Abstract
This study reports the first synthesis of a new type of ether-polyalphaolefin (DVE-PAO) base oil via free radical bulk copolymerization using triethylene glycol divinyl ether (DVE-3) and α-olefin in drip-feed mode. The characteristic structure of DVE-PAO was characterized by Fourier Transform Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonance Spectroscopy (NMR). The relative molecular weight and molecular weight distribution of DVE-PAO were determined using gel permeation chromatography (GPC). Structurally, it is a new type of base oil that integrates both polyalkylene glycol (PAG) and polyalphaolefin (PAO) structural units. The research shows that the viscosity of DVE-PAO base oil, the conversion rate of α-olefin, and the pour point of the copolymer increase with rising copolymerization temperature. Additionally, results from the rotating oxygen bomb test indicate that the oxidation stability of DVE-PAO also improves with increasing viscosity. Based on the principles of free radical copolymerization, this study provides a preliminary elucidation of the copolymerization patterns between the aforementioned double-ended vinyl ethers and α-olefins. Furthermore, the DVE-PAO base oil exhibits excellent miscibility with both mineral oils and polyalphaolefin (PAO) base oils. As a result, this ether-based polyalphaolefin is expected to find broad applications in the field of lubricants.
1. Introduction
The rapid development of modern industry has placed higher demands on the service life and operating environment of mechanical equipment, necessitating lubricants with superior overall performance. Although conventional mineral base oils remain one of the most important industrial base oils today, they fall short in meeting the requirements for low volatility, temperature resistance, oxidative stability, and viscosity–temperature characteristics. Consequently, many operating conditions necessitate the use of synthetic or semi-synthetic base oils [1]. In recent years, to meet the lubrication needs of mechanical equipment under various operating conditions, different types of synthetic or semi-synthetic base oils have emerged. Among these, polyalkylene glycol (PAG) base oils and polyalphaolefin (PAO) base oils have garnered increasing attention due to their superior performance.
PAG is a high-performance synthetic base oil obtained by ring-opening polymerization of epoxy compounds such as ethylene oxide, propylene oxide, and butane oxide. It has been successfully used in high-temperature lubricating oil, gear oil, compressor oil, anti-combustion hydraulic fluid, quenching fluid, and metalworking fluid because of its low friction coefficient, high viscosity index, wide temperature range, excellent low-temperature fluidity, excellent oxidation and thermal stability, no sludge, and other characteristics. However, the immiscibility of PAG with mineral oil, PAO, and other base oils greatly limits its application [2,3,4,5,6,7,8].
Compared with mineral oil of the same viscosity, PAO has the advantages of wide operating temperature range, high viscosity index, low pour point, high flash point, low evaporation loss, low toxicity, and good thermal oxidation stability [9,10], and is mainly used for engine oil, hydraulic oil, compressor oil, high temperature gear oil, and bearing oil [11]. However, the low polarity of PAO results in poor frictional properties and poor solubility for polar additives [12].
PAG and PAO base oils each have significant advantages; however, their inherent limitations hinder wider application, particularly due to miscibility issues. Research has been conducted to enhance the solubility of PAG in mineral oils by improving its synthesis methods [13]. Additionally, some studies have explored the enhancement of the frictional properties of base oils by adding PAG as an additive to PAO formulations [14,15]. To date, no study has focused on synthesizing new base oils that combine the characteristics of both PAG and PAO. Therefore, the goal is to create a product that not only addresses compatibility issues between it and mineral oils, PAO, and other base oils but also enhances tribological performance and polar additive solubility.
To address these shortcomings and synergize the benefits of PAG and PAO [1,2,3,4,5,6,7,8,12], this study draws inspiration from the free-radical copolymerization of α-olefins and unsaturated acid esters to synthesize polyester-polyalphaolefins [16,17]. We propose a novel approach utilizing triethylene glycol divinyl ether (DVE-3) and α-olefins to synthesize an ether-polyalphaolefin (DVE-PAO) base oil. Based on this, this research systematically investigates the synthetic reaction conditions, copolymerization mechanism, and miscibility of DVE-PAO base oils to solve the problems of poor compatibility with other base oils and polar additives and inadequate tribological performance.
2. Materials and Methods
2.1. Materials
Triethylene glycol divinyl ether (DVE-3, ≥99.7%) was purchased from Shanghai Dongda Chemical Co., Ltd., Shanghai, China. α-Dodecene was obtained from Shanghai Qixi International Trade Co., Ltd., Shanghai, China. Di-tert-butyl peroxide (DTBP, ≥99.5%) was acquired from Jiangyan Haixiang Chemical Co., Ltd., Taizhou, China. Antioxidant T501 (≥99.7%) was sourced from Shanghai Titan Scientific Co., Ltd., Shanghai, China. Antioxidant L57 (≥99.98%) was sourced from Dongguan Hongli Chemical Technology Co., Ltd., Dongguan, China. The PAG base oils, including KLP32, KLP46, KLP100, and KLP680, were obtained from Nanjing Kailian Chemical New Materials Co., Ltd., Nanjing, China. Mineral base oil 100N and polyalphaolefin base oils PAO10 and PAO20 were procured from Shenzhen Yefeng New Materials Co., Ltd., Shenzhen, China. Tetrahydrofuran (THF, ≥99.9%) and deuterium-labeled chloroform (CDCl3, ≥99.8%) were obtained from Shanghai Titan Technology Co., Ltd., Shanghai, China. All reagents were used as received without further purification.
2.2. Synthesis of DVE-PAO
A 1000 mL three-necked flask equipped with a mechanical stirrer, a reflux condenser, and a temperature sensor was charged with α-dodecene (6.00 eq, 3.00 mol, 504.96 g). The α-dodecene was heated to 120 ± 3 °C using an electric heating mantle and maintained at this temperature under continuous stirring at 300 rpm. A mixture of DVE-3 (1.00 eq, 0.50 mol, 101.13 g) and DTBP (0.41 eq, 0.205 mol, 30.00 g) was prepared in a 250 mL pressure-equalizing dropping funnel. The funnel was then attached to the condenser that was mounted on a three-necked flask. The mixture was added dropwise to the α-dodecene at a constant rate of one drop per 6 s (calibrated via pre-experiment). The addition was completed within 5.0 ± 0.2 h, followed by an additional hour reaction period at 120 ± 3 °C with stirring at 300 rpm.
The same procedure was repeated at 130 °C, 140 °C, 150 °C, and 160 °C, with all other parameters (reactant equivalents, addition rate, stirring speed) held constant.
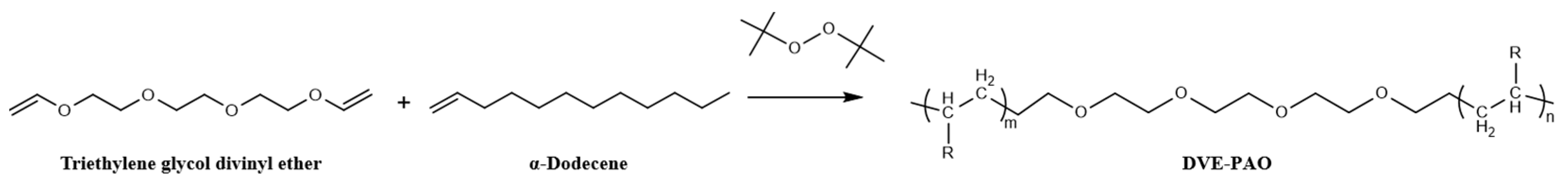
Upon completion of the reaction, the mixture was gradually heated to 230 ± 2 °C using an electric heating mantle and subjected to vacuum distillation to isolate unreacted α-dodecene, yielding the copolymerized product known as DVE-PAO base oil (Scheme 1). The conversion and yield were calculated based on the mass of α-dodecene that participated in the reaction.
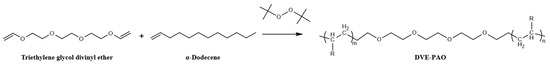
Scheme 1.
Synthesis reaction formula of DVE-PAO base oil.
A molar ratio of α-dodecene to DVE-3 of 6:1 (i.e., 3.00 mol α-dodecene and 0.50 mol DVE-3) was selected in the experimental design. This ratio ensured a significant excess of α-dodecene, allowing for the complete conversion of DVE-3 and minimizing the possibility of DVE-3 self-aggregation. The excess α-dodecene could be recovered by vacuum distillation and reused in subsequent polymerization cycles, a technologically feasible approach for industrial-scale implementation.
