Advances on Hydrogel Lubrication Modification Under Diverse Design Strategies
Abstract
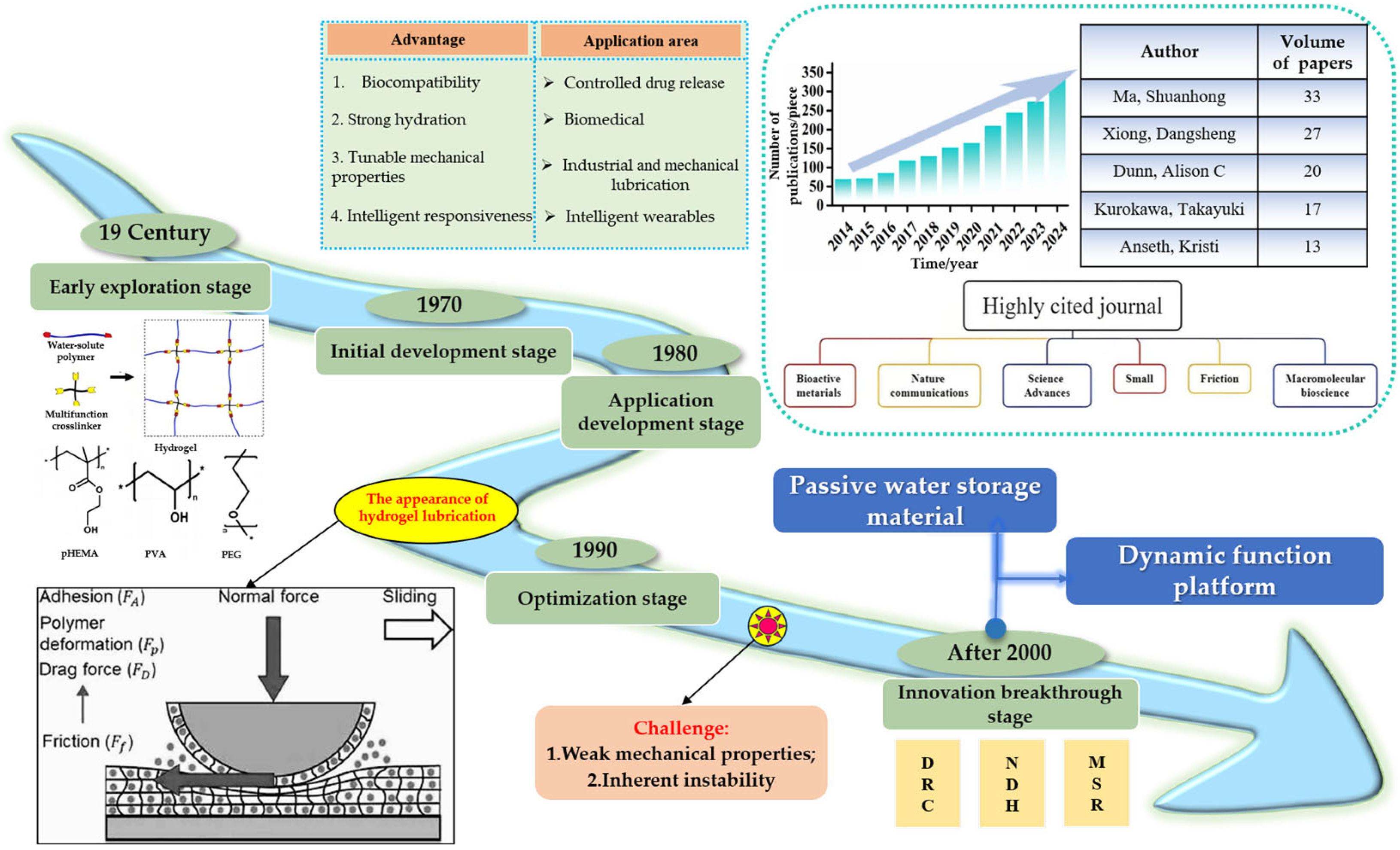
1. Introduction
2. Component Modification Strategy
2.1. Physical Component Doping Modification Strategy
2.1.1. Incorporation of Liposomes
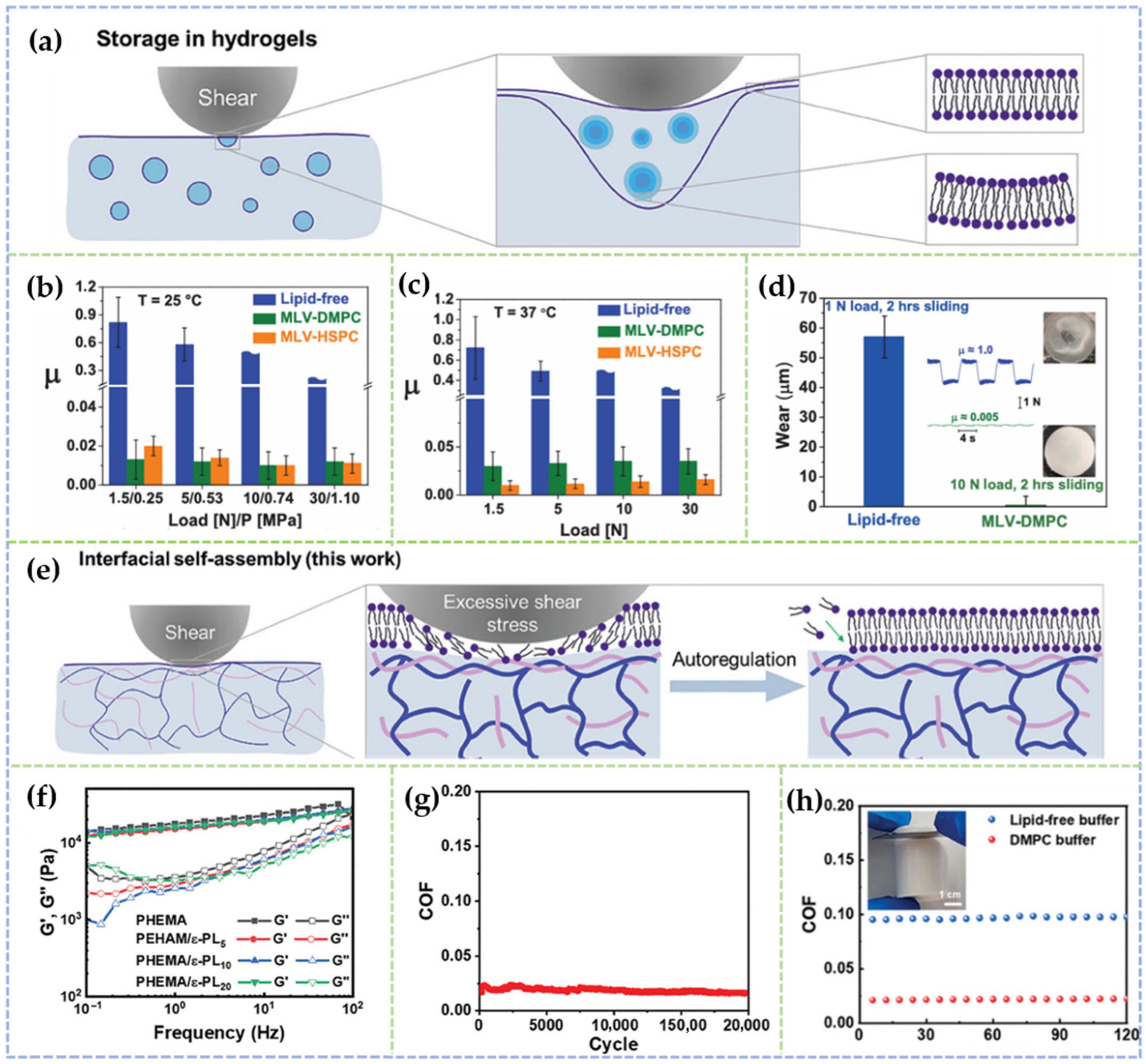
2.1.2. Doping of Nanoparticles
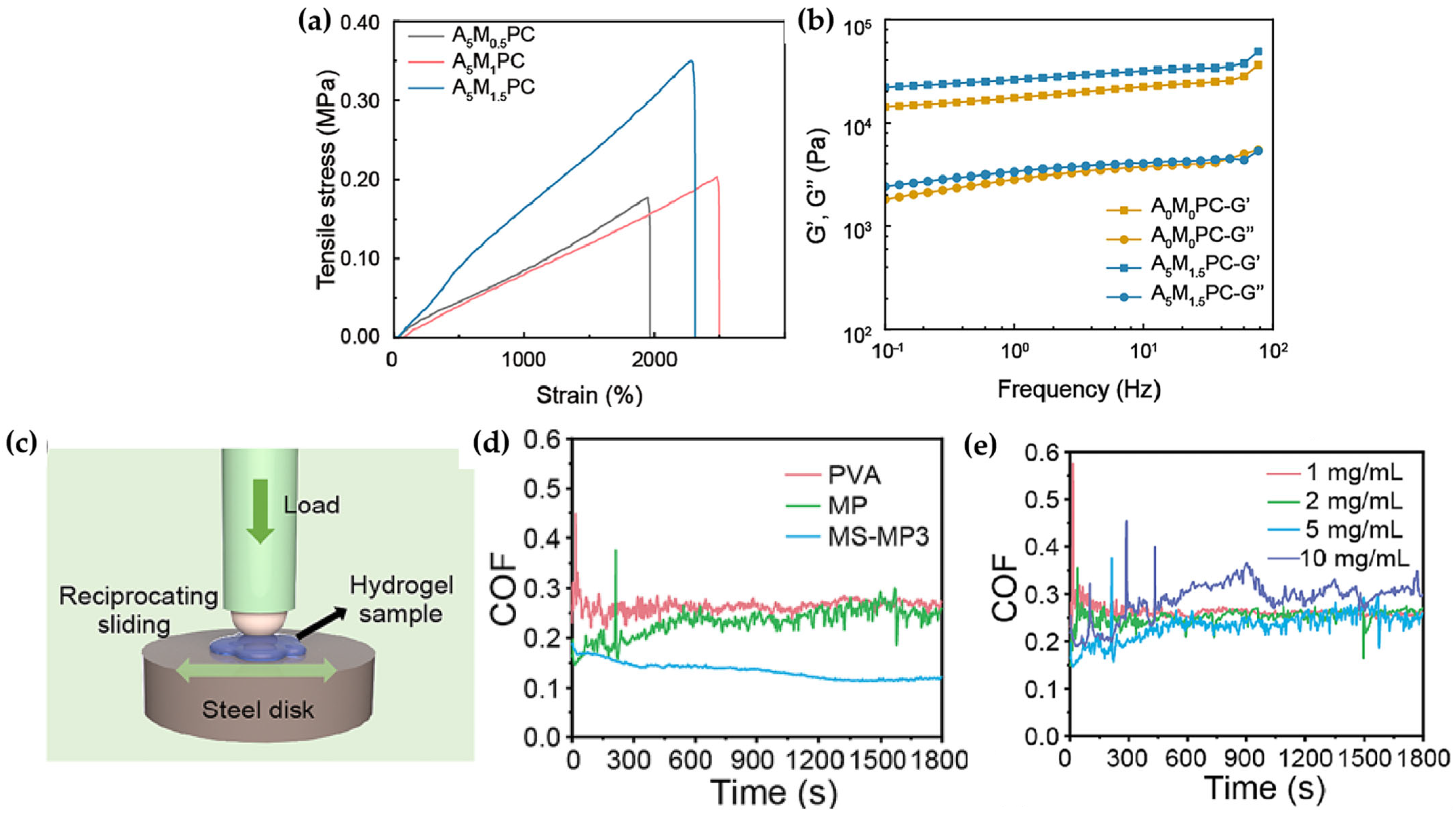
2.2. Introducing Functional Chemical Groups
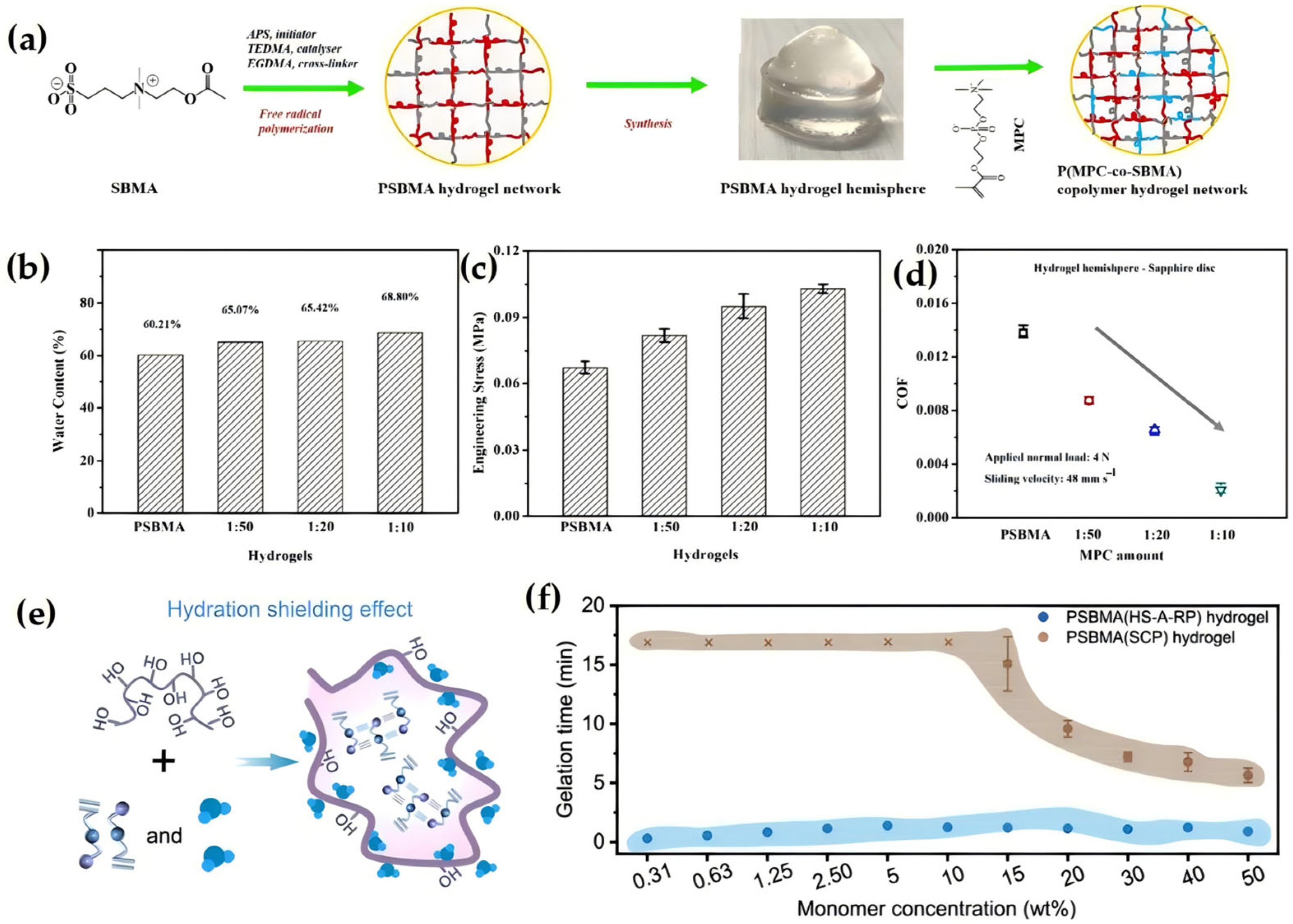
2.2.1. Introducing Zwitterionic Groups
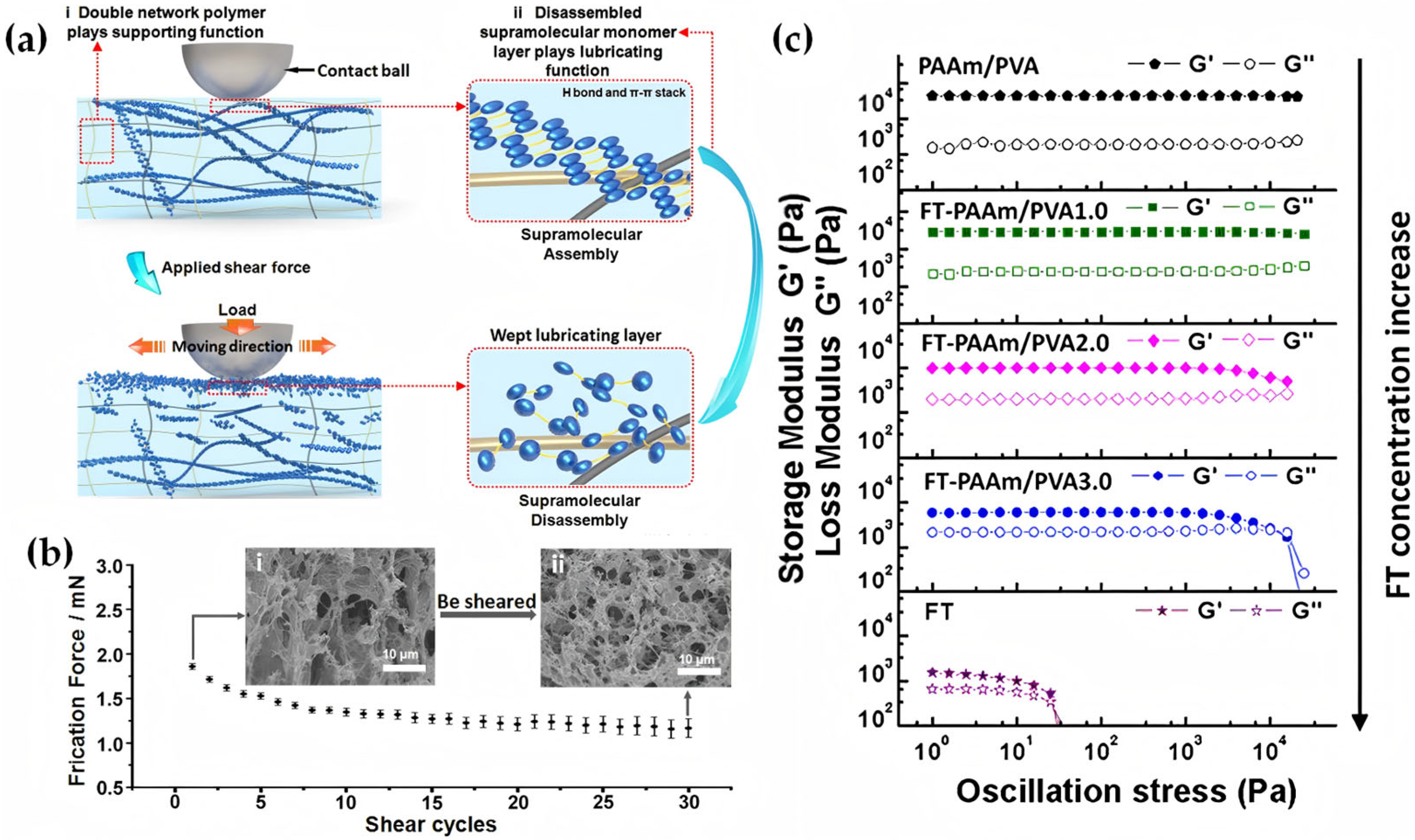
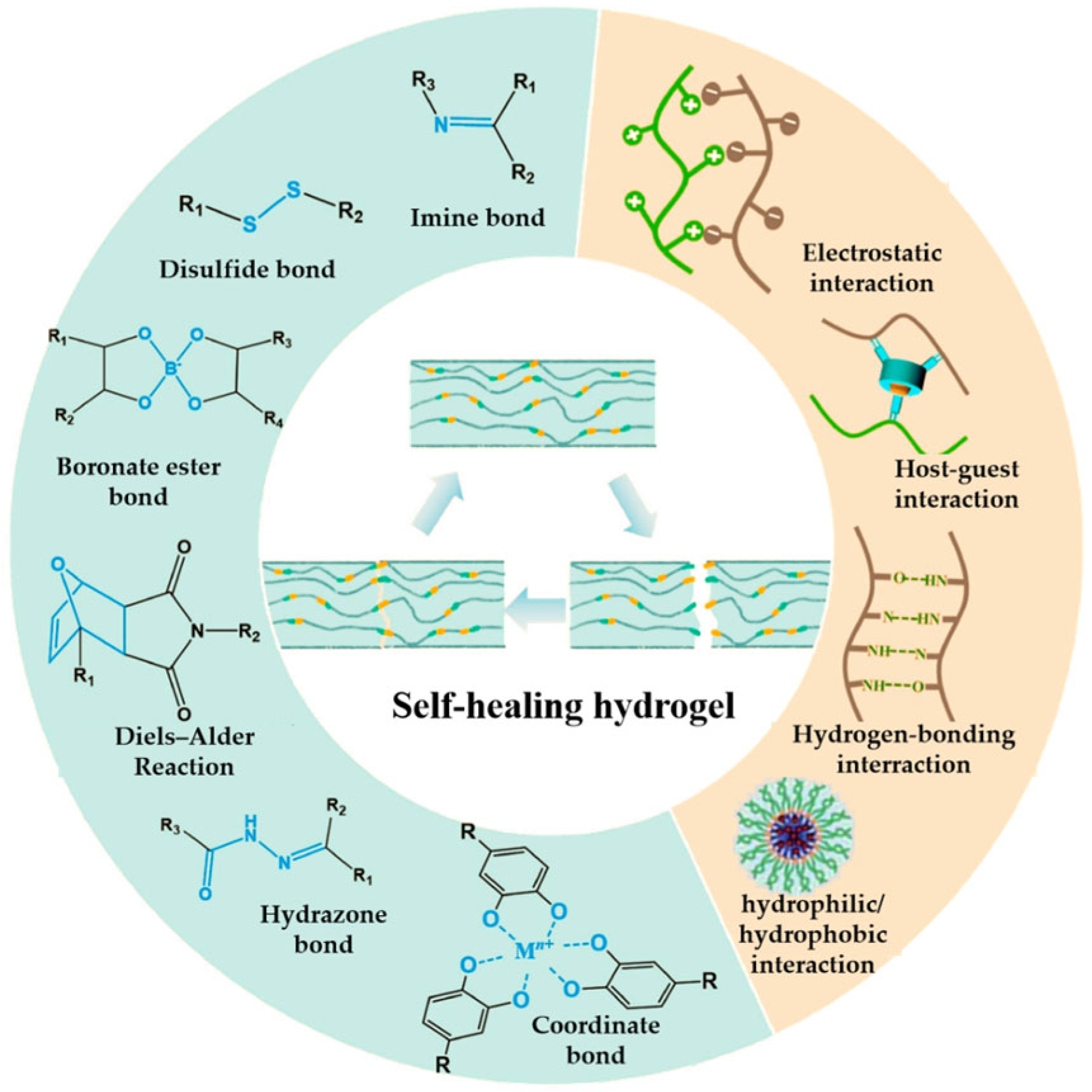
2.2.2. Introducing Dynamic Chemical Bonds
3. Multi-Scale Structure Regulation Strategy
3.1. Dual-Network Structure Design

3.2. Gradient Anisotropic/Heterogeneous Structure Design
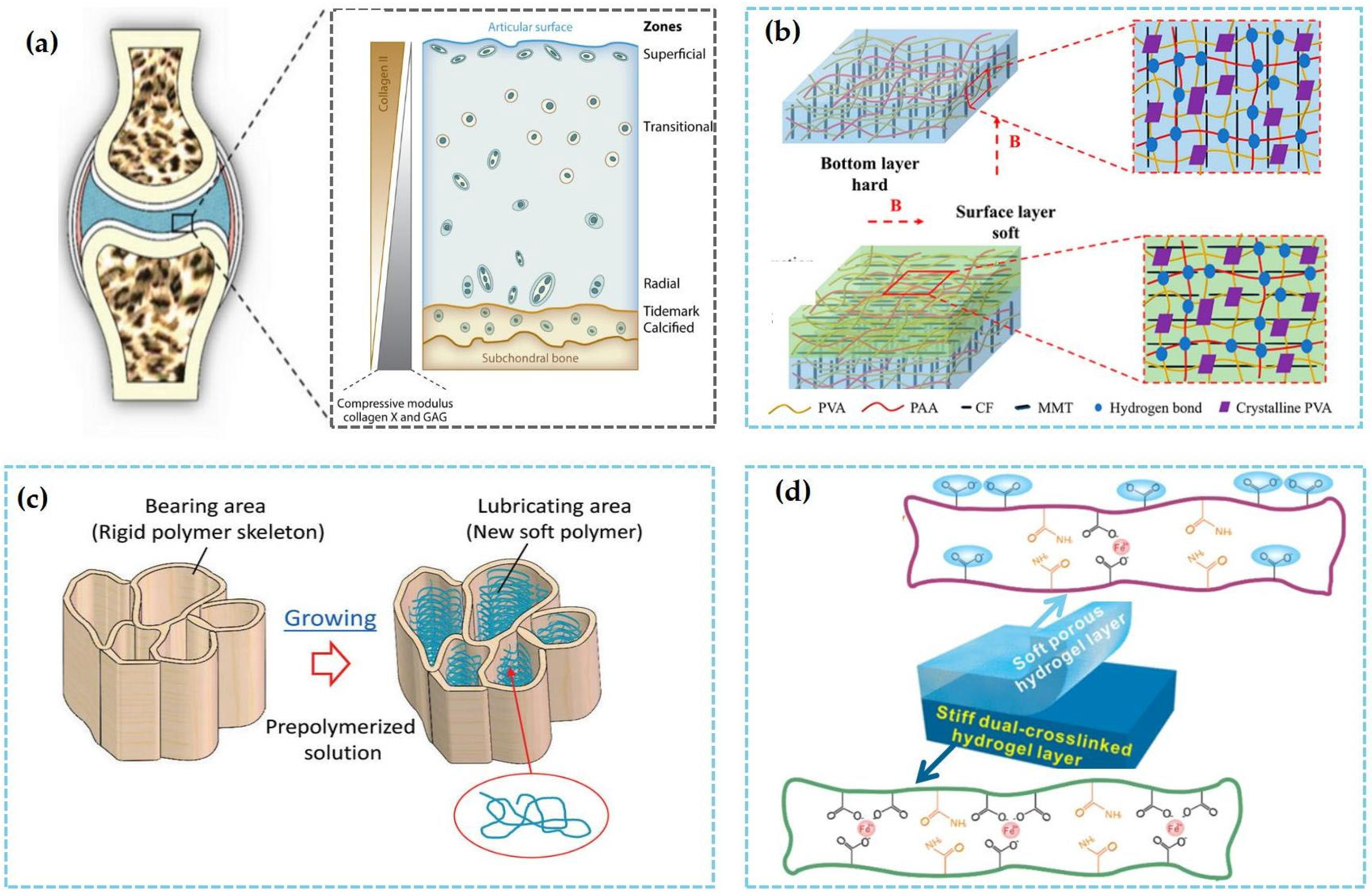
4. Surface and Interface Modification Strategy
4.1. Hydrogels Surface Modification (Hydrogel Paints)
4.2. Modification of the Friction Interfaces (Hydrogel Layers)
4.2.1. Interfacial Chemical Modification
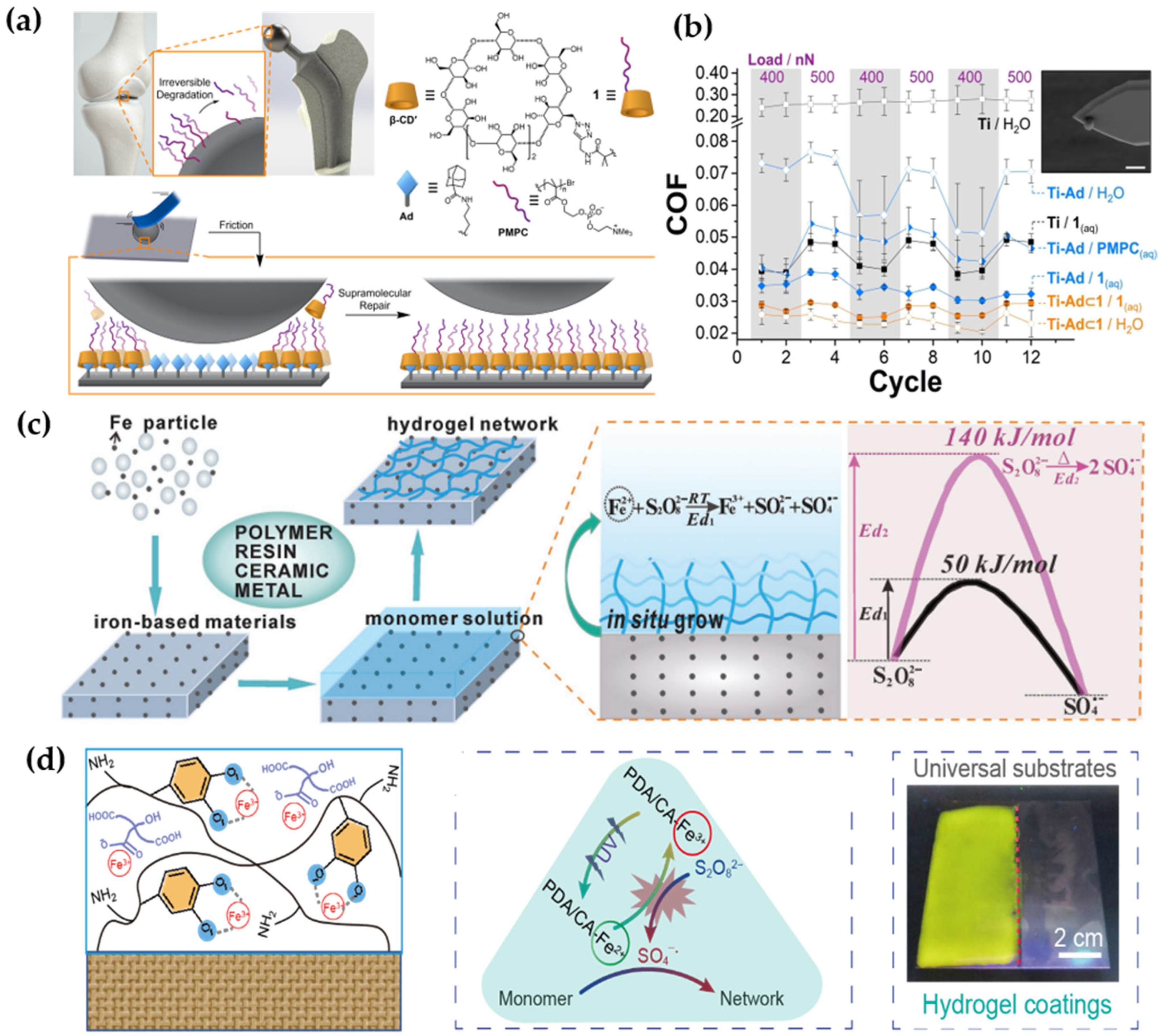
4.2.2. Interfacial Physical Modification
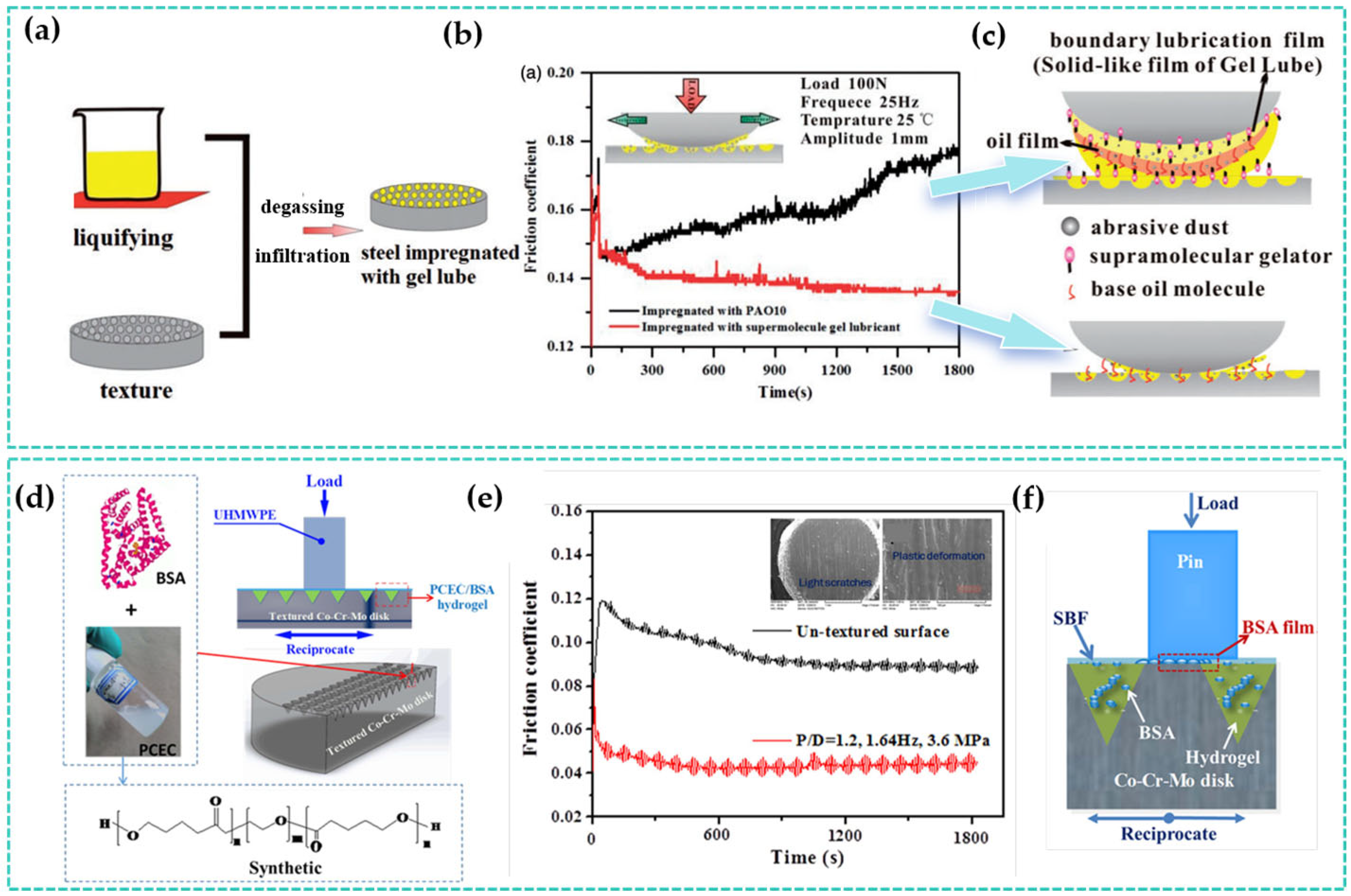
5. Summary and Outlook
- (1)
- The research lacks unified standard guidance and has not formed a systematic theoretical framework. Current research focuses on experiments with limited theoretical foundations, making it difficult to quantitatively predict friction characteristics based on hydrogel microstructures and tribological conditions.
- (2)
- The preparation of gels is difficult to industrialize. The preparation of high-quality hydrogel lubricants may involve complex cross-linking processes and precise condition control, which could increase production costs, limiting large-scale production.
- (3)
- The conducted research is mostly based on overly idealized laboratory conditions. Although the lubrication performance can be significantly improved by regulating the components and structure, issues related to long-term stability, environmental tolerance, and eco-friendliness are selectively ignored. Many studies may fail rapidly under real and complex working conditions, resulting in a low rate of successful application.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Hassan, M.; Zulkifli, S.A.; Hasnul, H.; Yusoff, A. Tribological advancement–strategies and effects towards emissions and global energy consumption. In Proceedings of the MATEC Web of Conferences, Abu Dhabi, United Arab Emirates, 20–22 November 2018; p. 00003. [Google Scholar] [CrossRef]
- Ji, F.; Zhao, W.; Liu, S. Study on Modes and Properties of Oil Supply for Space Liquid Lubrication System. Adv. Sci. Lett. 2011, 4, 2137–2141. [Google Scholar] [CrossRef]
- Li, F.; Ma, Y.; Chen, L.; Li, H.; Zhou, H.; Chen, J. In-situ polymerization of polyurethane/aniline oligomer functionalized graphene oxide composite coatings with enhanced mechanical, tribological and corrosion protection properties. Chem. Eng. J. 2021, 425, 130006. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Zheng, R.; Du, C.; Yu, T.; Li, K.; Bu, W.; Wang, D. Macroscale Superlubrication Achieved with Shear-Thinning Semisolid Lubricants. Adv. Mater. 2024, 36, e2412257. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Kantaros, A.; Ganetsos, T.; Petrescu, F.I.T. Transforming object design and creation: Biomaterials and contemporary manufacturing leading the way. Biomimetics 2024, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A.; Ganetsos, T. From static to dynamic: Smart materials pioneering additive manufacturing in regenerative medicine. Int. J. Mol. Sci. 2023, 24, 15748. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Y.; Yu, W.; Xu, L. Hydrogels for Lubrication: Synthesis, Properties, Mechanism, and Challenges. Lubricants 2024, 12, 186. [Google Scholar] [CrossRef]
