.S.; Bushe, N.A. Fundamentals of Non-Equilibrium Thermodynamics and Self-organization at Friction. In Self-organization during Friction: Advanced Surface Engineered Materials and Systems Design; Fox-Rabinovich, G.S., Totten, G., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, NW, USA, 2006; pp. 13–59. [Google Scholar]
- Gershman, I.S.; Bushe, N.A. Compatibility of tribo-systems. In Self-organization during Friction: Advanced Surface Engineered Materials and Systems Design; Fox-Rabinovich, G.S., Totten, G., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, NW, USA, 2006; pp. 59–81. [Google Scholar]
- Bouzakis, K.D.; Michailidis, N.; Scordaris, G.; Bouzakis, E.; Biermann, D.; M’Saoubi, R. Cutting with coated tools: Coating technologies, characterization methods and performance optimization. CIRP Ann. Manuf. Technol. 2012, 61, 703–723. [Google Scholar] [CrossRef]
- Ning, L.; Veldhuis, S.C.; Yamamoto, K. Investigation of wear behavior and chip formation for cutting tools with nano-multilayered TiAlCrN/NbN PVD coating. Int. J. Mach. Tools Manuf. 2008, 48, 656–665. [Google Scholar] [CrossRef]
- Astakhov, V.P. Tribology of Metal Cutting; Elsevier: London, UK, 2006. [Google Scholar]
- Wright, P.; Trent, E. Metal Cutting, 4th ed.; Butterworth-Heinemann: Boston, MA, USA, 2000. [Google Scholar]
- Fox-Rabinovich, G.S.; Kovalev, A.I.; Veldhuis, S.C.; Shuster, L.S.; Gershman, I.S. Surface Engineered Tool Materials for High Performance Machining. In Self-organization during Friction: Advanced Surface Engineered Materials and Systems Design; Fox-Rabinovich, G.S., Totten, G., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, NW, USA, 2006; pp. 231–297. [Google Scholar]
- Mah, T.I.; Mazdiyasni, K.S. Mechanical properties of mullite. J. Am. Ceram. Soc. 1983, 66, 699–703. [Google Scholar] [CrossRef]
- Hulse, C.O.; Pask, J.A. Analysis of deformation of fireclay refractory. J. Am. Ceram. Soc. 1966, 49, 312–318. [Google Scholar] [CrossRef]
- Lessing, P.A.; Gordon, R.S.; Mazdiyasni, K.S. Creep of polycrystalline mullite. J. Am. Ceram. Soc. 1975, 58. [Google Scholar] [CrossRef]
- Osendi, M.I.; Baudín, C. Mullitematerials: Microstructural evolution. J. Eur. Ceram. Soc. 1996, 16, 217–224. [Google Scholar]
- Aksay, I.A.; Dabbs, D.M.; Sarikaya, M. Mullite for structural, electronic and optical applications. J. Am. Ceram. Soc. 1991, 74, 2343–2358. [Google Scholar] [CrossRef]
- Hynes, A.P.; Doremus, R.H. High-temperature compressive creep of polycrystallinemullite. J. Am. Ceram. Soc. 1991, 74, 2469–2475. [Google Scholar]
- El Hakim, M.A.; Abad, M.D.; Abdelhameed, M.M.; Shalaby, M.A.; Veldhuis, S.C. Wear behavior of some cutting tool materials in hard turning of HSS. Tribol. Int. 2011, 44, 1174–1181. [Google Scholar]
- Fox-Rabinovich, G.; Kovalev, A.; Aguirre, M.H.; Yamamoto, K.; Gershman, I.; Rashkovskiy, A.; Endrino, J.L.; Beake, B.; Veldhuis, S.; Dosbaeva, G.; et al. Evolution of self-organization in nano-structured PVD coatings under extreme tribological conditions. Appl. Surf. Sci. 2014, 297, 22–32. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Endrino, J.L.; Agguire, M.H.; Beake, B.D.; Veldhuis, S.C.; Kovalev, A.I.; Gershman, I.S.; Yamamoto, K.; Losset, Y.; Wainstein, D.L.; et al. Mechanism of adaptability for the nano-structured TiAlCrSiYN-based hard PVD coatings under extreme frictional conditions. J. Appl. Phys. 2012, 111. [Google Scholar] [CrossRef]
- Beake, B.D.; Fox-Rabinovich, G.S.; Losset, Y.; Yamamoto, K.; Agguire, M.H.; Veldhuis, S.C.; Endrino, J.L.; Kovalev, A.I. Why can TiAlCrSiYN-based adaptive coatings deliver exceptional performance under extreme frictional conditions? Faraday Discuss. 2012, 156, 1–11. [Google Scholar] [CrossRef]
- Kovalev, A.; Wainstein, D.; Rashkovskiy, A.; Fox-Rabinovich, G.; Veldhuis, S.; Agguire, M.; Yamamoto, K. Investigation of electronic and atomic structure of tribofilms on the surface of cutting tools with TiAlCrSiYN and multilayer TiAlCrSiYN/TiAlCrN coatings during machining of hardened steels. Surf. Interface Anal. 2010, 42, 1368–1372. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Yamamoto, K.; Beake, B.D.; Kovalev, A.I.; Aguirre, M.H.; Veldhuis, S.C.; Dosbaeva, G.K.; Wainstein, D.L.; Biksa, A.; Rashkovskiy, A. Emergent behavior of nano-multilayered coatings during dry high speed machining of hardened tool steels. Surf. Coat. Technol. 2010, 204, 3425–3435. [Google Scholar] [CrossRef]