2.3. Product Analysis
We employed the following analytical techniques to characterize the structure of DVE-PAO base oils. First, we used a Thermo Scientific Nicolet iS50 high-resolution Fourier transform infrared (FTIR) spectroscopy (Thermo Fisher Scientific Inc., Waltham, MA, USA) to identify the functional groups present. Additionally, we utilized a Bruker AVANCE NEO 400 MHz nuclear magnetic resonance (NMR) spectroscopy system (Bruker (Beijing) Technology Co., Ltd., Beijing, China) to obtain hydrogen (¹H) spectra and carbon (13C) spectra, which enabled us to determine the structural characteristics of the compounds. Finally, an Agilent 1260 Infinity II gel permeation chromatography/size exclusion chromatography (GPC/SEC) system (Agilent Technologies, Inc., Santa Clara, CA, USA) was used to measure the relative molecular weight and molecular weight distribution of the DVE-PAO base oil. Further test conditions are described in the Supplementary Materials.
The kinematic viscosity at 40 °C and 100 °C, and viscosity index were determined using the Inglebert Technology (Shanghai) Co. (Shanghai, China) kinematic viscosity automatic tester (78ELB-KVD10) according to the GB/T 265-1988 method [18]. The pour point and turbidity point of the petroleum products were determined using the Petroleum Products Pour Point and Turbidity Point Tester (SYD-510D) from Shanghai Changji Geological Instrument Co. (Shanghai, China), according to the GB/T 3535-2006 method [19]. The rotary oxygen bomb time (RBOT) was determined using the automatic rotary oxygen bomb tester (SH0193C) from Shandong Shengtai Instrument Co. (Jinan, China) according to the NB/SH/T 0193-2022 method [20].
2.4. Miscibility Test
In this test, the copolymerization product DVE-PAO base oil, reference materials PAO20, KLP32, KLP100, and KLP680 were added to the colorimetric tube containing mineral oil 100N, polyalphaolefin base oil PAO10, PAG base oil KLP46, at the mass ratios of 1:1, 1:9, and 9:1, respectively. The mixtures were then shaken vigorously up and down 20 times and left to stand for observation. A solution was defined as miscible if it was clear and did not stratify, and as immiscible if it was opaque or appeared to stratify.
2.5. Tribological Test
The tribological performance of the DVE-PAO base oil, reference materials PAO 10, PAG base oils KLP46, and DVE-3 were evaluated using the MS-10A four-ball friction tester from Xiamen Tianji Automation Co., Ltd. (Xiamen, China), according to the NB/SH/T 0189-2017 method [21]. The main conditions were as follows: temperature of 75 ± 2 °C, load of 147 ± 2 N, speed of 1200 ± 60 r/min, and duration of 60 ± 1 min.
3. Results and Discussion
3.1. Characterization of DVE-PAO
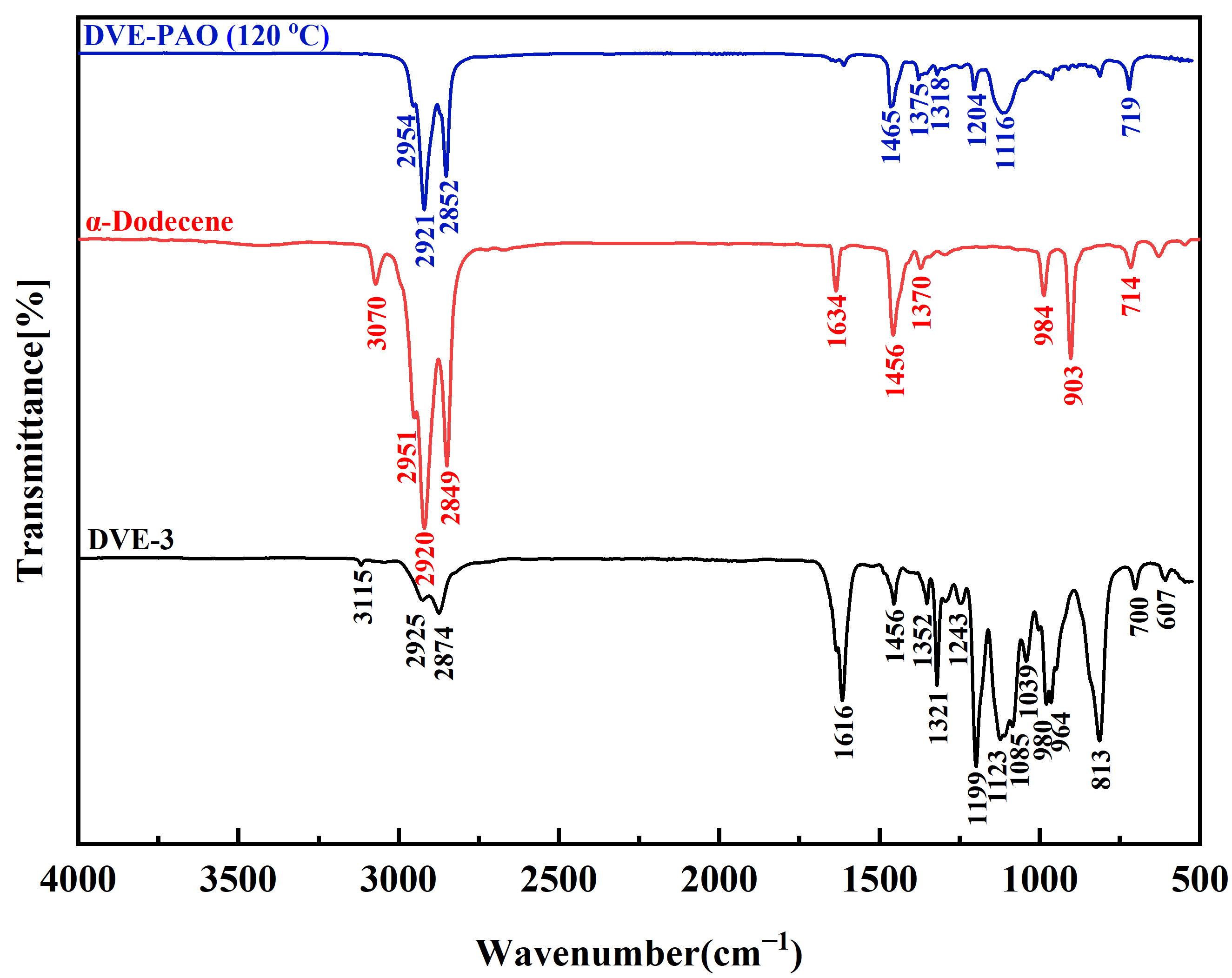
The FTIR spectra demonstrate that DVE-PAO copolymers synthesized across varying temperatures display highly consistent absorption profiles in both peak positions and intensities (Supplementary Materials). Owing to this spectral consistency, the copolymer synthesized at 120 °C was designated as a representative specimen for comparative FTIR analysis against DVE-3 and α-dodecene monomers.
As shown in Figure 1, the complete disappearance of characteristic peaks of DVE-3 and α-dodecene monomers was observed: the =C-H stretching vibration of terminal vinyl groups (~3070–3115 cm−1), the C=C stretching vibration (~1616–1634 cm−1), and the strong C-H out-of-plane bending vibrations of terminal olefins (~903–910 cm−1 and ~980–984 cm−1). These findings indicate full monomer conversion. Concurrently, peaks associated with the ether linkage (C-O-C of the fatty ether chain (-O-CH2-CH2-O-) stretching vibration at ~1100–1120 cm−1) and long-chain alkyl groups (C-H stretching vibrations of methyl and methyleneat at~2921–2954 cm−1, and ~2852 cm−1, C-H of methylene group bending at ~1456–1465 cm−1, and C-H of methyl group bending at ~1375 cm−1) showed relative intensity increase. These observations are consistent with the formation of a DVE-PAO copolymer backbone. The rocking vibration of methylene groups at ~719 cm−1 further supports long alkyl chains. Critically, no detectable signals corresponding to oxidation by-products (e.g., carbonyl or hydroxyl groups) were observed, ruling out side reactions. Collectively, the FTIR data demonstrate the successful copolymerization of DVE-3 with α-dodecene, yielding the DVE-PAO copolymer with the target molecular structure.
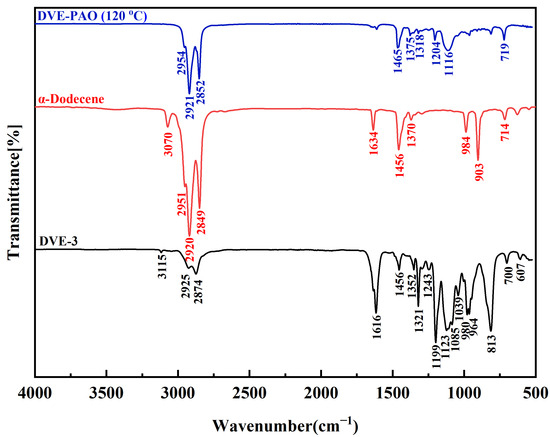
Figure 1.
FTIR spectrum of the DVE-PAO copolymer synthesized at 120 °C compared with the DVE-3 and α-dodecene monomers, measured in the wavenumber range of 4000 to 500 cm−1.