- Son, D.; Hwang, H.; Fontenot, J.F.; Lee, C.; Jung, J.P.; Kim, M. Tailoring physical properties of dual-network acrylamide hydrogel composites by engineering molecular structures of the cross-linked network. ACS Omega 2022, 7, 30028–30039. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Hydration lubrication in biomedical applications: From cartilage to hydrogels. Acc. Mater. Res. 2022, 3, 213–223. [Google Scholar] [CrossRef]
- Ma, Q.; Qi, P.; Dong, G. An experimental and molecular dynamics study of the superlubricity enabled by hydration lubrication. Appl. Surf. Sci. 2021, 553, 149590. [Google Scholar] [CrossRef]
- Qin, C.; Yang, H.; Lu, Y.; Li, B.; Ma, S.; Ma, Y.; Zhou, F. Tribology in Nature: Inspirations for Advanced Lubrication Materials. Adv. Mater. 2025, 2420626. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, T.; Espinosa-Marzal, R.M. Advances in understanding hydrogel lubrication. Colloids Interfaces 2020, 4, 54. [Google Scholar] [CrossRef]
- Wang, B.X.; Xu, W.; Yang, Z.; Wu, Y.; Pi, F. An overview on recent progress of the hydrogels: From material resources, properties, to functional applications. Macromol. Rapid Commun. 2022, 43, 2100785. [Google Scholar] [CrossRef]
- Hu, D.; Liu, D.; Hu, Y.; Wang, Y.; Lu, Y.; Bai, C.; Hossain, K.R.; Jiang, P.; Wang, X. Dual-physical network PVA hydrogel commensurate with articular cartilage bearing lubrication enabled by harnessing nanoscale crystalline domains. Nano Res. 2024, 17, 9784–9795. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.; Vélaz, I.; Peñas, F.; Zavala-Rivera, P.; Rosas-Durazo, A.; Maldonado-Arce, A.; Martínez-Barbosa, M. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydr. Polym. 2018, 195, 476–485. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Wang, J.; Guo, H.; Chen, Y.; Wang, N. Research on the thermal aging mechanism of polyvinyl alcohol hydrogel. Polymers 2024, 16, 2486. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Wei, L.; Bai, X.; Yuan, C.; Guo, Z.; Yang, Y. Environmentally Responsive Hydrogels and Composites Containing Hydrogels as Water-Based Lubricants. Gels 2025, 11, 526. [Google Scholar] [CrossRef]
- Kantaros, A. 3D printing in regenerative medicine: Technologies and resources utilized. Int. J. Mol. Sci. 2022, 23, 14621. [Google Scholar] [CrossRef] [PubMed]
- Protsak, I.S.; Morozov, Y.M. Fundamentals and Advances in Stimuli-Responsive Hydrogels and Their Applications: A Review. Gels 2025, 11, 30. [Google Scholar] [CrossRef]
- Langer, K.; Joensson, H.N. Rapid production and recovery of cell spheroids by automated droplet microfluidics. SLAS Technol. Transl. Life Sci. Innov. 2020, 25, 111–122. [Google Scholar] [CrossRef]
- Unadkat, H.V. Materiomics: Deciphering Topographic Cues for Cell-Surface Interactions; University of Twente: Enschede, The Netherlands, 2012. [Google Scholar]
- Gao, H.-L.; Lu, Y.; Mao, L.-B.; An, D.; Xu, L.; Gu, J.-T.; Long, F.; Yu, S.-H. A shape-memory scaffold for macroscale assembly of functional nanoscale building blocks. Mater. Horiz. 2014, 1, 69–73. [Google Scholar] [CrossRef]
- Fu, A.; Gwon, K.; Kim, M.; Tae, G.; Kornfield, J.A. Visible-light-initiated thiol–acrylate photopolymerization of heparin-based hydrogels. Biomacromolecules 2015, 16, 497–506. [Google Scholar] [CrossRef]
- Lin, X.; Wang, X.; Zeng, L.; Wu, Z.L.; Guo, H.; Hourdet, D. Stimuli-responsive toughening of hydrogels. Chem. Mater. 2021, 33, 7633–7656. [Google Scholar] [CrossRef]
- Chen, W.; Kumari, J.; Yuan, H.; Yang, F.; Kouwer, P.H. Toward tissue-like material properties: Inducing in situ adaptive behavior in fibrous hydrogels. Adv. Mater. 2022, 34, 2202057. [Google Scholar] [CrossRef] [PubMed]