- Kovalev, A.; Wainstein, D.; Fox-Rabinovich, G.; Veldhuis, S.; Yamamoto, K. Features of self-organization in nanostructuring PVD coatings on a base of polyvalent metal nitrides under severe tribological conditions. Surf. Interface Anal. 2008, 40, 881–884. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Veldhuis, S.C.; Dosbaeva, G.K.; Yamamoto, K.; Gershman, I.S.; Kovalev, A.; Beake, B.D.; Shuster, L.S. Nano-crystalline coating design for extreme applications based on the concept of complex adaptive behavior. J. Appl. Phys. 2008, 103. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.; Kovalev, A.; Gershman, I.; Korshunov, S.; Veldhuis, S.; Beake, B.; Endrino, J.; Wainstein, D.L. Features of self-organization in ion modified nano-crystalline PVD AlTiN coatings under severe tribological conditions. JAP 2007, 102. [Google Scholar] [CrossRef]
- Biksa, A.; Yamamoto, K.; Dosbaeva, G.; Veldhuis, S.C.; Fox-Rabinovich, G.S.; Wagg, T.; Elfizy, A.; Shusterd, L.S. Wear behavior of adaptive nano-multilayered AlTiN/MexN PVD coatings during machining of aerospace alloys. Tribol. Int. 2010, 43, 1491–1499. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Yamamoto, K.; Kovalev, A.I.; Veldhuis, S.C.; Ning, L.; Shuster, L.S. Wear behavior of adaptive nano-multilayered TiAlCrN/NbN coatings under dry high performance machining conditions. Surf. Coat. Technol. 2008, 202, 2015–2022. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Yamamoto, K.; Veldhuis, S.C.; Kovalev, A.; Shuster, L.S.; Ning, L. Self-adaptive wear behavior of nano-multilayered TiAlCrN/WN coatings under severe machining conditions. Surf. Coat. Technol. 2006, 201, 1852–1860. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.S.; Yamamoto, K.; Veldhuis, S.C.; Kovalev, A.; Dosbaeva, G.K. Tribological Adaptability of TiAlCrN PVD Coatings under High Performance Dry Machining Conditions. Surf. Coat. Technol. 2005, 200, 1804–1813. [Google Scholar] [CrossRef]
- Hovsepian, P.E.; Luo, Q.; Robinson, G.; Pittman, M.; Howarth, M.; Doerwald, D. TiAlN/VN superlattice structured PVD coatings: A new alternative in machining of aluminium alloys for aerospace and automotive components. Surf. Coat. Technol. 2006, 201, 265–270. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Hultman, L.; Clemens, H. Microstructural design of hard coatings. Prog. Mater. Sci. 2006, 51, 1032–1114. [Google Scholar] [CrossRef]
- Shalaby, M.A.; El Hakim, M.A.; Abdelhameed, M.M.; Krzanowski, J.E.; Veldhuis, S.C.; Dosbaeva, S.C. Wear mechanisms of several cutting tool materials in hard turning of high carbon-chromium tool steel. Tribol. Int. 2014, 70, 148–154. [Google Scholar] [CrossRef]
- Veldhuis, S.C.; Dosbaeva, G.K.; Yamamoto, K. Tribological compatibility and improvement of machining productivity and surface integrity. Tribol. Int. 2009, 42, 1004–1010. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fox-Rabinovich, G.S.; Gershman, I.; Hakim, M.A.E.; Shalaby, M.A.; Krzanowski, J.E.; Veldhuis, S.C. Tribofilm Formation As a Result of Complex Interaction at the Tool/Chip Interface during Cutting. Lubricants 2014, 2, 113-123. https://doi.org/10.3390/lubricants2030113
Fox-Rabinovich GS, Gershman I, Hakim MAE, Shalaby MA, Krzanowski JE, Veldhuis SC. Tribofilm Formation As a Result of Complex Interaction at the Tool/Chip Interface during Cutting. Lubricants. 2014; 2(3):113-123. https://doi.org/10.3390/lubricants2030113
Chicago/Turabian StyleFox-Rabinovich, German S., Iosif Gershman, Mohamed A. El Hakim, Mohamed A. Shalaby, James E. Krzanowski, and Stephen C. Veldhuis. 2014. "Tribofilm Formation As a Result of Complex Interaction at the Tool/Chip Interface during Cutting" Lubricants 2, no. 3: 113-123. https://doi.org/10.3390/lubricants2030113
APA StyleFox-Rabinovich, G. S., Gershman, I., Hakim, M. A. E., Shalaby, M. A., Krzanowski, J. E., & Veldhuis, S. C. (2014). Tribofilm Formation As a Result of Complex Interaction at the Tool/Chip Interface during Cutting. Lubricants, 2(3), 113-123. https://doi.org/10.3390/lubricants2030113