During this phase, 1H NMR and 13C NMR spectra in deuterated chloroform (CDCl3) were acquired for both the reaction raw materials (DVE-3 and α-dodecene) and the resulting DVE-PAO copolymers synthesized at polymerization temperatures of 120, 130, 140, 150, and 160 °C. All spectra are provided in Figures S17–S30 of the Supplementary Materials.
The 1H and 13C NMR spectra demonstrate that DVE-PAO copolymers synthesized at different temperatures display highly consistent spectral features in both chemical shifts and signal intensities. Owing to this consistency, the copolymer synthesized at 120 °C was selected as a representative sample for analysis (Figure 2 and Figure 3).
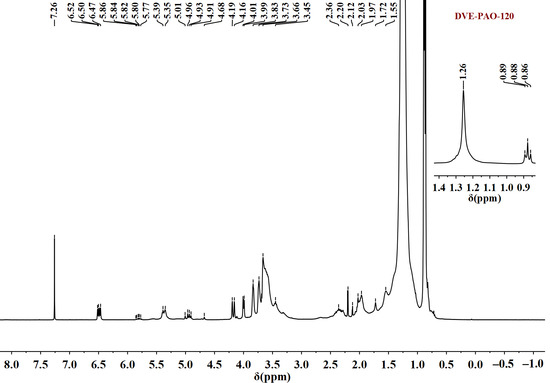
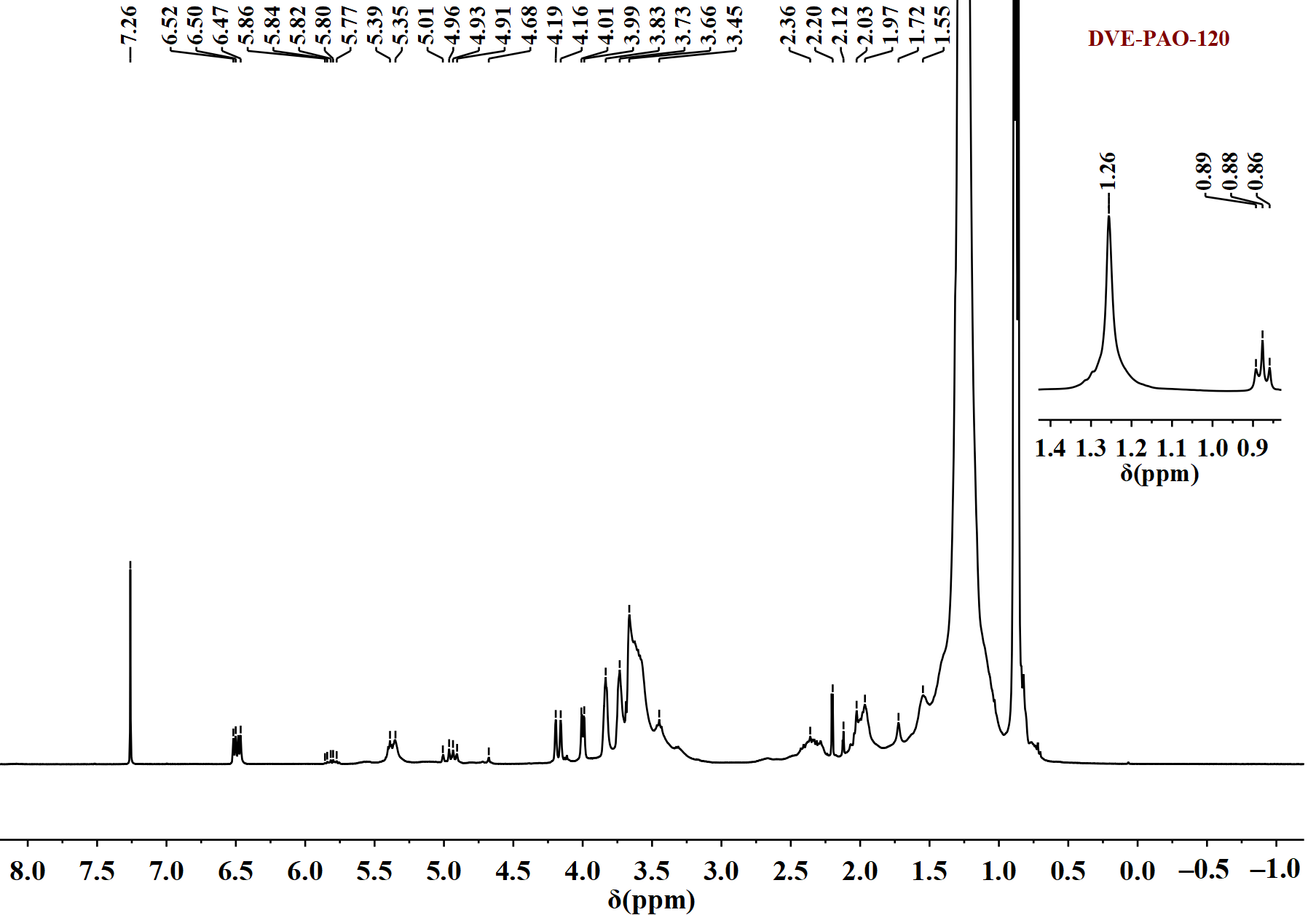
Figure 2.
1H NMR spectrum of DVE-PAO copolymer sample (synthesized at 120 °C) measured in the spectral range of −1 to 8.5 ppm.
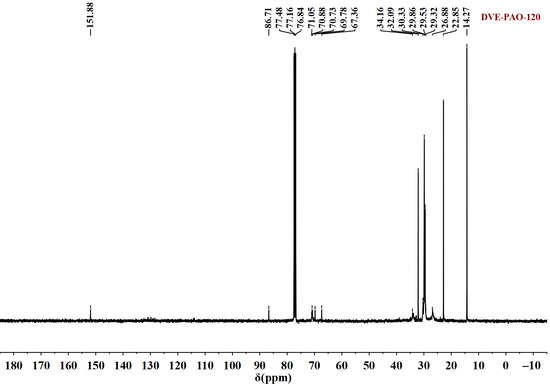
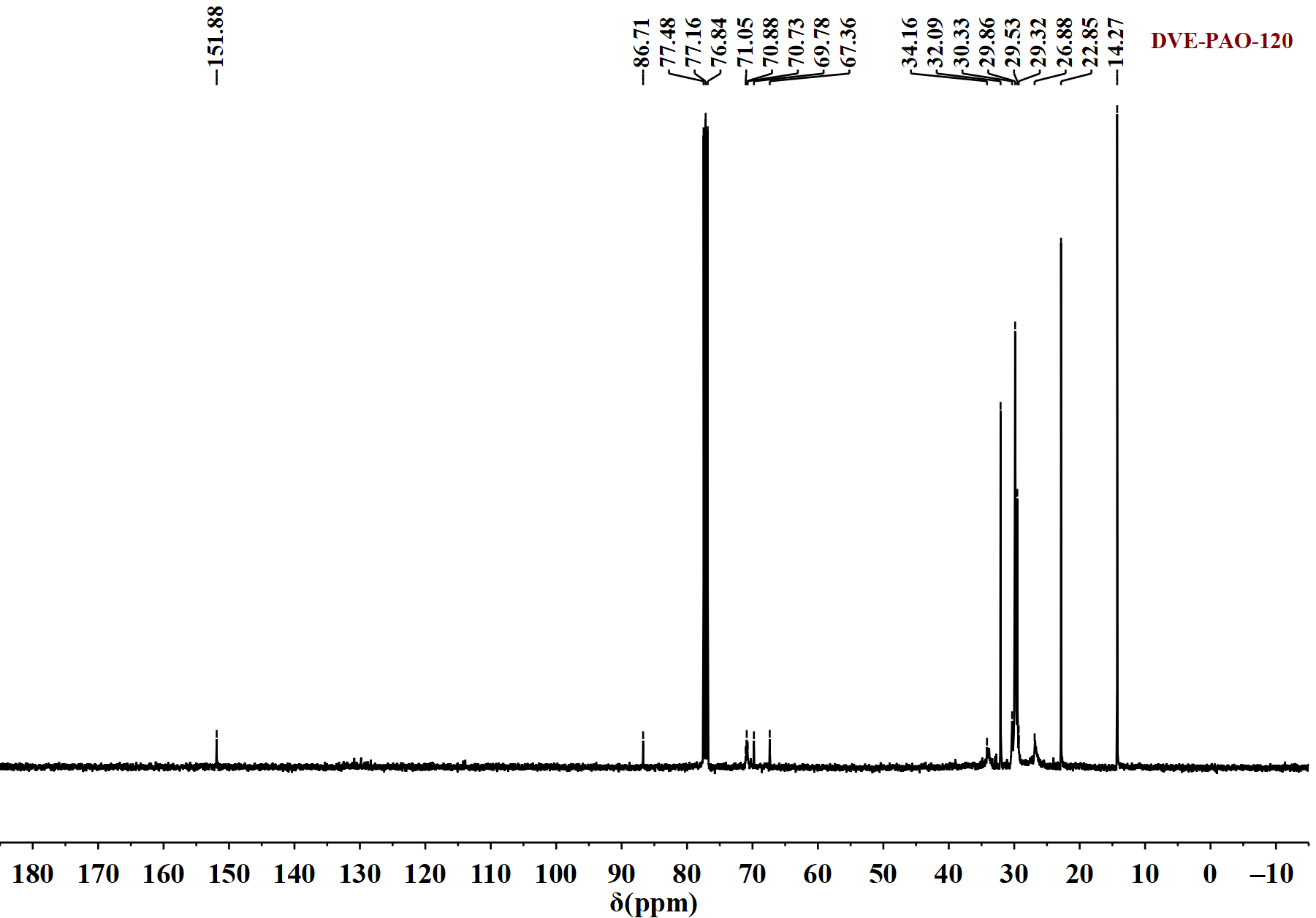
Figure 3.