- Michalicha, A.; Belcarz, A.; Giannakoudakis, D.A.; Staniszewska, M.; Barczak, M. Designing composite stimuli-responsive hydrogels for wound healing applications: The state-of-the-art and recent discoveries. Materials 2024, 17, 278. [Google Scholar] [CrossRef]
- Knipe, J.M.; Peppas, N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen. Biomater. 2014, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Liang, J.; Wang, Z.; Lin, F.; Zhuang, Y.; Saiding, Q.; Wang, F.; Deng, L.; Cui, W. Motion lubrication suppressed mechanical activation via hydrated fibrous gene patch for tendon healing. Sci. Adv. 2023, 9, eadc9375. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Q.; Fang, Z.; Gu, L.; Bian, L. Structurally dynamic hydrogels for biomedical applications: Pursuing a fine balance between macroscopic stability and microscopic dynamics. Chem. Rev. 2021, 121, 11149–11193. [Google Scholar] [CrossRef]
- Sun, M.; Li, H.; Hou, Y.; Huang, N.; Xia, X.; Zhu, H.; Xu, Q.; Lin, Y.; Xu, L. Multifunctional tendon-mimetic hydrogels. Sci. Adv. 2023, 9, eade6973. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sorkin, R.; Kampf, N.; Dror, Y.; Shimoni, E.; Klein, J. Origins of extreme boundary lubrication by phosphatidylcholine liposomes. Biomaterials 2013, 34, 5465–5475. [Google Scholar] [CrossRef]
- Petelska, A.D.; Kazimierska-Drobny, K.; Janicka, K.; Majewski, T.; Urbaniak, W. Understanding the unique role of phospholipids in the lubrication of natural joints: An interfacial tension study. Coatings 2019, 9, 264. [Google Scholar] [CrossRef]
- Lin, W.; Kluzek, M.; Iuster, N.; Shimoni, E.; Kampf, N.; Goldberg, R.; Klein, J. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science 2020, 370, 335–338. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Y.; Shen, J.; Cai, Z.; Zhao, C.; Chen, H.; Luo, X.; Hu, N.; Cui, W.; Huang, W. Injectable hydrogel microspheres with self-renewable hydration layers alleviate osteoarthritis. Sci. Adv. 2022, 8, eabl6449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, P.; Li, B.; Yang, S.; Zhao, T.; Meng, Y.; Li, Q.; Hao, J.; Wang, X. Innovating Lubrication with Polyelectrolyte Hydrogels: Sustained Performance Through Lipid Dynamics. Adv. Funct. Mater. 2025, 35, 2413712. [Google Scholar] [CrossRef]
- Cheng, R.; Yan, Y.; Liu, H.; Chen, H.; Pan, G.; Deng, L.; Cui, W. Mechanically enhanced lipo-hydrogel with controlled release of multi-type drugs for bone regeneration. Appl. Mater. Today 2018, 12, 294–308. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, J.; Huang, X.; Kang, W.; Zhou, G. A robust, low swelling, and lipid-lubricated hydrogel for bionic articular cartilage substitute. J. Colloid Interface Sci. 2023, 629, 467–477. [Google Scholar] [CrossRef]
- Dellatolas, I.; Bantawa, M.; Damerau, B.; Guo, M.; Divoux, T.; Del Gado, E.; Bischofberger, I. Local mechanism governs global reinforcement of nanofiller-hydrogel composites. ACS Nano 2023, 17, 20939–20948. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene oxide–chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Hu, F.; Lu, H.; Ye, Z.; Zhang, S.; Wang, W.; Gao, L. Slow-release lubrication of artificial joints using self-healing polyvinyl alcohol/polyethylene glycol/graphene oxide hydrogel. J. Mech. Behav. Biomed. Mater. 2021, 124, 104807. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Ye, L.; Coates, P.; Twigg, P. In situ cross-linking of poly (vinyl alcohol)/graphene oxide–polyethylene glycol nanocomposite hydrogels as artificial cartilage replacement: Intercalation structure, unconfined compressive behavior, and biotribological behaviors. J. Phys. Chem. C 2018, 122, 3157–3167. [Google Scholar] [CrossRef]
- He, X.; Cui, C.; Chen, Y.; Zhang, L.; Sheng, X.; Xie, D. MXene and polymer collision: Sparking the future of high-performance multifunctional coatings. Adv. Funct. Mater. 2024, 34, 2409675. [Google Scholar] [CrossRef]