13C NMR spectrum of DVE-PAO copolymer sample (synthesized at 120 °C) measured in the spectral range of −10 to 180 ppm.
The following information can be obtained from Figure 2. The signals observed at 0.86–0.89 ppm are attributed to the methyl protons of the α-dodecene segment. The signal at 1.26 ppm corresponds to the methylene protons (-CH2-) along the long alkyl chain of α-dodecene. The signals between 3.4 and 3.7 ppm arise from the methylene protons (-O-CH2-CH2-O-) within the DVE-3 moiety. The multiplet between 3.7 and 4.2 ppm is assigned to the terminal vinylic protons (=CH2) of DVE-3. Signals found in the 4.7–5.0 ppm range are assigned to the terminal vinylic protons (=CH2) of α-dodecene. The signals observed at 5.35–5.86 ppm correspond to the vinylic protons (=CH-) along the long alkyl chain of α-dodecene. Signals found in the 6.4–6.6 ppm range corresponding to the vinylic protons (=CH-) of DVE-3. The signal at 7.26 ppm is attributed to the solvent peak of CDCl3.
Figure 3 reveals the following structural information. The signals at 14.27 ppm and 20–35 ppm are assigned to the carbon atoms of the terminal (-CH3) and the methylene (-CH2-) groups in the α-dodecene segment, respectively. The resonances between 67 and 70.65 ppm correspond to the methylene carbons (-O-CH2-CH2-O-) of the DVE-3 moiety. The triplet observed at 76.84–77.48 ppm is attributed to the solvent peak of CDCl3. The appearance of weak carbon signals at 86 ppm and 151 ppm, attributable to the terminal vinyl groups (=CH2) and (=CH-) of DVE-3, respectively, indicates that a very minor fraction of DVE-3 molecules retained their unreacted vinyl groups at one terminus.
A comparison of the 1H and 13C NMR spectra of the starting materials (DVE-3 and α-dodecene) with those of the polymeric product DVE-PAO synthesized at 120 °C reveals that the spectra of DVE-PAO exhibit characteristic signals from both DVE-3 and α-dodecene, confirming the incorporation of structural units from both monomers into the product. Furthermore, weak signals corresponding to unreacted vinyl groups from DVE-3 are observed in both the 1H and 13C NMR spectra. This is because, as the free radical copolymerization of α-dodecene and DVE-3 progresses, the increasing molecular weight induces a progressive rise in steric hindrance. This may reduce the probability of simultaneous reaction at both vinyl groups of DVE-3 radicals with α-dodecene, resulting in a minor fraction of DVE-3 molecules retaining one unreacted vinyl group. In addition, extremely weak signals associated with the double bonds of α-dodecene are detectable in the 1H NMR spectrum; these signals indicate an extremely low concentration of such double bonds. Their presence is likely due to terminal double bonds formed during chain termination rather than residual α-dodecene (which was removed by distillation).
The FTIR and NMR spectra demonstrate that DVE-PAO copolymers synthesized at 120, 130, 140, 150, and 160 °C exhibit highly consistent spectral features in both chemical shifts and signal intensities. Owing to this consistency, the copolymer synthesized at 150 °C was selected as a representative sample for GPC analysis. GPC chromatogram, molecular weight distribution, cumulative distribution, and molecular weight data of DVE-PAO are shown in Figure 4 and Table 1. Further experimental details are provided in the Supplementary Materials.
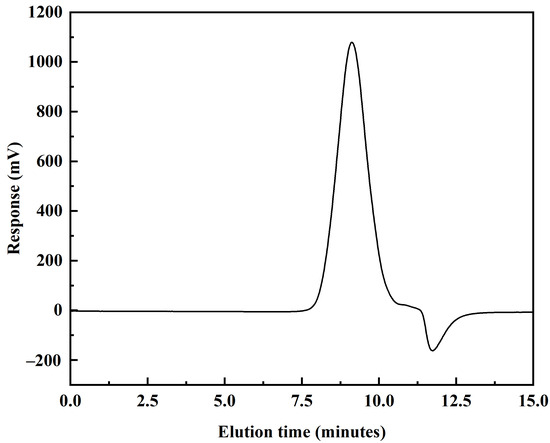
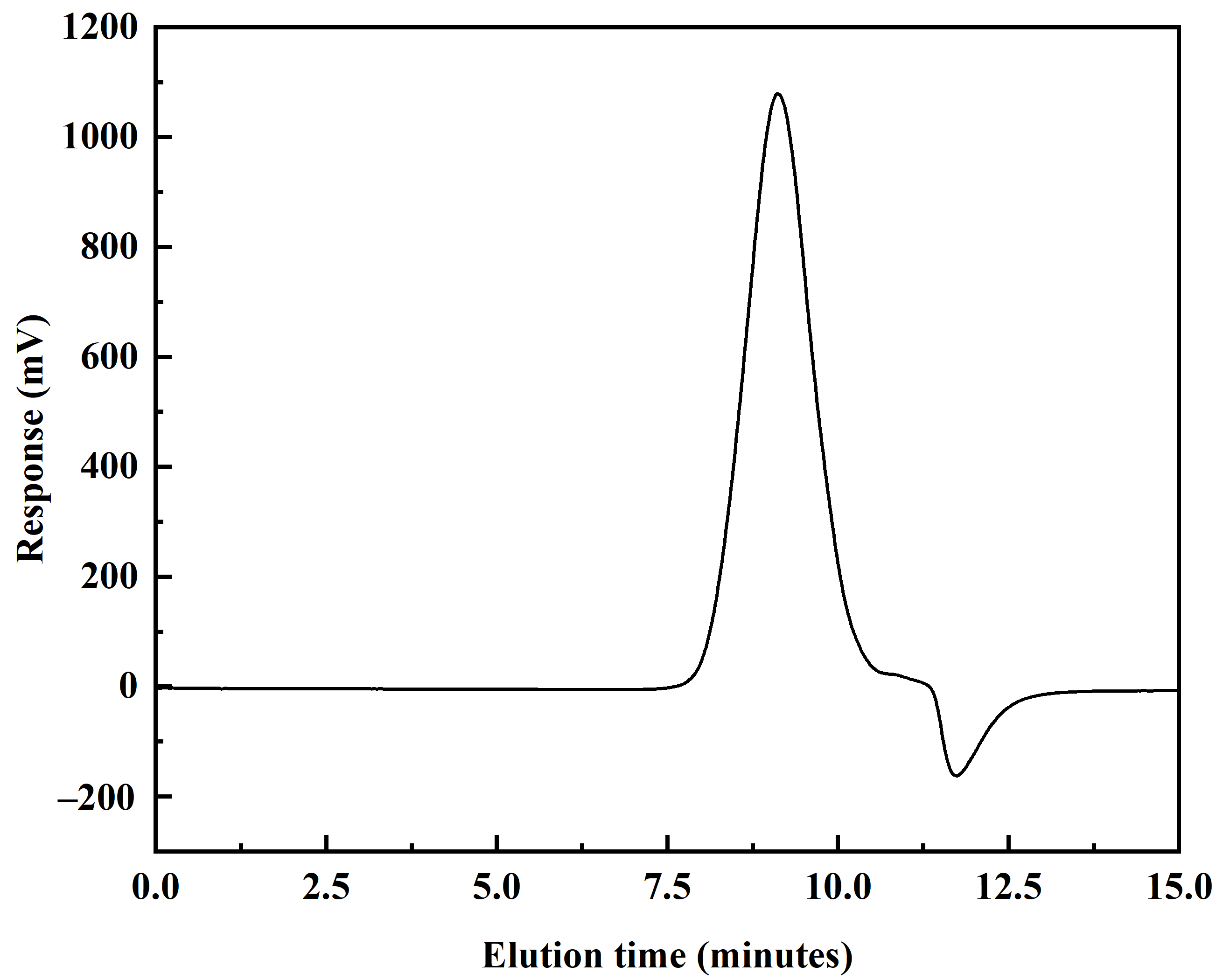
Figure 4.
GPC chromatogram of DVE-PAO copolymer synthesized at 150 °C.

Table 1.
Molecular weight of DVE-PAO copolymer synthesized at 150 °C.