- Oh, T.; Lee, S.; Kim, H.; Ko, T.Y.; Kim, S.J.; Koo, C.M. Fast and High-Yield Anhydrous Synthesis of Ti3C2Tx MXene with High Electrical Conductivity and Exceptional Mechanical Strength. Small 2022, 18, 2203767. [Google Scholar] [CrossRef]
- Miao, X.; Li, Z.; Liu, S.; Wang, J.; Yang, S. MXenes in tribology: Current status and perspectives. Adv. Powder Mater. 2023, 2, 100092. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, T.; Hao, X.; Liu, S.; Zou, Y.; Li, J.; Wu, W.; Chen, L.; Liu, X. Aramid Nanofiber/MXene-Reinforced Polyelectrolyte Hydrogels for Absorption-Dominated Electromagnetic Interference Shielding and Wearable Sensing. Nano-Micro Lett. 2025, 17, 271. [Google Scholar] [CrossRef]
- Hu, L.; Yu, X.; Hao, J.; Xu, L. pH-and near-infrared light-responsive, biomimetic hydrogels from aqueous dispersions of carbon nanotubes. Nano Res. 2024, 17, 3120–3129. [Google Scholar] [CrossRef]
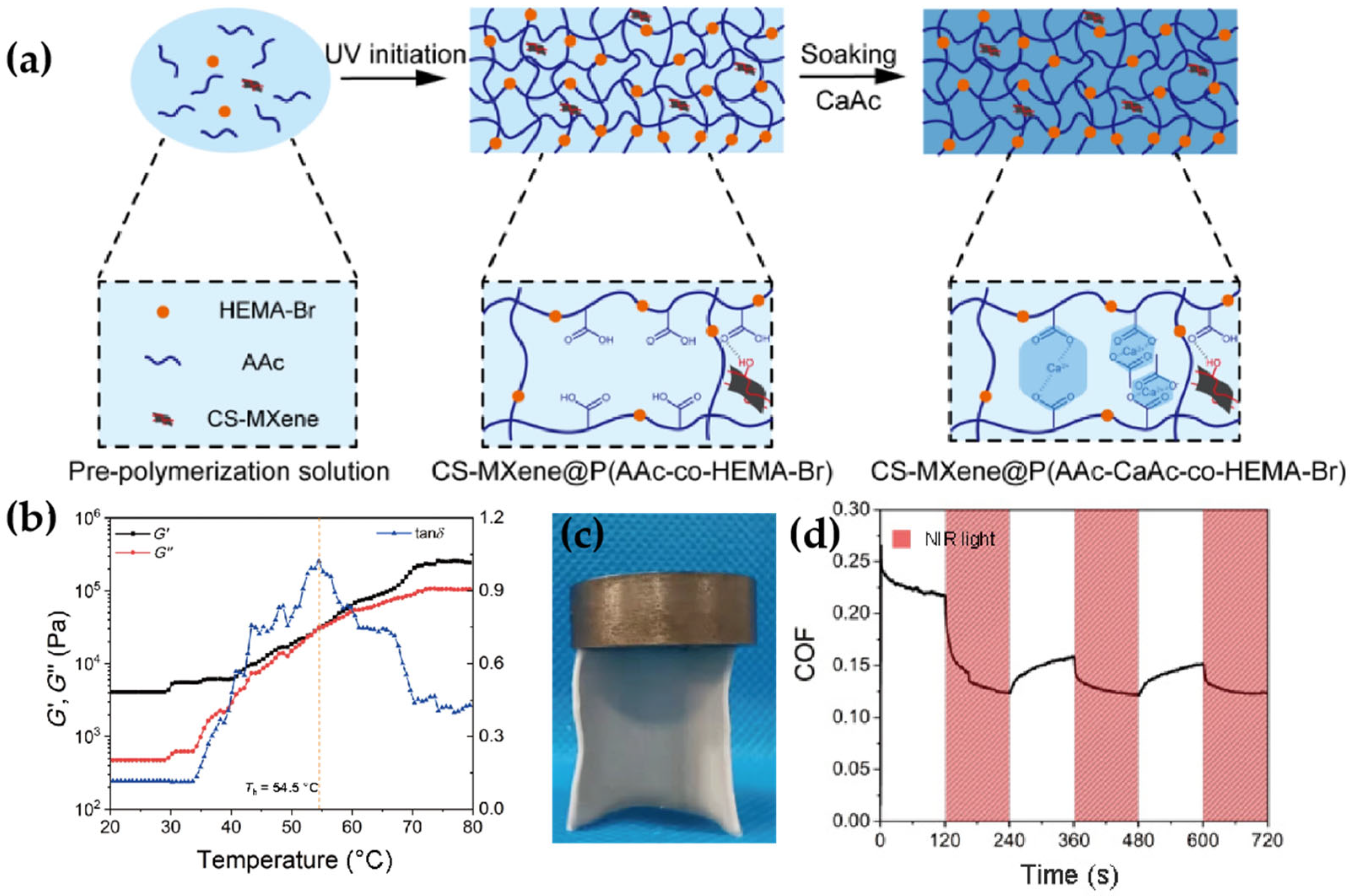
- Huang, Y.; Li, Z.; Wang, Y.; Gao, Q.; Hou, K.; Liu, S.; Wang, J.; Yang, S. Injectable and Self-Healing MXene-Reinforced pH-Responsive Hydrogel: Realizing Low-Friction and Durable Lubrication. ACS Sustain. Chem. Eng. 2024, 12, 18679–18690. [Google Scholar] [CrossRef]
- Miao, X.; Li, Z.; Hou, K.; Gao, Q.; Huang, Y.; Wang, J.; Yang, S. Bioinspired multi-crosslinking and solid–liquid composite lubricating MXene/PVA hydrogel based on salting out effect. Chem. Eng. J. 2023, 476, 146848. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, G.; Wu, P.; Guo, J.; Liu, X.; Zhou, F.; Liu, W. Controlling interfacial lubrication: Modulating MXene-based hydrogel properties with near-infrared light. Friction 2025, 13, 9440915. [Google Scholar] [CrossRef]
- Ma, S.; Scaraggi, M.; Lin, P.; Yu, B.; Wang, D.; Dini, D.; Zhou, F. Nanohydrogel Brushes for Switchable Underwater Adhesion. J. Phys. Chem. C 2017, 121, 8452–8463. [Google Scholar] [CrossRef]
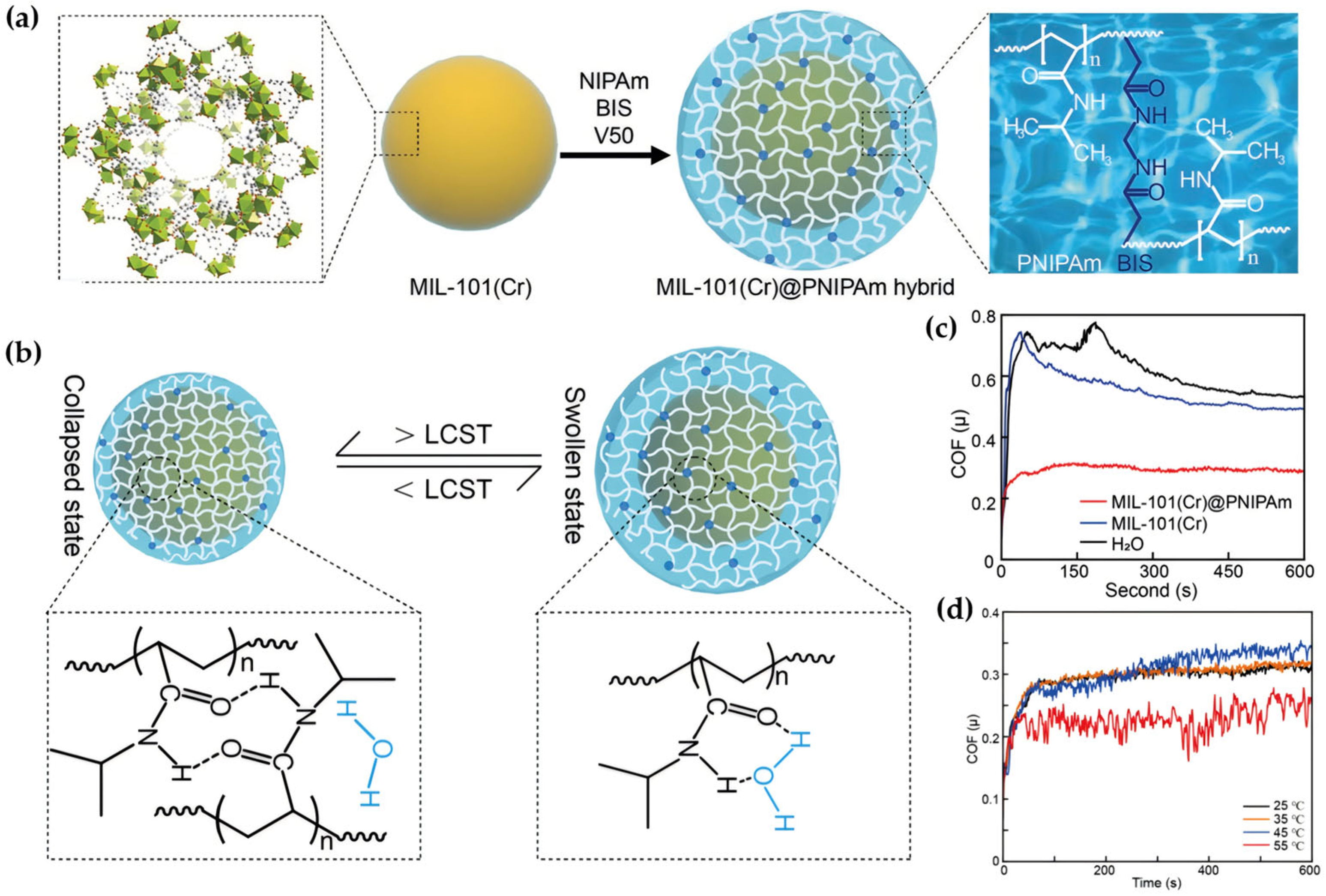
- Wu, W.; Liu, J.; Gong, P.; Li, Z.; Ke, C.; Qian, Y.; Luo, H.; Xiao, L.; Zhou, F.; Liu, W. Construction of Core-Shell NanoMOFs@microgel for Aqueous Lubrication and Thermal-Responsive Drug Release. Small 2022, 18, 2202510. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Liao, Z.; Zhang, R.; Song, S.; Yang, Y.; Xie, D.; Liu, X.; Wei, L.; Liu, Y.; Song, Y. Cellulose nanofibers/liquid metal hydrogels with high tensile strength, environmental adaptability and electromagnetic shielding for temperature monitoring and strain sensors. Carbohydr. Polym. 2025, 348, 122788. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, Y.; Luo, J. Macroscale superlubricity achieved between zwitterionic copolymer hydrogel and sapphire in water. Mater. Des. 2020, 188, 108441. [Google Scholar] [CrossRef]
- Hou, J.; Lin, Y.; Zhu, C.; Chen, Y.; Lin, R.; Lin, H.; Liu, D.; Guan, D.; Yu, B.; Wang, J.; et al. Zwitterion-Lubricated Hydrogel Microspheres Encapsulated with Metformin Ameliorate Age-Associated Osteoarthritis. Adv. Sci. 2024, 11, 2402477. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, V.K.; Singh, M. Zwitterionic polyelectrolytes: A review. e-Polymers 2007, 7, 030. [Google Scholar] [CrossRef]
- Raviv, U.; Giasson, S.; Kampf, N.; Gohy, J.-F.; Jérôme, R.; Klein, J. Lubrication by charged polymers. Nature 2003, 425, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-w.; Wang, J.; Yu, Y.; Yu, L.; Wang, Y.-x.; Ren, K.-f.; Ji, J. A polyzwitterion-based antifouling and flexible bilayer hydrogel coating. Compos. Part B Eng. 2022, 244, 110164. [Google Scholar] [CrossRef]
- Li, S.; Bai, Y.; Liu, X.; Zhang, Y.; Tang, Y.; Zhao, F.; Li, Q.; Guo, Z.; Feng, Z.; Dong, A. Bio-inspired robust, superhydrophilic and superlubric artificial vascular endothelium coating for anti-thromboinflammation on blood-contacting devices. Compos. Part B Eng. 2023, 257, 110670. [Google Scholar] [CrossRef]