As shown in Figure 4, the GPC chromatogram of the DVE-PAO base oil synthesized at 150 °C exhibits a well-defined, unimodal profile. This indicates that the product primarily consists of a single copolymer species. This result, combined with the FTIR and NMR analysis, provides further evidence for the successful copolymerization of DVE-3 and α-dodecene. The relative molecular weight data presented in Table 1 show that the resulting polymer possesses a dispersity index of 2.2. This value is indicative of a medium–broad molecular weight distribution, which falls within the typical range for free-radical polymerization products. Notably, the dispersity index of the DVE-PAO base oil synthesized in this work is lower than that generally observed for Group I, II, and III base oils; it aligns with the characteristic dispersity index range of Group IV PAO base oils.
3.2. Effect of Temperature on Copolymerization
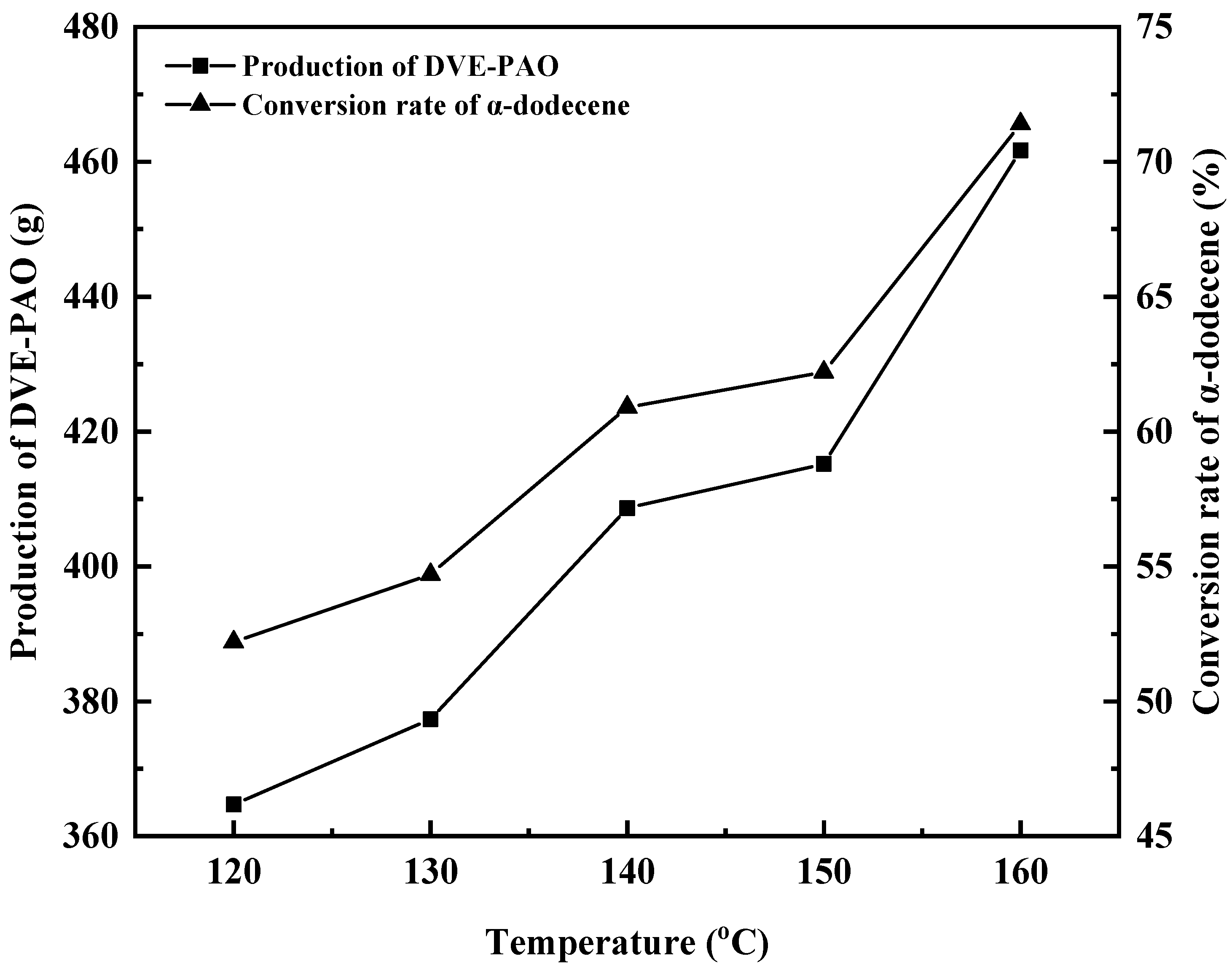
Under conditions where the molar ratio of α-dodecene to triethylene glycol divinyl ether was 6:1 (i.e., 3.00 moles of α-dodecene and 0.50 moles of DVE-3), the initiator dosage was 30.00 g (0.205 mol), and the dropwise copolymerization reaction time was 6 h, the effect of different copolymerization temperatures on the copolymerization reaction was investigated, as shown in Figure 5 and Figure 6.
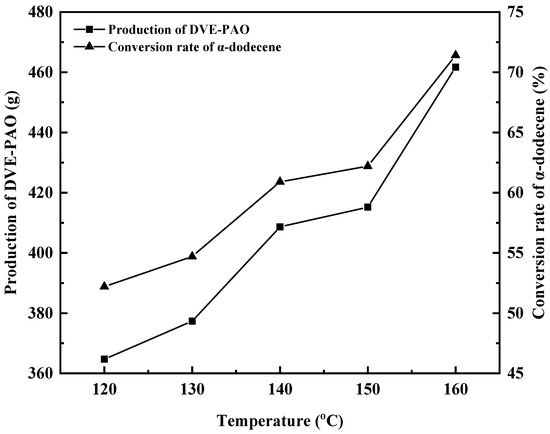
Figure 5.
Effect of copolymerization temperature on DVE-PAO production and α-dodecene conversion rate.
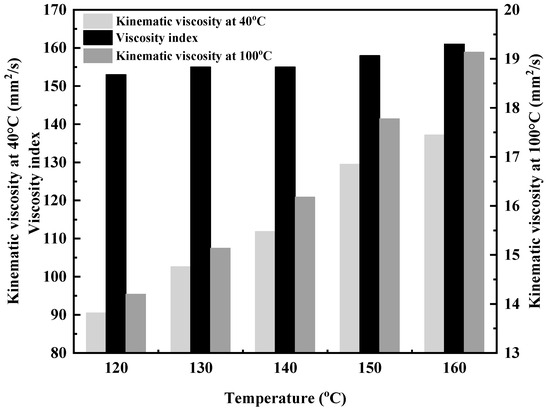
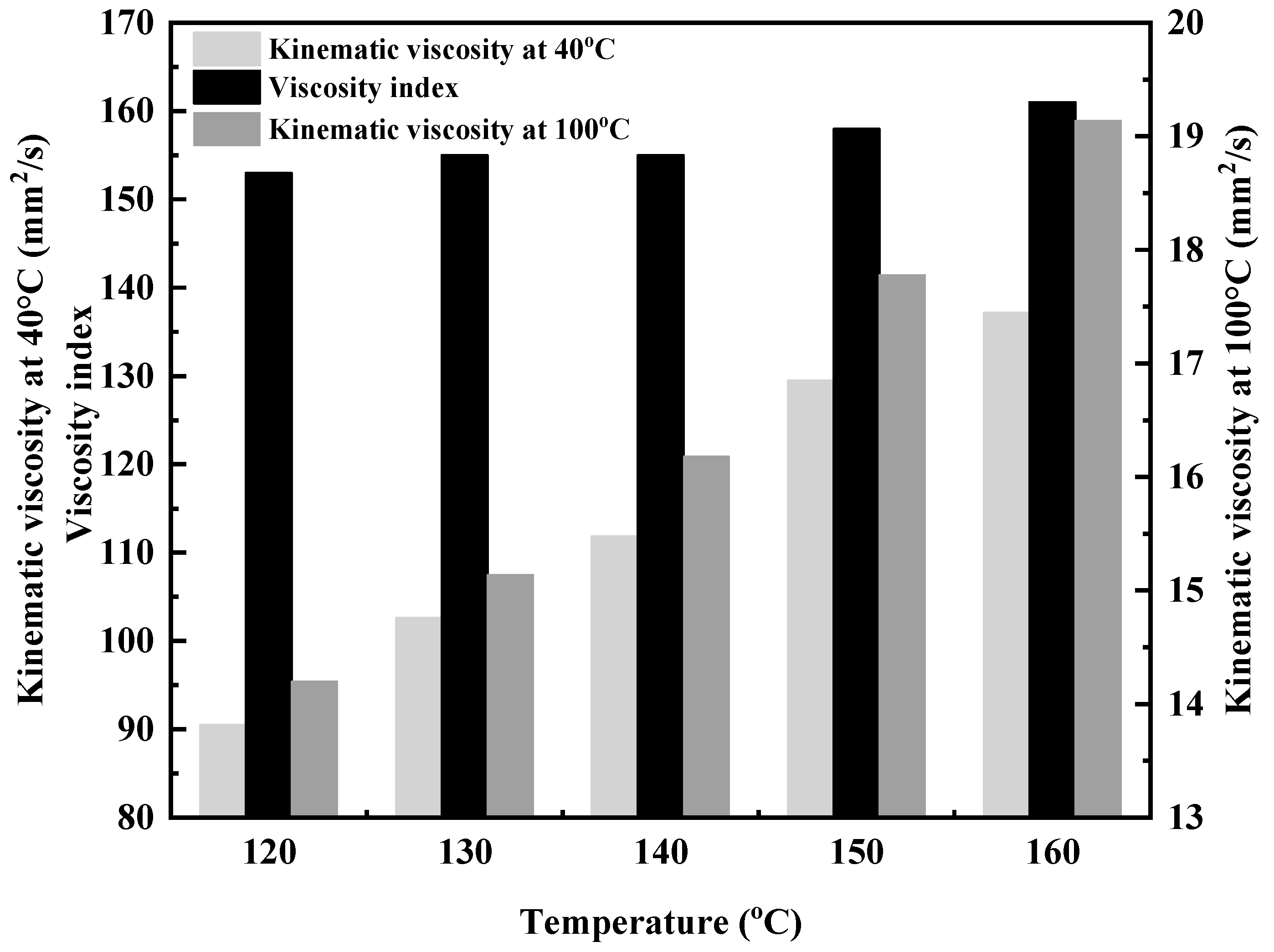
Figure 6.