- Du, W.; Sun, S.; Zhao, Z.; Zhao, B.; Zhang, X. Controllable transformation of UCST and LCST behaviors in polyampholyte hydrogels enabled by an association–disassociation theory-based switch mechanism. Mater. Horiz. 2025, 12, 587–598. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Zhang, Y.; Zhao, W.; Zhao, X.; Liu, Y.; Yu, B.; Ma, S.; Zhou, F. Scalable Preparation of Polyzwitterionic Hydrogels Based on Hydration Shielding-Accelerated Redox Self-Catalytic Polymerization (HS-A-RP). Angew. Chem. 2025, 64, e202424129. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.D.; Joiner, C.S.; Stoddart, J.F. Template-directed synthesis employing reversible imine bond formation. Chem. Soc. Rev. 2007, 36, 1705–1723. [Google Scholar] [CrossRef] [PubMed]
- Rollas, S.; Güniz Küçükgüzel, Ş. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef]
- Apostolides, D.E.; Patrickios, C.S. Dynamic covalent polymer hydrogels and organogels crosslinked through acylhydrazone bonds: Synthesis, characterization and applications. Polym. Int. 2018, 67, 627–649. [Google Scholar] [CrossRef]
- Pettignano, A.; Grijalvo, S.; Haering, M.; Eritja, R.; Tanchoux, N.; Quignard, F.; Díaz, D.D. Boronic acid-modified alginate enables direct formation of injectable, self-healing and multistimuli-responsive hydrogels. Chem. Commun. 2017, 53, 3350–3353. [Google Scholar] [CrossRef]
- Du, P.; Wu, M.; Liu, X.; Zheng, Z.; Wang, X.; Sun, P.; Joncheray, T.; Zhang, Y. Synthesis of linear polyurethane bearing pendant furan and cross-linked healable polyurethane containing Diels–Alder bonds. New J. Chem. 2014, 38, 770–776. [Google Scholar] [CrossRef]
- Li, D.-q.; Wang, S.-y.; Meng, Y.-j.; Guo, Z.-w.; Cheng, M.-m.; Li, J. Fabrication of self-healing pectin/chitosan hybrid hydrogel via Diels-Alder reactions for drug delivery with high swelling property, pH-responsiveness, and cytocompatibility. Carbohydr. Polym. 2021, 268, 118244. [Google Scholar] [CrossRef]
- Lu, H.; Wang, X.; Hao, J. Research progress on transient hydrogels. Chin. Sci. Bull. 2021, 66, 1733–1745. [Google Scholar] [CrossRef]
- Hou, S.; Wang, X.; Park, S.; Jin, X.; Ma, P.X. Rapid self-integrating, injectable hydrogel for tissue complex regeneration. Adv. Healthc. Mater. 2015, 4, 1491. [Google Scholar] [CrossRef]
- Xu, J.; Ren, X.; Gao, G. Salt-inactive hydrophobic association hydrogels with fatigue resistant and self-healing properties. Polymer 2018, 150, 194–203. [Google Scholar] [CrossRef]
- Sun, C.; Jia, H.; Lei, K.; Zhu, D.; Gao, Y.; Zheng, Z.; Wang, X. Self-healing hydrogels with stimuli responsiveness based on acylhydrazone bonds. Polymer 2019, 160, 246–253. [Google Scholar] [CrossRef]
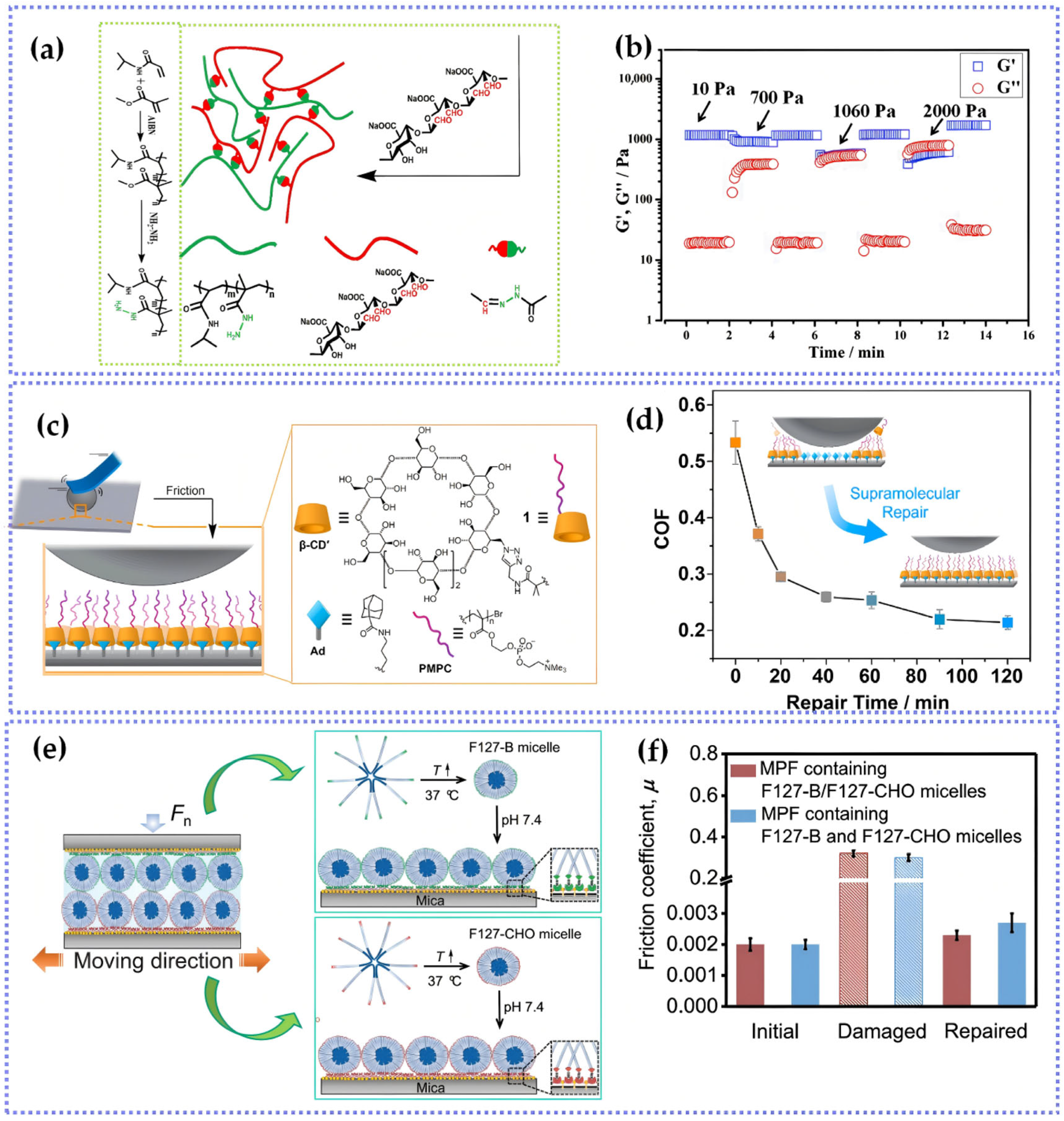
- Wang, Y.; Sun, Y.; Avestro, A.-J.; McGonigal, P.R.; Zhang, H. Supramolecular repair of hydration lubrication surfaces. Chem 2022, 8, 480–493. [Google Scholar] [CrossRef]
- Xiang, L.; Zhang, J.; Wang, W.; Wei, Z.; Chen, Y.; Zeng, H. Targeted Repair of Super-Lubricating Surfaces via Pairing Click Chemistry. Adv. Funct. Mater. 2023, 33, 2301593. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Jin, H.; Wang, S.; Song, W. Bioinspired supramolecular lubricating hydrogel induced by shear force. J. Am. Chem. Soc. 2018, 140, 3186–3189. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, K.; Yang, X.; Wang, J.; Liu, H.; Song, W.; Wang, S. Supramolecular Semi-Convertible Hydrogel Enabled Self-Healing Responsive Lubrication under Dynamic Shearing. CCS Chem. 2023, 5, 2482–2496. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, X.; Wang, X.; Liang, Y.; Liu, W.; Zhou, F. Biomimicking lubrication superior to fish skin using responsive hydrogels. NPG Asia Mater. 2014, 6, e136. [Google Scholar] [CrossRef]
- Wan, T.; Xu, M.; Chen, L.; Wu, D.; Cheng, W.; Li, R.; Zou, C. Synthesis and properties of a dual responsive hydrogel by inverse microemulsion polymerization. J. Chem. Sci. 2014, 126, 1623–1627. [Google Scholar] [CrossRef]
- Jahn, S.; Seror, J.; Klein, J. Lubrication of articular cartilage. Annu. Rev. Biomed. Eng. 2016, 18, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhao, W.; Zhang, Y.; Zhang, X.; Ma, Z.; Wang, R.; Wei, Q.; Ma, S.; Zhou, F. Recent progress of bioinspired cartilage hydrogel lubrication materials. Biosurf. Biotribol. 2022, 8, 225–243. [Google Scholar] [CrossRef]
- Morgese, G.; Benetti, E.M.; Zenobi-Wong, M. Molecularly engineered biolubricants for articular cartilage. Adv. Healthc. Mater. 2018, 7, 1701463. [Google Scholar] [CrossRef]
- Wang, X.; Lin, J.; Li, Z.; Ma, Y.; Zhang, X.; He, Q.; Wu, Q.; Yan, Y.; Wei, W.; Yao, X. Identification of an ultrathin osteochondral interface tissue with specific nanostructure at the human knee joint. Nano Lett. 2022, 22, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Furey, M.J.; Burkhardt, B.M. Biotribology: Friction, wear, and lubrication of natural synovial joints. Lubr. Sci. 1997, 9, 255–271. [Google Scholar] [CrossRef]
- Walker, P.; Dowson, D.; Longfield, M.; Wright, V. Lubrication of human joints. Ann. Rheum. Dis. 1969, 28, 194. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, K.; Tang, C.; Liu, Z.; Fan, J.; Qin, G.; Cui, W.; Zhu, L.; Chen, Q. Recent progress in double network elastomers: One plus one is greater than two. Adv. Funct. Mater. 2022, 32, 2110244. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.-N.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-strong and tough poly (vinyl alcohol)/poly (acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 6, 8105–8114. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Chen, H.; Yan, H.; Huang, L.; Yang, J.; Zheng, J. A novel design strategy for fully physically linked double network hydrogels with tough, fatigue resistant, and self-healing properties. Adv. Funct. Mater. 2015, 25, 1598–1607. [Google Scholar] [CrossRef]
- Feng, H.; Wang, S.; Chen, K.; Zhang, X.; Feng, C.; Li, X.; Zhang, D. Dual-network nanocomposite robust hydrogel with excellent durability properties as cartilage replacement. Tribol. Int. 2024, 194, 109518. [Google Scholar] [CrossRef]
- Shi, F.-K.; Zhong, M.; Zhang, L.Q.; Liu, X.Y.; Xie, X.M. Robust and self-healable nanocomposite physical hydrogel facilitated by the synergy of ternary crosslinking points in a single network. J. Mater. Chem. B 2016, 4, 6221–6227. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Zhao, X.; Yu, B.; Zhang, L.; Ma, S.; Zhou, F. Superior anti-swelling and durably lubricious bio-hydrogels via robust crystalline domain construction for diverse biodevice coating. Matter 2025, 102317, in press. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Y.; Li, C.; Guan, G.; Wang, F.; Gao, J.; Wang, L. Gradient scaffolds in bone-soft tissue interface engineering: Structural characteristics, fabrication techniques, and emerging trends. J. Orthop. Translat. 2025, 50, 333–353. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, S.; Zhou, J.; Chen, W.; Liu, T.; Yang, S.; Long, S.; Li, X. Achieving swollen yet strengthened hydrogels by reorganizing multiphase network structure. Adv. Funct. Mater. 2023, 33, 2213549. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, H.; Yang, H.-B.; Yao, X.; Qin, H.; Cong, H.-P.; Yu, S.-H. Hierarchically aligned heterogeneous core-sheath hydrogels. Nat. Commun. 2025, 16, 400. [Google Scholar] [CrossRef]
- Hasan, M.M.; Johnson, C.L.; Dunn, A.C. Soft contact mechanics with gradient-stiffness surfaces. Langmuir 2022, 38, 9454–9465. [Google Scholar] [CrossRef]