Effect of copolymerization temperature on the kinematic viscosity of DVE-PAO.
As shown in Figure 5, when the polymerization temperature was 120, 130, 140, 150, and 160 °C, the conversion of α-dodecene reached 52.20%, 54.70%, 60.90%, 62.20%, and 71.40%, respectively; the yields of the DVE-PAO copolymer were 364.71 g, 377.34 g, 408.65 g, 415.21 g, and 461.67 g, respectively. With increasing reaction temperature, the conversion rate of α-dodecene gradually increases, and the yield and production of DVE-PAO also improve.
As shown in Figure 6, the viscosity indices of the copolymer products obtained under reaction conditions of 120–160 °C are all above 150, demonstrating excellent viscosity–temperature properties [22,23]. This indicates that the viscosity of the DVE-PAO base oil is virtually unaffected by temperature, exhibiting insensitivity to temperature changes. Furthermore, as the reaction temperature increases, both the 40 °C kinematic viscosity and the 100 °C kinematic viscosity of the copolymer products show an upward trend, and the viscosity index also increases. This is attributed to the increased proportion of α-olefins in the copolymer, which enhances the proportion of its linear structure [24,25].
The dropwise addition reaction method employed in this study enables both DVE-3 and the initiator to remain at low concentrations always, thereby facilitating continuous initiation. This approach effectively suppresses the likelihood of DVE-3 self-polymerization (which has a reactivity ratio significantly greater than 1), ensuring that the DVE radical primarily engages in chain-growth reactions with the α-dodecene radical, which has a reactivity ratio much less than 1. At lower temperatures, the concentration of the α-dodecene radical is relatively low, causing the chain-growth reaction with the DVE radical to proceed relatively slowly. This slower reaction translates to a smaller molecular weight for the resulting copolymer, resulting in lower viscosity. Conversely, at higher reaction temperatures, the increased concentration of α-dodecene radical accelerates the chain-growth reaction with DVE radical, leading to a higher molecular weight for the copolymer and consequently higher viscosity. The production of DVE-PAO and the conversion rate of the copolymerization reaction follow a similar trend. At lower temperatures, the conversion rate of α-dodecene is relatively low, leading to lower copolymer production. In contrast, at higher temperatures, the conversion rate of α-dodecene increases, resulting in greater copolymer production.
3.3. Relationship Between Kinematic Viscosity and Oxidation Resistance of Copolymer
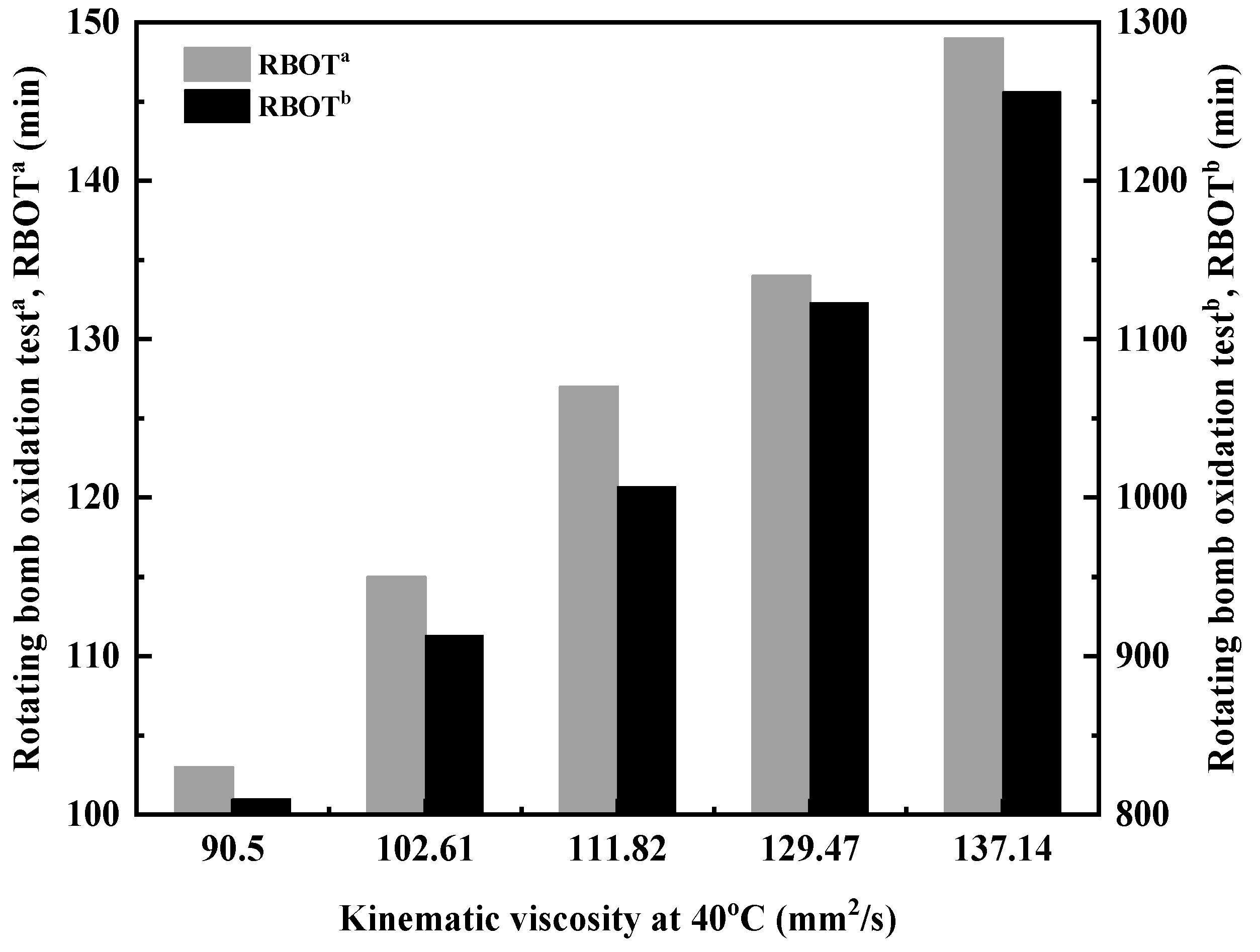
As shown in Figure 7, the oxidation resistance of ether-polyalphaolefin copolymer base oils is outstanding. In the absence of antioxidants, the rotating bomb oxidation test (RBOT) can exceed 100 min, with the duration increasing as viscosity rises. This improvement is attributed to the decrease in the relative proportion of residual double bonds in the copolymer molecules, which occurs as the molecular weight of the copolymer increases [26,27]. Preliminary tests have demonstrated that when amine and phenol-based composite antioxidants are added, the RBOT duration can reach 800–1200 min, significantly enhancing antioxidant performance.
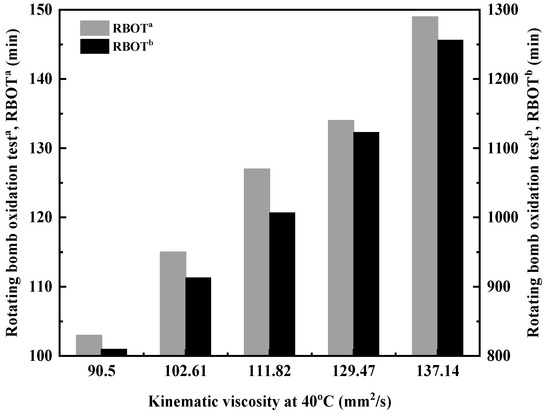
Figure 7.