- Chen, F.; Li, X.; Yu, Y.; Li, Q.; Lin, H.; Xu, L.; Shum, H.C. Phase-separation facilitated one-step fabrication of multiscale heterogeneous two-aqueous-phase gel. Nat. Commun. 2023, 14, 2793. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Chen, K.; Feng, C.; Wang, D.; Qi, J.; Li, X.; Zhao, X.; Chai, Z.; Zhang, D. Bilayer hydrogels with low friction and high load-bearing capacity by mimicking the oriented hierarchical structure of cartilage. ACS Appl. Mater. Interfaces 2022, 14, 52347–52358. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Yu, B.; Ma, S.; Yang, H.; Liu, L.; Yu, J.; Pei, X.; Cai, M.; Zhou, F. Vertical and Horizontal Double Gradient Design for Super-Slippery and High-Bearing Hydrogel Skeleton. Adv. Funct. Mater. 2024, 34, 2400360. [Google Scholar] [CrossRef]
- Qu, M.; Liu, H.; Yan, C.; Ma, S.; Cai, M.; Ma, Z.; Zhou, F. Layered hydrogel with controllable surface dissociation for durable lubrication. Chem. Mater. 2020, 32, 7805–7813. [Google Scholar] [CrossRef]
- Ma, S.; Liu, L.; Zhao, W.; Li, R.; Zhao, X.; Zhang, Y.; Yu, B.; Liu, Y.; Zhou, F. Earthworm inspired lubricant self-pumping hydrogel with sustained lubricity at high loading. Nat. Commun. 2025, 16, 398. [Google Scholar] [CrossRef]
- Cosimi, A.; Stöbener, D.D.; Nickl, P.; Schusterbauer, R.; Donskyi, I.S.; Weinhart, M. Interfacial Nanoengineering of Hydrogel Surfaces via Block Copolymer Self-Assembly. ACS Appl. Mater. Interfaces 2025, 17, 10073–10086. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016, 15, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, Y.; Ran, M.; Chen, H.; Han, J.; Zhai, J.; Wang, Z.; Ning, C.; Shi, Z.; Yu, P. Hydrogel coatings of implants for pathological bone repair. Adv. Healthc. Mater. 2024, 13, 2401296. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, J.; Yang, C.; Yang, X.; Wei, J.; Xia, Y.; Gong, X.; Suo, Z. Hydrogel paint. Adv. Mater. 2019, 31, 1903062. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Deng, Z.; Chen, Y.; Yu, Q.; Tang, W.; Sun, T.L.; Zhang, Y.S.; Yue, K. Tough bonding, on-demand debonding, and facile rebonding between hydrogels and diverse metal surfaces. Adv. Mater. 2019, 31, 1904732. [Google Scholar] [CrossRef]
- Imbia, A.S.; Ounkaew, A.; Mao, X.; Zeng, H.; Liu, Y.; Narain, R. Mussel-inspired polymer-based coating technology for antifouling and antibacterial properties. Langmuir 2024, 40, 10957–10965. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Han, X.; Pan, Y.; Wang, P.; Wang, T.; Lu, T. A universal strategy for tough adhesion of wet soft material. Adv. Funct. Mater. 2020, 30, 2003207. [Google Scholar] [CrossRef]
- Yang, Z.; He, Y.; Liao, S.; Ma, Y.; Tao, X.; Wang, Y. Renatured hydrogel painting. Sci. Adv. 2021, 7, eabf9117. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, E.; Öğütcü, M. The texture, sensory properties and stability of cookies prepared with wax oleogels. Food Funct. 2015, 6, 1194–1204. [Google Scholar] [CrossRef]
- Jang, A.; Bae, W.; Hwang, H.-S.; Lee, H.G.; Lee, S. Evaluation of canola oil oleogels with candelilla wax as an alternative to shortening in baked goods. Food Chem. 2015, 187, 525–529. [Google Scholar] [CrossRef]
- Iwanaga, K.; Sumizawa, T.; Miyazaki, M.; Kakemi, M. Characterization of organogel as a novel oral controlled release formulation for lipophilic compounds. Int. J. Pharm. 2010, 388, 123–128. [Google Scholar] [CrossRef]