Relationship between kinematic viscosity and oxidation resistance of DVE-PAO. RBOTa: rotating bomb oxidation test of DVE-PAO at 150 °C without antioxidant; RBOTb: rotating bomb oxidation test of DVE-PAO at 150 °C with the addition of a composite antioxidant package (T501 + L57, each at 0.25%).
3.4. Comparison of the Physicochemical Properties of DVE-PAO Base Oil with PAO and PAG Base Oils
This paper compares and analyzes the basic performance of DVE-PAO base oil with commercially available PAO20 base oil and PAG base oils KLP32, KLP100, and KLP680. The results are shown in Table 2. Visualization of miscibility tests is provided in the Supplementary Materials (Figures S1–S9).

Table 2.
Comparison of the physicochemical properties of DVE-PAO with PAO and PAG.
As shown in Table 2, the viscosity index of DVE-PAO base oil is similar to that of KLP32, higher than PAO20, but lower than KLP100 and KLP680. Its pour point is similar to that of KLP680, but lower than PAO20, KLP32, and KLP100.
As is well known, PAG base oils such as KLP32, KLP100, and KLP680 are immiscible with mineral oils and PAO base oils [1,5]. However, the ether-polyalphaolefin base oil synthesized in this study incorporates structural units from both PAG and PAO base oils, thereby exhibiting miscibility with mineral oils and PAO base oils. This enables it to be combined with mineral oils and PAO base oils, which will undoubtedly enhance the lubricating properties, micropitting resistance, and thermal conductivity of lubricants while meeting the low additive requirements of lubricants [1,9,10].
3.5. Comparison of Tribological Performance of DVE-PAO with PAO, PAG, and DVE-3
In this work, the wear scar morphologies, wear scar diameter (WSD), average friction force, and average coefficient of friction (COF) of DVE-PAO base oil were compared to those of commercial-grade PAO10, PAG base oil KLP46, and DVE-3 using a four-ball friction tester. The results are listed in Table 3 and Table 4.

Table 3.
Wear morphologies of balls lubricated with DVE-PAO, PAO, PAG, and DVE-3 after tribological tests.

Table 4.
Comparison of the tribological performance of DVE-PAO with PAO, PAG, and DVE-3.
The four-ball tribological analysis (Table 3 and Table 4) demonstrates that DVE-PAO base oil exhibits superior anti-wear and friction-reduction properties compared to commercial references. Samples of DVE-PAO synthesized at 120–160 °C all exhibited the lowest wear scar diameters (WSDs) at the test temperature (0.487–0.518 mm), which were significantly lower than those of PAO10 (0.859 mm), DVE-3 (0.876 mm), and KLP46 (0.557 mm). DVE-PAO maintained consistently lower average friction forces (2.035–2.428 N) versus KLP46 (3.387 N) and DVE-3 (2.515 N), indicating enhanced lubricity. The average COFs of DVE-PAO (0.041–0.049) were lower than KLP46 (0.069) and DVE-3 (0.051), confirming its efficiency in boundary lubrication. Notably, samples of DVE-PAO synthesized at 140 °C, 150 °C, and 160 °C reached better performance with a WSD of 0.487–0.494 mm and average COFs of 0.041–0.042. In summary, preliminary results confirm that DVE-PAO base oil possesses both good friction-reducing and anti-wear properties. This is consistent with the predicted behavior. DVE-PAO exhibits different properties at different temperatures, with a general trend that higher polymerization temperatures result in better friction-reducing and anti-wear properties. The tribological properties of DVE-PAO base oil also indirectly confirm that DVE-PAO has combined the characteristics of both PAG and PAO base oils.
4. Conclusions
This paper reports the first synthesis of a novel DVE-PAO base oil using α-olefins and triethylene glycol divinyl ether as raw materials, thereby combining the characteristics of both PAG and PAO base oils. The DVE-PAO base oils synthesized at 120, 130, 140, 150, and 160 °C, respectively, exhibit excellent miscibility with mineral oil and PAO base oil, along with superior antioxidant performance, good pour point, viscosity index, friction-reducing and anti-wear properties, making it suitable for blending with other base oils or for standalone use in hydraulic oils, gear oils, and compressor oils. Additionally, this paper preliminarily elucidates the reaction patterns of the copolymerization of the diene vinyl ether monomer DVE-3 and α-olefins, and the effect of different copolymerization temperatures on the copolymerization reaction was investigated. The research shows that the copolymer’s viscosity, viscosity index, antioxidant performance, friction-reducing and anti-wear properties, and pour point, as well as the conversion rate of α-olefin, increase with rising copolymerization temperature. These studies provide a theoretical and practical foundation for future research into copolymerization reactions involving such diene vinyl ether structural compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/lubricants13090404/s1, Figure S1: Miscibility of DVE-PAO (120 °C) sample in 100N, KLP46, and PAO10 at mass ratios of 1:1, 1:9, and 9:1. (a) Miscibility of DVE-PAO in 100N at mass ratios of 1:1, 1:9, and 9:1. (b) Miscibility of DVE-PAO in KLP46 at mass ratios of 1:1, 1:9, and 9:1. (c) Miscibility of DVE-PAO in PAO10 at mass ratios of 1:1, 1:9, and 9:1; Figure S2: Miscibility of DVE-PAO (130 °C) sample in 100N, KLP46, and PAO10 at mass ratios of 1:1, 1:9, and 9:1. (a) Miscibility of DVE-PAO in 100N at mass ratios of 1:1, 1:9, and 9:1. (b) Miscibility of DVE-PAO in KLP46 at mass ratios of 1:1, 1:9, and 9:1. (c) Miscibility of DVE-PAO in PAO10 at mass ratios of 1:1, 1:9, and 9:1; Figure S3: Miscibility of DVE-PAO (140 °C) sample in 100N, KLP46, and PAO10 at mass ratios of 1:1, 1:9, and 9:1. (a) Miscibility of DVE-PAO in 100N at mass ratios of 1:1, 1:9, and 9:1. (b) Miscibility of DVE-PAO in KLP46 at mass ratios of 1:1, 1:9, and 9:1. (c) Miscibility of DVE-PAO in PAO10 at mass ratios of 1:1, 1:9, and 9:1; Figure S4: Miscibility of DVE-PAO (150 °C) sample in 100N, KLP46, and PAO10 at mass ratios of 1:1, 1:9, and 9:1. (a) Miscibility of DVE-PAO in 100N at mass ratios of 1:1, 1:9, and 9:1. (b) Miscibility of DVE-PAO in KLP46 at mass ratios of 1:1, 1:9, and 9:1. (c) Miscibility of DVE-PAO in PAO10 at mass ratios of 1:1, 1:9, and 9:1; Figure S5: Miscibility of DVE-PAO (160 °C) sample in 100N, KLP46, and PAO10 at mass ratios of 1:1, 1:9, and 9:1. (a) Miscibility of DVE-PAO in 100N at mass ratios of 1:1, 1:9, and 9:1. (b) Miscibility of DVE-PAO in KLP46 at mass ratios of 1:1, 1:9, and 9:1. (c) Miscibility of DVE-PAO in PAO10 at mass ratios of 1:1, 1:9, and 9:1; Figure S6: Miscibility of PAO20 sample in 100N, KLP46, and PAO10 at mass ratios of 1:1, 1:9, and 9:1. (a) Miscibility of PAO20 in 100N at mass ratios of 1:1, 1:9, and 9:1. (b) Miscibility of PAO20 in KLP46 at mass ratios of 1:1, 1:9, and 9:1. (c) Miscibility of PAO20 in PAO10 at mass ratios of 1:1, 1:9, and 9:1; Figure S7: Miscibility of KLP32 sample in 100N, KLP46, and PAO10 at mass ratios of 1:1, 1:9, and 9:1. (a) Miscibility of KLP32 in 100N at mass ratios of 1:1, 1:9, and 9:1. (b) Miscibility of KLP32 in KLP46 at mass ratios of 1:1, 1:9, and 9:1. (c) Miscibility of KLP32 in PAO10 at mass ratios of 1:1, 1:9, and 9:1; Figure S8: Miscibility of KLP100 sample in 100N, KLP46, and PAO10 at mass ratios of 1:1, 1:9, and 9:1. (a) Miscibility of KLP100 in 100N at mass ratios of 1:1, 1:9, and 9:1. (b) Miscibility of KLP100 in KLP46 at mass ratios of 1:1, 1:9, and 9:1. (c) Miscibility of KLP100 in PAO10 at mass ratios of 1:1, 1:9, and 9:1; Figure S9: Miscibility of KLP680 sample in 100N, KLP46, and PAO10 at mass ratios of 1:1, 1:9, and 9:1. (a) Miscibility of KLP680 in 100N at mass ratios of 1:1, 1:9, and 9:1. (b) Miscibility of KLP680 in KLP46 at mass ratios of 1:1, 1:9, and 9:1. (c) Miscibility of KLP680 in PAO10 at mass ratios of 1:1, 1:9, and 9:1; Figure S10: FTIR spectrum of DVE-3 monomer in the wavenumber range of 4000 to 500 cm−1; Figure S11: FTIR spectrum of α-dodecene monomer in the wavenumber range of 4000 to 500 cm−1; Figure S12: FTIR spectrum of DVE-PAO copolymer synthesized at 120 °C in the wavenumber range of 4000 to 500 cm−1; Figure S13: FTIR spectrum of DVE-PAO copolymer synthesized at 130 °C in the wavenumber range of 4000 to 500 cm−1; Figure S14: FTIR spectrum of DVE-PAO copolymer synthesized at 140 °C in the wavenumber range of 4000 to 500 cm−1; Figure S15: FTIR spectrum of DVE-PAO copolymer synthesized at 150 °C in the wavenumber range of 4000 to 500 cm−1; Figure S16: FTIR spectrum of DVE-PAO copolymer synthesized at 160 °C in the wavenumber range of 4000 to 500 cm−1; Figure S17: 1H NMR spectrum of DVE-3; Figure S18: 1H NMR spectrum of α-dodecene; Figure S19: 1H NMR spectrum of DVE-PAO copolymer synthesized at 120 °C; Figure S20: 1H NMR spectrum of DVE-PAO copolymer synthesized at 130 °C; Figure S21: 1H NMR spectrum of DVE-PAO copolymer synthesized at 140 °C; Figure S22: 1H NMR spectrum of DVE-PAO copolymer synthesized at 150 °C; Figure S23: 1H NMR spectrum of DVE-PAO copolymer synthesized at 160 °C; Figure S24: 13C NMR spectrum of DVE-3; Figure S25: 13C NMR spectrum of α-dodecene; Figure S26: 13C NMR spectrum of DVE-PAO copolymer synthesized at 120 °C; Figure S27: 13C NMR spectrum of DVE-PAO copolymer synthesized at 130 °C; Figure S28: 13C NMR spectrum of DVE-PAO copolymer synthesized at 140 °C; Figure S29: 13C NMR spectrum of DVE-PAO copolymer synthesized at 150 °C; Figure S30: 13C NMR spectrum of DVE-PAO copolymer synthesized at 160 °C; Figure S31: The GPC sample injection report of DVE-PAO copolymer synthesized at 150 °C.
Author Contributions
Conceptualization, W.E.; methodology, W.E.; validation, L.H. and W.E.; formal analysis, L.H.; investigation, L.H.; resources, W.E.; data curation, L.H.; Visualization, L.H.; writing—original draft preparation, L.H.; writing—review and editing, W.E.; supervision, W.E.; project administration, W.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Tianshan Elite” Leading Talent Project, grant number TSYCLJ2023011; and the Key Research and Development Project of the Xinjiang Uygur Autonomous Region, grant number 2022B01047-1.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Z.; Xue, W.; Shi, J.; Han, S.; Yan, J. Recent advances in polyalkylene glycol base oil. Res. Chem. Intermed. 2024, 50, 1515–1539. [Google Scholar] [CrossRef]
- Shubkin Ronald, L. Synthetic Lubricants and High-Performance Functional Fluids, 1st ed.; Marcel Dekker: New York, NY, USA, 1993; pp. 10l–123. [Google Scholar]
- Leslie, R.R. Synthetics Mineral Oils and Bio-Based Lubricants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 119–138. [Google Scholar]
- Leslie, R.R. Synthetics Mineral Oils and Bio-based Lubricants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 123–148. [Google Scholar]
- Gangopadhyay, A.; Cuthbert, J. Opportunities and Challenges with Polyalkylene Glycol for Engine Oil Application. Lubricants 2018, 6, 72. [Google Scholar] [CrossRef]
- Totten, G.E.; Webster, G.M.; Bishop, R.J.; Sloan, W.E. Anhydrous Polyalkylene Glycol Hydraulic Fluids (No. 2000-01-2557). In Proceedings of the International Off-Highway & Powerplant Congress & Exposition, Milwaukee, WI, USA, 11–13 September 2000. [Google Scholar]
- Brown, W.L. The Role of Polyalkylene Glycols in Synthetic Metal Working Fluids. Lubr. Eng. 1988, 44, 168–171. [Google Scholar]
- Khemchandani, G.; Greaves, M. Novel polyalkylene glycol-based hydraulic fluids. Iron Steel Tech. 2010, 7, 66–71. [Google Scholar]
- Gajewski, J.B.; Gogowski, M.J. Additives contents in PAG and synthetic motor base oils and their effect on electrostatic phenomena in a rotating shaft-oil-lip seal system. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 1561–1566. [Google Scholar] [CrossRef]
- Gajewski, J.B.; Głogowski, M.J. Anti-wear additive content in fully synthetic PAO and PAG base oils and its effect on electrostatic and tribological phenomena in a rotating shaft-oil-lip seal system. J. Phys. Conf. Ser. 2013, 418, 012045. [Google Scholar] [CrossRef]
- Gajewski, J.B.; Głogowski, M.J. Influence of additives blended with motor base oils on the braking torque under an auxiliary external DC electric field. J. Electrostat. 2013, 71, 1100–1103. [Google Scholar] [CrossRef]
- Boffa, A.B.; Bidwell, T.R. Wear Control with Dispersants Employing Poly Alpha-Olefin Polymers. U.S. Patent 5972853, 26 October 1999. [Google Scholar]
- Greaves, M.; Zaugg-Hoozemans, E.; Khelidj, N.; van Voorst, R.; Meertens, R. Performance properties of oil-soluble synthetic polyalkylene glycols. Lubr. Sci. 2012, 24, 251–262. [Google Scholar] [CrossRef]
- Greaves, M.; Topolovec Miklozic, K. Film forming behaviour of oil soluble polyalkylene glycols. Ind. Lubr. Tribol. 2015, 67, 133–138. [Google Scholar] [CrossRef]
- Greaves, M. Pressure viscosity coefficients and traction properties of synthetic lubricants for wind turbine gear systems. Lubr. Sci. 2012, 24, 75–83. [Google Scholar] [CrossRef]
- Jackov, T.; Filipovic, J.M.; Petrovic-Jackov, D. Synthetic lubricants based on copolymers of n-butyl methacrylate and α-olefins. Chem. Ind. 2002, 56, 526–528. [Google Scholar] [CrossRef][Green Version]
- Schweißinger, E.C.; Maier, S.K.; Nothdurft, K.; Groß-Onnebrink, Y.; Janssen, D.; Pletsch, H.; Hilf, S.; Kleinschmidt, D.; Babik, S. Acrylate-Olefin Copolymers as High Viscosity Base Fluids. U.S. Patent 11981877, 14 May 2024. [Google Scholar]
- GB/T 265-1988; Petroleum Products–Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. Standards Press of China (SINOPEC): Beijing, China, 1988.
- GB/T 3535-2006; Petroleum Products–Determination of Pour Point. Standards Press of China (SINOPEC): Beijing, China, 2006.
- NB/SH/T 0193-2022; Lubricanting Oils–Determination of Oxidation Stability–Rotating Pressure Vessel Method. Standards Press of China (SINOPEC): Beijing, China, 2022.
- NB/SH/T 0189-2017; Standard Test Method for Wear Preventive Characteristics of Lubricating Fluid–Four-Ball Method. Standards Press of China (SINOPEC): Beijing, China, 2017.
- Chen, P.; Liu, D.; Wang, X.; Zhang, Q.; Chu, X. Rapid determination of viscosity and viscosity index of lube base oil based on near-infrared spectroscopy and new transformation formula. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122079. [Google Scholar] [CrossRef] [PubMed]
- Verdier, S.; Coutinho, J.A.; Silva, A.M.; Alkilde, O.F.; Hansen, J.A. A critical approach to viscosity index. Fuel 2009, 88, 2199–2206. [Google Scholar] [CrossRef]
- Panwar, P.; Schweissinger, E.; Maier, S.; Hilf, S.; Sirak, S.; Martini, A. Effect of polymer structure and chemistry on viscosity index, thickening efficiency, and traction coefficient of lubricants. J. Mol. Liq. 2022, 359, 119215. [Google Scholar] [CrossRef]
- Dong, S.Q.; Mi, P.K.; Xu, S.; Zhang, J.; Zhao, R.D. Preparation and characterization of single-component poly-α-olefin oil base stocks. Energy Fuel 2019, 33, 9796–9804. [Google Scholar] [CrossRef]
- Singh, H.; Gulati, I.B. Influence of base oil refining on the performance of viscosity index improvers. Wear 1987, 118, 33–56. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Perez, J.M.; Erhan, S.Z. Effects of hydroprocessing on structure and properties of base oils using NMR. Fuel Process. Technol. 2008, 89, 984–991. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).