- Borgheti-Cardoso, L.N.; Kooijmans, S.A.; Fens, M.H.; Van der Meel, R.; Vicentini, F.T.; Fantini, M.C.; Bentley, M.V.L.; Schiffelers, R.M. In situ gelling liquid crystalline system as local siRNA delivery system. Mol. Pharm. 2017, 14, 1681–1690. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, H.; Meng, J.; Yang, G.; Liu, X.; Wang, S.; Jiang, L. Grooved organogel surfaces towards anisotropic sliding of water droplets. Adv. Mater. 2014, 26, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Rajarethinem, P.S.; Grędowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W.H.; Van de Walle, D.; Dewettinck, K. Edible applications of shellac oleogels: Spreads, chocolate paste and cakes. Food Funct. 2014, 5, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Öǧütcü, M.; Yılmaz, E. Oleogels of virgin olive oil with carnauba wax and monoglyceride as spreadable products. Grasas Y Aceites 2014, 65, e040. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, J.; Lee, J.; Hwang, H.S.; Lee, S. Utilization of oleogels as a replacement for solid fat in aerated baked goods: Physicochemical, rheological, and tomographic characterization. J. Food Sci. 2017, 82, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Moriano, M.E.; Alamprese, C. Organogels as novel ingredients for low saturated fat ice creams. LWT 2017, 86, 371–376. [Google Scholar] [CrossRef]
- Panagiotopoulou, E.; Moschakis, T.; Katsanidis, E. Sunflower oil organogels and organogel-in-water emulsions (part II): Implementation in frankfurter sausages. LWT 2016, 73, 351–356. [Google Scholar] [CrossRef]
- Ma, S.; Yan, C.; Cai, M.; Yang, J.; Wang, X.; Zhou, F.; Liu, W. Continuous surface polymerization via Fe(II)-mediated redox reaction for thick hydrogel coatings on versatile substrates. Adv. Mater. 2018, 30, 1803371. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Ma, S.; Ma, Z.; Yu, B.; Cai, M.; Zhou, F. A universal strategy for growing a tenacious hydrogel coating from a sticky initiation layer. Adv. Mater. 2022, 34, 2108889. [Google Scholar] [CrossRef]
- Sato, T.; Dunderdale, G.J.; Urata, C.; Hozumi, A. Sol–gel preparation of initiator layers for surface-initiated ATRP: Large-scale formation of polymer brushes is not a dream. Macromolecules 2018, 51, 10065–10073. [Google Scholar] [CrossRef]
- Xian, C.; Yuan, Q.; Bao, Z.; Liu, G.; Wu, J. Progress on intelligent hydrogels based on RAFT polymerization: Design strategy, fabrication and the applications for controlled drug delivery. Chin. Chem. Lett. 2020, 31, 19–27. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, J.; Yang, F.; Xu, H.; Guo, G.; Wang, Y. Enzyme-Immobilized Surface-Catalyzed Cross-Linking: Creating Multifunctional Double Network Hydrogel Coatings on Diverse Substrates. Adv. Funct. Mater. 2024, 34, 2312465. [Google Scholar] [CrossRef]
- Yu, Q.; Ma, Z.; Cai, M.; Zhou, F.; Liu, W. Tribological behavior of laser textured steel impregnated with supramolecular gel lubricant. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2017, 231, 1151–1159. [Google Scholar] [CrossRef]
- Guo, J.; Mei, T.; Li, Y.; Ren, S.; Hafezi, M.; Lu, H.; Li, Y.; Dong, G. Sustained-release application of PCEC hydrogel on laser-textured surface lubrication. Mater. Res. Express 2018, 5, 065315. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, Y.; An, K.; Ni, C.; Zhang, H.; Ren, Y.; Yang, Z. Advances on Hydrogel Lubrication Modification Under Diverse Design Strategies. Lubricants 2025, 13, 373. https://doi.org/10.3390/lubricants13090373
Xu Y, Wang Y, An K, Ni C, Zhang H, Ren Y, Yang Z. Advances on Hydrogel Lubrication Modification Under Diverse Design Strategies. Lubricants. 2025; 13(9):373. https://doi.org/10.3390/lubricants13090373
Chicago/Turabian StyleXu, Ying, Youqiang Wang, Kai An, Chenbing Ni, Haiyang Zhang, Yibing Ren, and Ziyi Yang. 2025. "Advances on Hydrogel Lubrication Modification Under Diverse Design Strategies" Lubricants 13, no. 9: 373. https://doi.org/10.3390/lubricants13090373
APA StyleXu, Y., Wang, Y., An, K., Ni, C., Zhang, H., Ren, Y., & Yang, Z. (2025). Advances on Hydrogel Lubrication Modification Under Diverse Design Strategies. Lubricants, 13(9), 373. https://doi.org/10.3390/lubricants13090373








